Abstract
Introduction: Exportin 1 (XPO1) is a well characterized and essential nucleo-cytoplasmic transport protein in the karyopherin family, and is responsible for the nuclear export of over 200 cargo proteins, including the major tumor suppressor proteins (TSPs) p53, p21, FOXO and the translation regulator elF4E. XPO1 is overexpressed in numerous cancer types including solid and hematological malignancies, often correlating with poor prognosis. Recently, a novel class of Selective Inhibitors of Nuclear Export (SINE) compounds, selinexor (KPT-330) and the second generation KPT-8602, have been developed for the treatment of advanced cancers. We have previously shown that selinexor has marked activity in AML and DLBCL pre-clinical models.
The BCL-2 family of anti-apoptotic proteins are deregulated and linked to maintenance and survival in AML and DLBCL. For its translation, the mRNA for BCL-2 is transported from the nucleus to the cytoplasm by forming a complex with XPO1 cargo, elF4E. Other important mRNAs exported from the nucleus via this mechanism include BCL6 and MYC. We hypothesize that SINE compounds inhibit XPO1/elF4E-mediated nuclear-cytoplasmic transport by covalently binding to the XPO1 cargo binding site and that in the absence of protein translation, BCL-2, BCL6 and MYC levels rapidly decline. Venetoclax (VEN; ABT-199) is a potent, selective inhibitor of BCL-2. In vitro, AML cells acquire resistance to VEN over time, often due to up-regulation of another BCL-2 family anti-apoptotic protein, MCL-1. MCL-1 is regulated by the anti-apoptotic transcription factor and XPO1 cargo NF-kB. We have previously shown that SINE compounds significantly decreased MCL-1 levels, presumably via inactivation of NF-kB.
The goal of this study was to test whether SINE compounds will synergize with VEN via BCL-2 modulation and whether the combination would diminish MCL-1 mediated resistance to BCL-2 inhibition in DLBCL and AML models, respectively.
Methods: BH3 profiling was performed in a sample of cell lines using a cytochrome c release assay to identify anti-apoptotic dependencies. The effects of SINE compounds and VEN as single agents or in combination on cell viability were performed in AML (K-562, MOLM-13, MV-4-11, and U-937) and DLBCL cell lines (SU-DHL-6, DoHH-2 and Toledo). Whole cell protein lysates were extracted 24 hours after treatment for immunoblot analysis. The activity of SINE compounds (5 mg/kg) and VEN (25 mg/kg) as single agents, or in combination were measured in AML (MV-4-11) and DLBCL (DoHH-2 and Toledo) xenografts in NSGS and nude mice, respectively. Tumor growth and survival were measured throughout these animal studies. Tumor tissue was collected at the end of treatment for flow cytometric analysis, western blotting and immunohistochemistry (IHC).
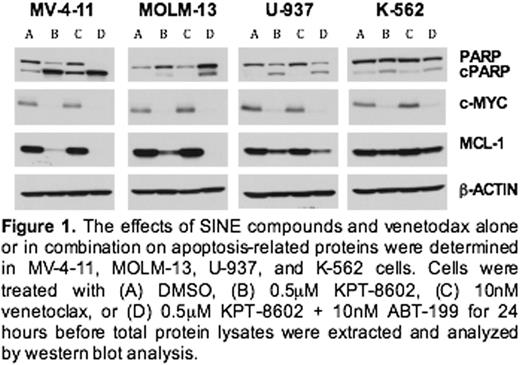
Results: By employing BH3 profiling, we identified AML cell lines that were dependent (MV-4-11 and MOLM-13) and not dependent (U-937 and K-562) on MCL-1. Dose response analysis demonstrated that each of the AML cell lines was sensitive to the SINE compounds, while VEN only reduced viability in the MV-4-11 and MOLM-13 cells. Additionally, there was enhanced growth inhibition when the SINE compounds were combined with VEN in the MCL-1 dependent cells. SINE compound treatment synergistically decreased c-MYC protein levels in all 4 AML cell lines with the combination treatment (Figure 1), whereas PARP cleavage was only enhanced with the combination in the MV-4-11 and MOLM-13 cells. Likewise, MCL-1 is reduced in the presence of SINE compound or SINE compound-VEN combinations. In DLBCL xenograft studies (DoHH-2 and Toledo), combination of selinexor with VEN was synergistic for tumor reduction and increased animal survival when compared to either single agent alone. By IHC we observed a concomitant reduction in BCL-2 and BCL-6 and an increase in cleaved caspase 3 in DLBCL tumors after combination treatment.
Conclusions:
SINE compound-VEN combinations show enhanced antitumor effect, with reduction of oncogenic activity. SINE compounds reduce MCL-1 in VEN-resistant cells. As MCL-1 driven anti-apoptotic machinery is responsible for resistance to inhibition of BCL-2 in DLBCL and AML, SINE compound regulation of MCL-1 may lead to rescue of VEN resistance. SINE compounds and VEN are excellent candidate partners for combination therapies in AML and DLBCL.
Friedlander:Karyopharm Therapeutics: Employment. Chang:Karyopharm Therapeutics: Employment, Equity Ownership. Kashyap:Karyopharm Therapeutics: Employment, Equity Ownership. Argueta:Karyopharm Therapeutics: Employment, Equity Ownership. Klebanov:Karyopharm Therapeutics: Employment, Equity Ownership. Senapedis:Karyopharm Therapeutics: Employment, Equity Ownership. Baloglu:Karyopharm Therapeutics: Employment, Equity Ownership. Lee:Karyopharm Therapeutics: Employment, Equity Ownership. Shacham:Karyopharm Therapeutics: Employment, Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Savona:TG Therapeutics: Research Funding; Amgen Inc.: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Sunesis: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees; Ariad: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal