Abstract
INTRODUCTION: Despite the limited number of patients with chronic myelomonocytic leukemia (CMML) included in the clinical trials that resulted in the approval of hypomethylating agents (HMA) in patients with myelodysplastic syndromes (MDS), HMA (azacitidine and decitabine) remain the most commonly used therapeutic intervention for patients with CMML. We present our experience with HMA in the treatment of CMML.
METHODS: We retrospectively reviewed 153 patients diagnosed with CMML following the 2006 WHO classification and treated with frontline HMA between 2004 and 2015 at a single institution. Clinical and demographic data were obtained from our electronic database. Response was assessed by modified 2006 International Working Group (IWG) criteria. Statistical analyses were performed with the IBM SPSS Statistics 23.0 software. Overall survival (OS) and leukemia free survival were defined as the time between treatment onset and death or leukemia (or last contact), respectively. Data were summarized using median, range, and percentage. Censored endpoints were estimated by the nonparametric Kaplan-Meier method and compared using the log-rank test. All tests were 2-sided with significance set at p<0.05.
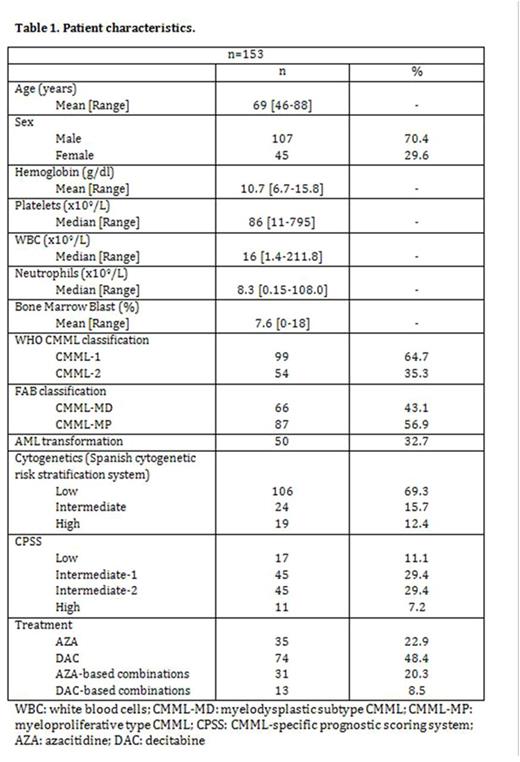
RESULTS: Baseline characteristics are shown in Table 1. 45 (30%) patients were female. Median age at diagnosis was 69 years (range 50-88). According to the CMML-specific prognostic scoring system (CPSS), 17 patients (11%) belonged to the low risk group, 45 (29%) to the intermediate-1 risk group, 45 (29%) to the intermediate-2 risk group, and 11 (7%) to the high risk group. Following the FAB criteria, 66 of the patients (43%) had CMML-MD and 87 (57%) had CMML-MP. By WHO classification, 99 patients (65%) were CMML-1 and 54 patients (35%) were CMML-2. Karyotype was available in 150 patients (abnormal in 50 [33%]), and according to the Spanish Haematological Cytogenetics Working Group, 106 (69%) belonged to the good-risk category, 24 (16%) to the intermediate-risk category, and 19 (12%) to the poor-risk category. 35 (23%) patients received azacitidine in monotherapy (AZA), 74 (48%) decitabine in monotherapy (DAC), and 44 (29%) were treated with combinations.
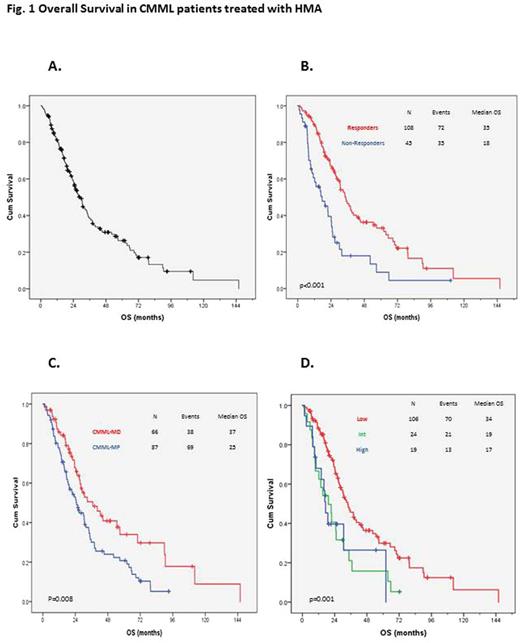
The overall response rate (ORR) was 71% (108/153), with 56% (86/153) of the patients achieving complete response (CR). The median duration of response was 10 months (range 1-86 months). No differences were observed in ORR or CR between CMML-MD and CMML-MP (73% vs 69%, p=0.613, for ORR and 61% vs 53%, p=0.340, for CR), CMML-1 and CMML-2 (68% vs 76%, p=0.285; 56% vs 57%, p=0.825) or CPSS score (p=0.133). Median OS was 23 months (Fig.1A), and the cumulative probability of AML transformation was 30% at 5 years. There were statistically significant OS differences between responders and non-responders: 35 vs. 18 months, respectively (p<0.01) (Fig.1B); CMML-MD and CMML-MP: 37 months vs. 25 (p=0.008) (Fig.1C); and good, intermediate, and poor risk cytogenetics: 34, 19, and 17 months, respectively (p=0.001) (Fig.1D). No differences were observed in terms of OS between CMML-1 and CMML-2: 30 and 29 months (p=0.974).
Considering only the patients treated with AZA or DAC in monotherapy, CR was significantly higher in those patients treated with DAC (74.3%) when compared with patients treated with AZA (37.1%) (p < 0.001). Despite the fact that DAC shows a trend toward be better than AZA in terms of ORR (79.7% and 62.9%, respectively; p=0.06), leukemia free survival (LFS) (median 22 and 12 months, p=0.367) and OS (median 34 and 24 months, p=0.231), no statistical differences were observed. The only difference observed between groups was hemoglobin level (11.4 g/dl vs 10.4 g/dl, p=0.027). No other significant differences in terms of white blood cells, platelets, neutrophils, number of bone marrow blast, CMML-1 vs CMML-2, CMML-MD vs CMML-MP, CPSS and cytogenetic risk groups were observed between patients treated with AZA vs DAC.
CONCLUSION: Here, we present the largest series of CMML treated with HMA. As previously published, HMA are clinically effective in the treatment of patients with CMML, providing ORR of 71% with 56% of CR. Better OS was observed in responders compared with non-responders, in CMML-MD compared with CMML-MP, and in those with low risk cytogenetics. In our cohort, CR was significantly higher in those patients treated with DAC when compared with patients treated with AZA. These results need to be confirmed in larger numbers of patients.
Jabbour:ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; BMS: Consultancy. Cortes:ARIAD: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Teva: Research Funding. DiNardo:Celgene: Research Funding; Novartis: Research Funding; Agios: Research Funding; Abbvie: Research Funding; Daiichi Sankyo: Research Funding. Konopleva:Cellectis: Research Funding; Calithera: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal