Abstract
Introduction:
Recent clinical trials with T cells engineered to express 2nd generation CD19 chimeric antigen receptors (CARs) unprecedented anti-leukemic responses. We have developed a novel CD19CAR with a new scFv in the 41BBz format (CAT-41BBz CAR) which confers enhanced cytotoxicity and cytokine secretion in response to stimulation with CD19+ targets in vitro as well as equivalent in vivo anti-tumour efficacy to the FMC63 41BBZ CAR in use in clinical studies. We have designed, optimized and validated GMP-grade CAR T cell production using this novel CAR. Based on these data, we have recently initiated a Phase I clinical study (CARPALL) of this novel CAR in pediatric patients with relapsed ALL and other CD19+ hematological malignancies to determine the safety profile and durability of responses to CD19CART therapy. This will be critical in determining whether CD19CAR T cells are best used as a stand-alone therapy or as a bridge to stem cell transplant (SCT).
Methods:
We initially optimized our GMP production methodology in terms of activation method, cytokine milieu and expansion conditions on healthy donor peripheral blood mononuclear cells (PBMCs) to give optimal transduction efficiency and preserve early memory subsets within the CAR T cell product. We have subsequently validated this methodology using unstimulated leucaphereses from 5 lymphopenic patients with ALL. PBMCs were activated with anti-CD3/CD28 microbeads (Dynabeads CTS) and then lentivirally transduced with the CAT CAR vector. T cells were then expanded in the WAVE bioreactor before bead removal on a magnetic system and cryopreservation.
Patients on study receive lymphodepletion with fludarabine and cyclophosphamide followed by a single dose of 106 CAR+ T cells/kg and are then monitored as an in-patient for 14 days post infusion for toxicities such as cytokine release syndrome or neurotoxicity. The primary end-points of the study are toxicity and the proportion of patients achieving molecular CR at 1 month post CD19CAR T cell infusion. Following this, patients undergo intensive monitoring of disease status for a total of 2 years post infusion. To determine the durability of responses, patients achieving a molecular CR will be monitored closely for the re-emergence of molecular level disease without additional consolidative therapy or SCT
Results:
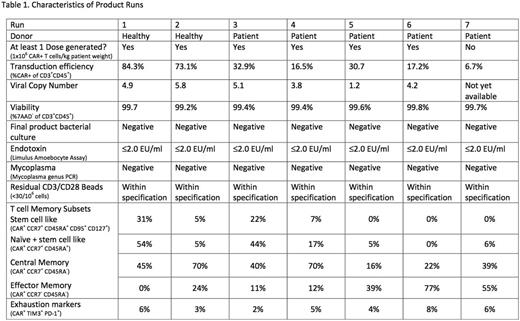
We were able to generate the target dose of 1x106 CAR+ T cells/kg in 6 of 7 production runs (involving 2 healthy donors and 5 patients) to date, all of which met sterility release criteria. Transduction efficiency was on average 37% (range 7-84%, see table 1). Mean viral copy was 4.2 (range 1.2-5.8). Memory T cells of stem cell-like phenotype (CAR+ CCR7+ CD45RA+ CD95+ CD127+) formed a mean of 9% (range 0-31%), central memory T cells (CAR+ CCR7+ CD45RA-) formed a mean of 43% (range 16-70%) and effector memory T cells formed a mean of 31% (range 0-77%) of the final CAR T cell product. The percentage of CAR T cells expressing dual exhaustion markers (TIM3+ PD-1+) was on average 5% (range 2-8%). So far 2 patients have been treated.
Conclusions
We have optimized and successfully validated a robust GMP production method for CD19CAR T cells lentivirally transduced with a novel CD19CAR. Preliminary results of therapy with CAT-41BBz CAR T cells in initial patients on the clinical study will be presented.
Qasim:Autolus: Consultancy, Equity Ownership, Research Funding; Cellectis: Research Funding; Calimmune: Research Funding; Catapult: Research Funding. Pule:Autolus Ltd: Employment, Equity Ownership, Research Funding; UCL Business: Patents & Royalties; Amgen: Honoraria; Roche: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal