Abstract
Introduction: Autophagy is a process whereby cells digest their own organelles in conditions of stress, such as low nutrient concentration, hypoxia or exposure to chemotherapy. It is well established that acute myeloid leukemia (AML) cells presiding within a hypoxic microenvironment are markedly less sensitive to cytarabine chemotherapy than normoxic cells. We hypothesized that AML cells survive and mediate chemoresistance by invoking autophagy mediators, specifically light chain 3B protein (LC3B). LC3B is a key regulator in the fusion of autophagosomes and lysosomes, giving rise to autophagolysosomes.
Methods: Human AML cells (HEL, HL60) were grown under normoxic (21% O2) and hypoxic (1% O2) conditions for 24-72 hours. Cells were treated with cytarabine chemotherapy and/or the autophagy inhibitors, bafilomycin A1 (Baf) and chloroquine (CQ). Viability was determined using trypan blue cell counting and tetrazolium dye MTT. Apoptosis and cell death were measured by flow cytometry for annexin-FITC and propidium iodide. Autophagy was assessed by flow cytometry using Cyto-ID Green Dye (Enzo Life Sciences), fluorescent microscropy for acridine orange dye accumulation, and western blot analysis. HEL cells were also transfected with an anti-LC3B siRNA and non-target sequences or anti-GAPDH siRNA as negative and positive controls, respectively. Real-time PCR with LC3B-specific probes, along with Western blotting with a rabbit anti-LC3B primary antibody, were performed to assess for changes in LC3B mRNA and protein levels.
Results: Autophagy in human AML cell lines was significantly increased following 24-72 hours of low oxygenation (1% O2) as compared with normoxia and was a predominantly late response to prolonged hypoxia (> 48 hours). We determined that AML cells exposed to chronic hypoxia were able to overcome an initial growth restriction with a corresponding increase in LC3B levels by Western blotting. Treatment of AML with ARA-C under hypoxia resulted in further dose-dependent increases in autophagic vesicles in AML cells consistent with enhanced autophagy induction. In contrast, exposure of hypoxic HEL cells to the autophagy inhibitors (Baf, CQ), led to arrest in autophagic flux (via higher levels of the LC3BII isoform expression as compared with LC3B I) and decreased autophagic vesicle density (as measured by fluorescent microscopy for acridine orange). Combination treatment with autophagy inhibitors (Baf, CQ) and ARA-C chemotherapy significantly enhanced apoptosis and cell death of AML cells under hypoxia as compared with single agent therapy.
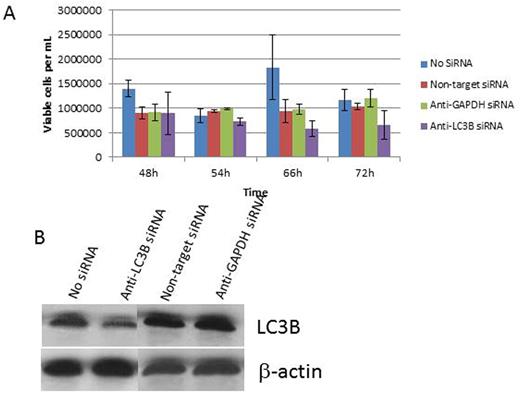
To further confirm these effects were LC3B dependent, we used anti-LC3B versus nonspecific siRNA to knockdown protein expression in HEL cells. HEL cells with anti-LC3B siRNA exhibited markedly decreased cell viability under prolonged hypoxia as compared to control siRNA transfected cells. Cells also demonstrated enhanced cell death following ARA-C treatment under hypoxia with the most pronounced effects ranging from 48h up to 72h from the start of treatment (Figure 1).
Conclusions: Our experiments demonstrate that the autophagy pathway is upregulated in human AML cells under hypoxic conditions and likely confers a rescue pathway under such conditions to promote leukemia survival. Using pharmacological inhibitors of autophagy and anti-LC3B siRNA technology, we show that targeting the autophagy pathway can render hypoxic AML cells more susceptible to cell death and enhance sensitivity to cytarabine chemotherapy. These preclinical results show promise for further exploring autophagy as a therapeutic target in acute myeloid leukemia.
A: Graph showing decreased cell viability and increased chemosensitivity in the anti-LC3B siRNA-treated HEL-Luc cells. This effect is not as pronounced in the control groups, and is most pronounced after the 48h time point.
B: Western blot showing LC3B protein knockdown compared to control groups
A: Graph showing decreased cell viability and increased chemosensitivity in the anti-LC3B siRNA-treated HEL-Luc cells. This effect is not as pronounced in the control groups, and is most pronounced after the 48h time point.
B: Western blot showing LC3B protein knockdown compared to control groups
Scott:Immunogen: Research Funding. Wang:Immunogen: Research Funding; Incyte: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal