Abstract
Background: Haplo-HSCT after depletion of α/β T and B cells is a suitable and effective option for those children with acute leukemia (AL) who need an allograft and lacking an immediately available HLA-identical donor. With this approach, recipients can benefit immediately after transplantation from the anti-leukemia effect mediated by donor natural killer (NK) and γd T cells, which can also protect against infections. A further improvement of the results achievable with this platform could achieved with a faster adaptive T-cell immunity recovery, which play a key role to augment the graft-versus-leukemia effect and the capacity to fight infections. In light of these considerations, we designed a phase I/II trial aimed at testing the safety and efficacy of post-transplant infusion of donor-derived T cells transduced with the new iC9 suicide gene (BPX-501) in children with either malignant or non-malignant disorders (NCT02065869). Remarkably, after the activation and transduction with the retroviral iC9 construct, BPX501 cells switch the phenotype towards a preferential CD45RO pattern.
Patients and methods: The phase I portion of the trial consisted of a classical 3+3 design with 3 cohorts, receiving escalating doses of BPX-501 cells of 2.5x105, 5x105, and 1x106 cells/kg, respectively. Patients included in the phase II portion were planned to receive the recommended dose identified during the phase I part of the study.Enrollment of patients started in December 2014; so far, 25 patients with AL in morphological complete remission (CR) have been enrolled. Twenty patients had acute lymphoblastic leukemia (ALL) and 5 acute myeloid leukemia (AML). Details on patient, donor and transplant characteristics are reported in table 1. All patients transplanted in CR1 had either poor cytogenetic/molecular characteristics or high levels of minimal residual disease at the end of induction therapy, both factors predicting a high relapse rate. All patients were given a fully myeloablative conditioning regimen (table 1). Before haplo-HSCT, children received rabbit anti-thymocyte globulin (ATG NEOVII, 12 mg/Kg over 3 days, from day -4 to day -2) to prevent both graft-versus-host disease (GvHD) and graft failure, and Rituximab (200 mg/ m2 on day -1) to prevent EBV-related lymphoproliferative disorders. No post-transplantation GvHD prophylaxis was administered.
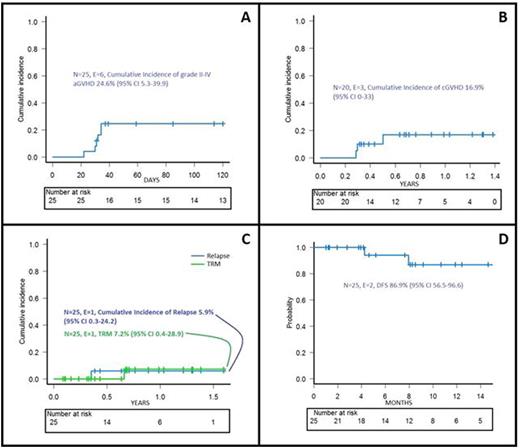
Results: All patients engrafted and no secondary graft failure was recorded. Median time to neutrophil and platelet recovery was 18 days (range 10-22) and 11 days (range 9-13), respectively. Once documented the engraftment of donor cells, BPX-501 T lymphocytes were infused at a median time of 17 days (range 13-52) after the allograft. Two patients were enrolled in the phase I portion of the study; one each received 2.5x105 and 1x106 cells/kg. The remaining 23 children were treated in the phase II, where the recommended dose was 1x106 cells/kg. However, since we did not observe any acute GvHD requiring the infusion of the dimerizing agent (Rimiducid/AP1903) activating iC9 gene in the first 15 children receiving 1x106 cells/kg, we decided to emend the protocol to further increase the BPX501 cell dose infused to 2 and 4x106 cells/kg. Thus, the last 6 patients were enrolled in these 2 last dose levels (3 patients each). Six and 3 patients developed grade II-IV acute and chronic GvHD, respectively. In one child, given 4x106 cells/kg, we infused rimiducid for steroid-resistant grade II skin acute GvHD, with complete resolution of the disease in 24 hours. The cumulative incidence of grade II-III acute and chronic GvHD are shown in figure 1A and B, respectively. Median follow-up of these 25 children is 8 months (range 1-19 months). One of them died due to chronic GvHD-associated bronchiolitis obliterans and one child with ALL transplanted in CR2 relapsed; the cumulative incidence of non-relapse mortality and leukemia recurrence are shown in figure 1C. The probability of disease-free survival at 15 months is 87% (figure 1D). Once infused, BPX501 cells expanded and persisted over time in both peripheral blood and bone marrow.
Conclusion: Overall, these data indicate that the infusion of BPX-501 cells in children with AL given selectively manipulated haplo-HSCT results in low non-relapse mortality and chronic GvHD. Although the median observation time is still limited, the cumulative incidence of disease recurrence is promising.
Stanson:Bellicum pharmaceuticals: Employment. Moseley:Bellicum Pharmaceuticals: Employment, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal