Abstract
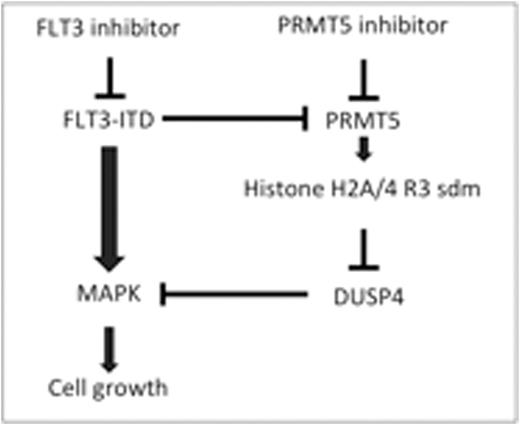
Background: The FLT3-ITD (internal tandem duplication) is the most frequently observed molecular defect in acute myeloid leukemia (AML) and has been shown to be a poor prognostic factor in several studies. Many inhibitors of FLT3-ITD have been developed and tested in clinical trials; while a recent randomized study of PK412 (FLT3 inhibitor) showed significant clinical benefit in AML patients, many other studies have shown only modest effect. We and others have shown that the protein arginine methyltransferase 5 (PRMT5) is required to maintain self-renewal in many types of stem cells; we found that PRMT5 is expressed at much higher levels in hematopoietic stem cells than in differentiated cells and moreover, the expression of PRMT5 is increased in most acute myeloid leukemia cells and PRMT5 is also overexpressed in lymphoma and ovarian cancer cells. While we have been to define roles for PRMT5 in myeloproliferative disorders and normal hematopoietic stem and progenitor cells. The role of PRMT5 in AML has not been extensively studied. The mitogen activated protein kinases (MAPK) pathway is known to abnormally enhance the proliferation signal downstream of the FLT3-ITD. The dual-specificity mitogen activated protein kinase phosphatase-2 gene (DUSP4/MK-2) specifically dephosphorylates and inactivates MAPK. The role of DUSP4 in AML also is not clear. Here we reported that the role of PRMT5 and DUSP4 in AML with FLT3-ITD.
Objective: The aim of the present study was to investigate whether the FLT3-ITD affects histone modification and/or the expression level of DUSP4 via effects on PRMT5 itself and also to explore whether the combination of a FLT3 inhibitor and a PRMT5 inhibitor could have synergistic anti-leukemic effects.
Results: We showed that overexpression of FLT3-ITD increased the level of phospho-PRMT5 in 293T cells and that several FLT3 inhibitors reduced the phosphorylation of PRMT5 in MV4-11 cells. The FLT3-ITD binds PRMT5 when expressed in 293T cells and FLT3, FLT3-ITD, and FLT3-D835Y can directly phosphorylate PRMT5 in an in vitro kinase assay but this phosphorylation is inhibited by the AC220, in a dose-dependent fashion. Histone H2A arginine symmetric methylation, which is known to be a target of PRMT5, increases after treatment with CEP701 and AC220. These results suggest that the FLT3 inhibitor can enhance PRMT5 enzyme activity, and that FLT3-ITD inhibits PRMT5 enzymatic activity. We also showed that there was an enrichment of histone H2AR3/H4R symmetric di-methylation (sdm) at the promoter region of DUSP4. We also showed that absent PRMT5 increased the protein expression of DUSP4 and FLT3-ITD signaling suppression with AC220 decreased it through histone modification with Chip assay. We are able to show that PRMT5 inhibitor and sh-PRMT5 suppressed the cytokine signaling such as p-STAT5, p-Erk, and p-AKT that are known as signaling pathway that FLT3-ITD enhanced and DUSP4 suppressed. The PRMT5 was an essential gene for maintenance of MV4-11 cells. The sh-DUSP4 was able to partially rescue MV4-11 cells with treatment of sh-PRMT5. We showed that a FLT3 inhibitor and a PRMT5 inhibitor have synergistic anti-leukemic effects in MV4-11 cells.
Conclusion: The FLT3-ITD affects the expression of DUSP4 through epigenetic modification such as the methylation status of histone H2AR3/H4R3 arginine symmetric methylation via PRMT5 in AML. The combination of an FLT3 inhibitor and PRMT5 inhibitor may have synergistic anti-leukemic effects in AML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal