Abstract
Introduction -TP53 is the most extensively studied gene in cancer and is associated with a poor outcome. The clinical implication of TP53 gene mutations in adult ALL has not been evaluated.
Methods - We screened for TP53 mutations in 119 newly diagnosed patients with ALL who were treated with HCVAD-based regimens (2012-2016) and evaluated the predictive and prognostic value of TP53mutations. Bone marrow samples underwent targeted amplicon-based next-generation sequencing (NGS) based mutation analysis.
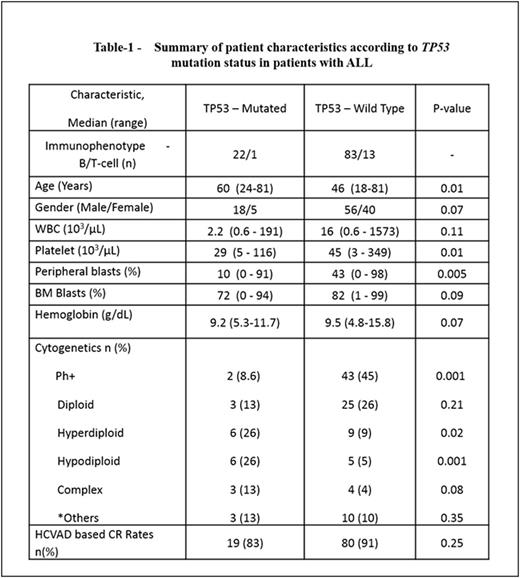
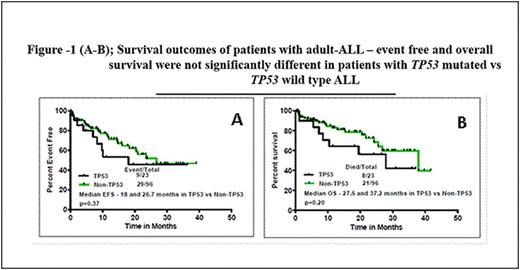
Results - Among 119 patients with adult ALL, we identified 23 patients (19%, 22 B-cell; 1 T-cell) with TP53 mutations (TP53mut); 96 had wild type TP53 (TP53wt; 83 B-cell; 13 T-cell). BCR/ABL1 rearrangement was noted in 2/23 TP53mut ALL and 43/96 TP53wt ALL (p=0.001). Of the 23 patients with TP53mut, 20 were missense, 2 were nonsense mutations and 1 was an insertion/ deletion, spanning exons 2-10. The most common pattern of amino acid substitution,in 10 of the 23 patients, was a substitution of arginine to histidine or proline on different codons. The median TP53 mutant allelic frequency was 42.2% (range, 1.4 - 93.8). Seven patients (30%) with TP53muthad concurrent mutations: NRAS in 2, BRAF, NOTCH1, TET2, DNMT3A, and EZH2 - 1 each. Among patients with TP53wt, 40 patients (42%) had mutations in other genes: 9 JAK2, 7 NRAS, 5 KRAS, 5 NOTCH1, 3 IDH2, 2 each with DNMT3A, ASXL1, FLT3, PTPN11, and 1 with TET2. The clinical characteristics, pattern of mutations, response to therapy, and outcomes of patients with TP53mut ALL (n=23) vs. TP53wt ALL(n=96) were compared (Table 1 and Figure-1). Patients with TP53mutALL were significantly older at presentation (median age 60 years [24-81] versus 46 years [18-81]; p=0.01) and had significantly lower platelet counts (29 x 109 /L versus 45 x 109/L; p=0.01) and lower peripheral blood blast percentages (10% versus 43%; p=0.005). Distribution of chromosomal aberrations was significantly different among the two groups: t(9;22) was observed at a much lower rate in TP53mut ALL, while hyper and hypodiploidy were more commonly encountered in TP53mut ALL There was no significant difference in the complete remission (83% in TP53mut ALL versus 91% in TP53wt ALL, p=0.25) and minimal residual disease (66% in TP53mut ALL versus 59% in TP53wt ALL, p=0.23) rates between the 2 groups. Overall, the median follow-up was 11.3 months (range, 0.2 to 41 months). There was no statistically significant difference in outcome between patients with and without TP5mutation. (Figure -1 A-B). The 3-year event-free (EFS) and overall survival (OS) rates were 45% and 42% in patients with TP53mutand 46% and 59% in those with TP53wt. The median EFS was 18 and 26.7 months (p=0.37) respectively. The median OS was 27.5 and 37.2 months (p=0.20), respectively. Furthermore, no significant difference was observed when only patients with Philadelphia-negative B-ALL were assessed.
Conclusions -TP53 mutations are seen in about 19% of adult with newly diagnosed ALL. Patients tend to be older with higher incidence of hypodiploidy. TP53 mutations had no significant negative impact on outcome in patients treated with Hyper-CVAD based regimens. Further studies are underway at our institution to expand the cohort and identify the relevance of TP53mutations in adult ALL in the era of novel chemoimmunotherapy.
*3 patients in TP53 - Mutated group had 1 patient each with miscellaneous/insufficient metaphases/ not done while 6 patients had miscellaneous aberrations and 4 with insufficient metaphases TP53wild type respectively
Konopleva:Calithera: Research Funding; Cellectis: Research Funding. Jain:Incyte: Research Funding; Abbvie: Research Funding; Pharmacyclics: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Consultancy, Honoraria, Research Funding; Novimmune: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Infinity: Research Funding; Servier: Consultancy, Honoraria; BMS: Research Funding; Seattle Genetics: Research Funding; Genentech: Research Funding; Celgene: Research Funding; Novartis: Consultancy, Honoraria. Wierda:Novartis: Research Funding; Gilead: Research Funding; Genentech: Research Funding; Acerta: Research Funding; Abbvie: Research Funding. O'Brien:Janssen: Consultancy, Honoraria; Pharmacyclics, LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal