Abstract
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare, aggressive hematologic malignancy which originates from the precursors of plasmacytoid dendritic cells. Data on the biology of BPDCN are limited, patient outcomes have historically been poor and there remains no established standard of care. CD123/IL3Rα is overexpressed in nearly 100% of patients with BPDCN. Considering the urgent unmet medical need for patients with BPDCN, targeting CD123 emerged as an attractive therapeutic target given its accessibility (cell surface) and differential expression (markedly over-expressed on BDPCN blasts as compared to normal hematopoietic stem cell compartment).
UCART123 (CD123CAR/RQR8+_TCRαβ-_T-cells) are genetically modified allogeneic T-cells (obtained from healthy volunteer donors by leukapheresis) containing (i) an anti-CD123 CAR (CD123 scFv-41BB-CD3z) and (ii) an RQR8 depletion ligand that confers susceptibility to rituximab. The cell surface expression of the T cell receptor (TCR) is depleted through the inactivation of the TCRa constant (TRAC) gene using Cellectis' TALEN® technology. In this study, we examined efficacy of this first allogenic anti-CD123 CAR-T cells in pre-clinical models of BPDCN.
We first analysed the level of expression of CD123 in the bone marrow of 8 patients with newly diagnosed BPDCN, and compared with CD123 expression on blasts of 28 newly diagnosed AML patients. CD123 expression levels were significantly higher in BPDCN (mean fluorescence intensity (MFI) range 3,484-17,937) compared to AML (MFI range 360-5,073) (p<0.01).
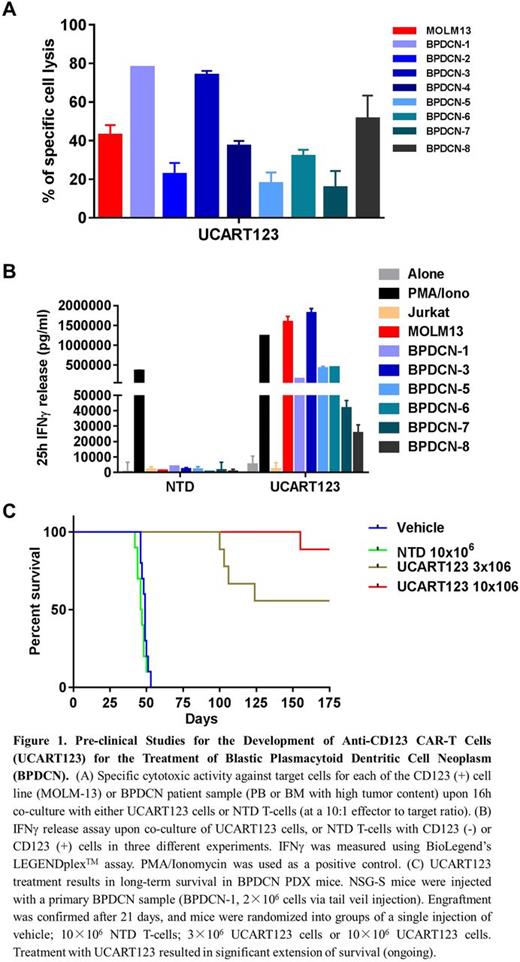
In vitro cytotoxic activity of UCART123 cells was evaluated by co-culturing UCART123 cells with primary human BPDCN, at a 10:1 effector to target ratio, using flow cytometry. The results show significant cytotoxic activity of UCART123 cells against BPDCN samples compared to cells co-cultured with non-transduced TCRab- T-cells (NTD) (Fig. 1A).
We next evaluated antigen-specific UCART123 cell degranulation by staining of CD107α, a lysosomal associated membrane protein residing in cytolytic granule membranes. The results indicated that specific degranulation of UCART123 cells is observed upon co-culture with CD123 (+) target cells.
The capacity of UCART123 cells to secrete cytokines, in particular IFNγ, in response to specific stimulation with CD123 (+) BPDCN cells was examined using a BioLegend's LEGENDplexTM assay. UCART123 cells stimulated by CD123 (+) BPDCN cells, but not NTD cells, secreted high levels of IFNγ in the culture supernatants (Fig. 1B).
To evaluate in vivo anti-tumor activity of UCART123 cells, we established patient-derived xenograft (PDX) from a patient with relapsed BPDCN in NSG-SGM3 mice. Upon engraftment, mice were randomized into 4 groups (9 mice/group). Mice received either a single tail vein injection of vehicle, 10×106 NTD T-cells, and 3×106 or 10×106 UCART123 cells. All mice in vehicle-treated group and in the group that received 10×106 NTD cells died by day 53, with high tumor burden of BPDCN detected in peripheral blood, spleen and bone marrow. Both cell doses of UCART123 significantly extended mice survival (Fig. 1C) and reduced or eliminated circulating BPDCN cells. 57 days after UCART123 cells injection, one mouse from UCART123 10×106 group was sacrificed. UCART123 cells were detected in spleen (16.4% CAR+ cells) and bone marrow (1.1% CAR+ cells) using human CD5 antibody or CD123-Fc protein.
In summary, UCART123 causes specific killing of BPDCN cells, associated with antigen-specific T-cell degranulation and robust levels of IFNg production. Our preliminary data indicate persistence of UCART123 cells in vivo in an NSG-S model of primary BPDCN. Most importantly, UCART123 therapy results in BPDCN eradication and long-term disease-free survival in primary BPDCN PDX mice. Additional PDX studies are ongoing and will be presented. These results demonstrate pre-clinical proof-of principle of high anti-BPDCN activity of UCART123 allogeneic CAR T-cells, and warrant further clinical testing of this approach in human clinical trials in BPDCN.
Galetto:Cellectis SA: Employment. Gouble:Cellectis: Employment. Smith:Cellectis SA: Employment. Lane:Stemline Therapeutics: Research Funding; N-of-1: Consultancy. Guzman:Cellectis: Research Funding. Konopleva:Cellectis: Research Funding; Calithera: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal