Key Points
Because extravascular FIX is physiologically important, FIXs circulating levels do not independently predict hemostatic potential.
A saphenous vein hemophilia B mouse model shows that 7 days postinfusion FIX-Fc and FIX provide equal hemostatic protection.
Abstract
FIX binds tightly to collagen IV. Furthermore, a FIX mutant, FIXK5R, which binds better than wild-type FIX to collagen IV, provides better hemostasis than wild-type FIX, long after both are undetectable in the plasma. There is also credible evidence of extravascular FIX. Here, we use the saphenous vein bleeding model to compare the efficacy of recombinant FIXFc (Alprolix) and wild-type FIX (BeneFIX) in hemophilia B mice 7 days postinfusion. Although the terminal half-life of Alprolix is significantly longer than that of BeneFIX, at equal doses Alprolix is not better at controlling bleeding 7 days postinfusion, presumably because of the extravascular FIX. Both BeneFIX and Alprolix exhibit a linear response in clotting efficacy up to 150 IU/kg, where they appear to saturate an extravascular compartment, because there is no additional prophylactic benefit from higher doses. A robust pool of extravascular FIX is clearly observed surrounding blood vessels, localized to the same region as collagen IV, in 2 representative human tissues: liver and skeletal muscle. We see no increased risk for thrombosis at 250 IU/kg FIX at 6 hours postinfusion. In summary, 7 days postinfusion into hemophilia B mice, BeneFIX and Alprolix are hemostatically indistinguishable despite the latter’s increased half-life. We predict that doses of FIX ∼3 times higher than the currently recommended 40 to 50 IU/kg will, because of FIX’s large extravascular compartment, efficiently prolong prophylactic hemostasis without thrombotic risk.
Introduction
Hemophilia management traditionally involves treating severe bleeding episodes when they occur (on-demand therapy). However, this approach does not prevent intra-articular microbleeds, which eventually result in joint debilitation. Therefore, the World Federation of Hemophilia recommends that on-demand treatment be replaced by prophylactic maintenance.1 The goal of prophylaxis has been to maintain a plasma trough level greater than 1% of normal, but it is now recognized that 5% to 15% of normal is required to prevent spontaneous bleeding.2
The new target level of 5% to 15% is based on correlations between Factor VIII (FVIII) levels and bleeding events. A clinical trial correlating bleeding and plasma levels of FIX3 has never been performed, although recently presented data examining a large database of US men with mild and moderate hemophilia support a similar target for hemophilia B.4 Because current practice requires prophylactic infusions of clotting factors 2 times per week, several companies have designed FIX molecules that have longer terminal half-lives. One of these drugs, FIXFc (Alprolix), is approved for prophylaxis when administered every 7 to 10 days.5-7 The published half-life data of these “longer-acting” molecules are impressive, but there is no evidence that they provide better clinical outcomes. Moreover, the extravascular role played by FIX has not been adequately considered. FIX plasma concentration remains the standard surrogate endpoint for hemostatic efficacy in hemophilia B patients, despite evidence that extravascular FIX plays an important role in coagulation. This evidence for the importance of extravascular FIX includes the following points:
First, FIX binds reversibly and saturably to collagen IV, which is found mainly in the basement membranes of all tissues.8
Second, we previously described a mutation of FIX (lysine-5 to alanine-5, designated FIXK5A) with reduced affinity for collagen IV. We later created a knock-in mouse expressing FIXK5A. This mouse exhibits a bleeding diathesis even though its circulating level of FIXK5A is about 20% higher than normal.9 The specific activity of this FIXK5A in vitro is indistinguishable from that of wild-type FIX (FIXWT); this suggests an important hemostatic role for FIX bound to extravascular collagen IV.
Third, hemophilia B mice, injected with a FIX (FIXK5R) that binds more tightly to collagen IV, exhibit better hemostasis 7 days postinfusion than do mice injected with an equivalent amount of FIXWT. This improved hemostasis persists despite the fact that their circulating FIX levels reach undetectable levels at 3 days postinfusion.10
Last, there is evidence of a large extravascular reservoir of FIX. As Stern et al demonstrated, administering increasing amounts of bovine FIX to baboons rapidly displaced the endogenous extravascular stores of baboon FIX into the plasma.11
In aggregate, these data suggest that collagen IV–bound FIX plays an important role in hemostasis. In this study, we compare FIXFc (Alprolix) and FIXWT (BeneFIX) to test the hypothesis that simply increasing FIX’s plasma half-life may not confer a clinical advantage in prophylactic hemophilia treatment.
Methods
All mouse experiments were Institutional Animal Care and Use Committee–approved (University of North Carolina Office of Animal Care and Use #13-144) and followed U.S. Public Health Service guidelines for animal care and use. The hemophilia B mice were made in our laboratory12 and were in a C57BL-6 background. The normal control mice were from Jackson Labs and had the same genetic background as the hemophilia B mice.
Proteins
Alprolix was purchased from Diversified Biologicals (Miami, FL). BeneFIX was provided by Pfizer Inc. Both proteins were reconstituted per manufacturer’s instructions immediately before use. A new vial was opened for each infusion.
Bleeding assay
A saphenous vein bleeding assay was used.13 The FIX was infused into the right saphenous vein of hemophilia B mice. After 7 days, the left saphenous vein was exposed and partially transected. Upon cessation of bleeding, the clot was physically dislodged, and the site was observed for repeat clotting. The total number of clots formed over 30 minutes was recorded. Increased numbers of reformed clots equate to better hemostatic control. For testing the hypothesis that the 2 populations are the same, the Mann-Whitney test was used. Variance equivalence was tested by the Brown-Forsythe test. All statistical analysis employed Mathematica version 10.3.
Thrombosis assays
Intravital fluorescence imaging of venous thrombogenesis was performed as described in Cooley.14 Alexa Fluor 647–labeled antifibrin monoclonal antibody was used to monitor fibrin levels. Rhodamine 6G (0.5 mg/kg) was used to label platelets. Fluorophores were injected 5 minutes prior to thrombus induction. The femoral vein was exposed, and a surface-applied electrolytic injury was delivered for 30 seconds via a blunt wire at 1.5 V of anodal current. The vessel injury site was shutter-illuminated with 532-nm and 650-nm defocused lasers, and fluorescence emission was captured via time-lapse digital video microscopy at original magnification ×100 for 60 minutes. The relative intensity of each fluorophore was quantitated at 2-minute intervals, normalizing for animal body weight and the amount of injected fluorophore-labeled platelets to yield a “relative intensity” for each fluorophore.14
Histology
Human tissues, obtained from the University of North Carolina Translational Pathology core facility and classified as exempt by the Institutional Review Board, were paraffin embedded and sectioned at 5 µm. For immunohistochemistry, heat-induced epitope retrieval15 was performed, and endogenous peroxidases were blocked using 3% H2O2. Sections were then blocked using 10% normal goat serum; incubated in Anti-Human Factor IX (1:1600; Green Mountain Antibodies GMA-101), Anti-Human Factor X (FX) (1:100; Green Mountain Antibodies GMA-540), GMA-213 Anti-Factor VII (FVII) Antibody 1:500, or Anti-Human Collagen IV (1:500; Abcam ab6586) overnight at 4°C; and then incubated in Biotinylated Goat Anti-Mouse Immunoglobulin G (1:500; Jackson ImmunoResearch 115-065-166) or Biotinylated Goat Anti-Rabbit Immunoglobulin G (1:500; Jackson ImmunoResearch 111-065-144) for 1 hour at room temperature. Finally, staining was visualized using a Vectastain ABC Elite Kit (Vector Labs PK-6100) followed by 3,3′-diaminobenzidine. The images were captured with a Nikon Optiphot-2 with plan-apo lenses and an Olympus DP-70 camera.
Results
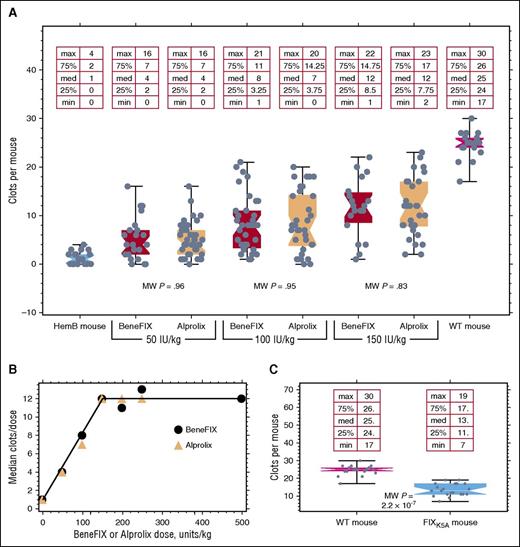
Figure 1A shows the results of saphenous vein bleeding in hemophilia B mice 7 days postinfusion. Both Alprolix and BeneFIX, whether infused at 50, 100, or 150 IU/kg, elicit almost identical hemostatic efficacies. At each dose of BeneFIX and Alprolix, Mann-Whitney P values for the differences between population medians are >.83. At any dose, the variance equivalence between Alprolix and BeneFIX is not significant. However, the variance of either Alprolix or BeneFIX differs significantly from the values found in the WT mice (P < .02). Table 1 compares the Mann-Whitney test values for differences between doses within groups 7 days postinfusion. Table 1 uses the subscript letters and numbers to indicate the infused protein and its dose: FIXB50 and FIXA50 indicate doses of 50 IU/kg; FIXB100 and FIXA100 indicate doses of 100 IU/kg, and so on. The P value for the comparison of Alprolix at 100 IU/kg vs 150 IU/kg is >.05 simply because of the number of mice employed; in the Alprolix comparison, 53 mice were used in combination between the 2 doses, while 64 mice were used to compare the same BeneFIX dose. The smallest sample size was 53 total mice for comparing FIXA100 with FIXA150. Comparing the P values between incremental doses for the rising portion of the dose response curve with that for the flat portion of the curve reveals that, for both BeneFIX and Alprolix, each increasing dose is statistically significant up to 150 IU/kg. Beyond 150 IU/kg, no statistically significant improvement in hemostatic protection is afforded.
Comparison of BeneFIX and Alprolix at different doses in Hemophilia B mice. Each point in (A) represents the number of times that clotting can recur in a hemophilic mouse with a saphenous vein injury 7 days postinfusion with either Alprolix or BeneFIX. The P values shown within the plot are from Mann-Whitney tests. (B) Plot of median values for the number of clots formed at different doses of BeneFIX or Alprolix. The medians were taken from the data shown in (A) together with additional similar data from higher doses. The line connecting the points has no theoretical meaning but serves to draw attention to the proportional increase. (C) Mice expressing FIXK5A at 120% of WT levels are about 50% as active in the saphenous vein bleeding model as are WT mice. The in vitro activity of FIXK5A is indistinguishable from FIXWT in an activated partial thromboplastin time assay. HemB, hemophilia B.
Comparison of BeneFIX and Alprolix at different doses in Hemophilia B mice. Each point in (A) represents the number of times that clotting can recur in a hemophilic mouse with a saphenous vein injury 7 days postinfusion with either Alprolix or BeneFIX. The P values shown within the plot are from Mann-Whitney tests. (B) Plot of median values for the number of clots formed at different doses of BeneFIX or Alprolix. The medians were taken from the data shown in (A) together with additional similar data from higher doses. The line connecting the points has no theoretical meaning but serves to draw attention to the proportional increase. (C) Mice expressing FIXK5A at 120% of WT levels are about 50% as active in the saphenous vein bleeding model as are WT mice. The in vitro activity of FIXK5A is indistinguishable from FIXWT in an activated partial thromboplastin time assay. HemB, hemophilia B.
Mann-Whitney comparison of Alprolix (A) or BeneFIX (B) at different doses: B50 = 50 IU BeneFIX and similar
| Comparison . | P value . |
|---|---|
| FIXKO vs FIXB50 | .0008 |
| FIXB50 vs FIXB100 | .043 |
| FIXB100 vs FIXB150 | .027 |
| FIXB50 vs FIXB150 | .00016 |
| FIXB150 vs FIXB200 | .82 |
| FIXB200 vs FIXB250 | .88 |
| FIXKO vs FIXA50 | .00008 |
| FIXA50 vs FIXA100 | .02 |
| FIXA100 vs FIXA150 | .08 |
| FIXA50 vs FIXA150 | .0002 |
| FIXA150 vs FIXA200 | .91 |
| FIXA200 vs FIXA250 | .86 |
| Comparison . | P value . |
|---|---|
| FIXKO vs FIXB50 | .0008 |
| FIXB50 vs FIXB100 | .043 |
| FIXB100 vs FIXB150 | .027 |
| FIXB50 vs FIXB150 | .00016 |
| FIXB150 vs FIXB200 | .82 |
| FIXB200 vs FIXB250 | .88 |
| FIXKO vs FIXA50 | .00008 |
| FIXA50 vs FIXA100 | .02 |
| FIXA100 vs FIXA150 | .08 |
| FIXA50 vs FIXA150 | .0002 |
| FIXA150 vs FIXA200 | .91 |
| FIXA200 vs FIXA250 | .86 |
Figure 1B shows the dose response curve of the median number of times hemophilia B mice clot 7 days postinfusion to doses of BeneFIX at 50, 100, 150, 200, 250, and 500 IU/kg and to Alprolix at doses of 50, 100, 150, and 250 IU/kg. The distribution of the data points is clearly seen in Figure 1A; Figure 1B replots the data from Figure 1A with additional doses of either BeneFIX or Alprolix. It is clear from Figure 1A-B that hemostatic function is directly dose-proportional until 150 IU/kg, beyond which there is no additional benefit from higher doses. (The line in Figure 1B is drawn to help the reader see the proportional increase with doses up to ∼150 IU/kg.) Note that at 7 days postinfusion of 150 IU/kg, no BeneFIX antigen was detectable (<1 ng/mL, the limit of detection) in the plasma, whereas 98 ± 8 ng/mL (n = 5) of Alprolix remained.
We previously reported that hemophilia B mice expressing a FIX variant, FIXK5A, that binds poorly to collagen IV had a mild bleeding diathesis.16 The tail bleed model was used for those experiments, and too few mice were used for robust statistical analysis. We expanded these experiments by using more mice (the minimum number of animals used for any dose of either FIX construct was 19) and by evaluating the coagulation status with the saphenous vein model instead of the tail bleed model. Figure 1C shows the reduced clotting efficacy of human FIXK5A in these mice. Clearly, although FIXK5A displays normal activity in an in vitro activated partial thromboplastin time assay,17,18 the FIXK5A knock-in mice show reduced clotting; this is despite the knock-in FIXK5A mice having slightly higher plasma levels of FIXK5A than the WT mice have of FIXWT.
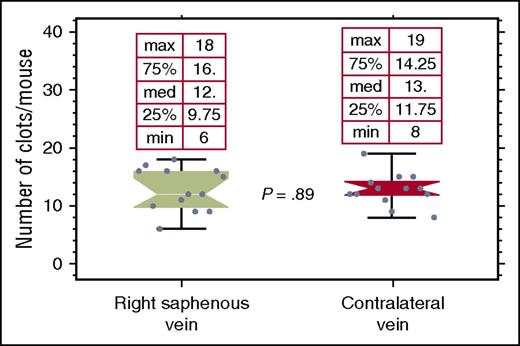
In this experiment, we used a saphenous vein bleeding model instead of the tail bleeding experiment of the prior study.9,10 Although the hemophilia B mice show undetectable circulating levels of FIX 7 days after an infusion of BeneFIX, an injured saphenous vein will nevertheless form clots on day 7. If clotting at the site of an injury were due to residual, nondetectable levels of circulating FIX, then one would expect the limit of clot recurrence at that wound to represent either the consumption of the residual circulating FIX, or conversely, exhaustion of some local factor(s) required at the wound site for clotting to reoccur. To distinguish these 2 possibilities, we examined the clotting function at the contralateral saphenous vein, transected after clotting ceased at the original injured vein 7 days postinfusion of 150 IU/kg BeneFIX. Figure 2 demonstrates that the subsequently injured contralateral vein clots just as well as did the original saphenous vein before the latter ceased clotting. This finding argues that long-term clotting is mediated, not by circulating FIX, but by FIX sequestered in the extravascular tissue, presumably due to collagen IV binding. Furthermore, hemophilia B mice subjected to saphenous vein injury typically clot 0 to 2 times. If a 500-IU/kg bolus is administered 10 minutes after clotting ceases, 5.9 ± 0.9 minutes elapses before clotting at the injury resumes, despite the fact that, in mice, the blood circulates the body completely every 7 to 9 seconds.19 Taken together, these results suggest that it is not circulating FIX but sequestered FIX that is responsible for coagulation, and that the 5.9 minutes represents the time necessary to accumulate sufficient FIX in the extravascular tissue to support clotting.
Hemophilia B mice, that had been infused with 150 IU/kg BeneFIX 7 days previously, were subjected to the saphenous vein injury. After the vein ceased clotting, the contralateral saphenous vein was transected. The data show that there is no significant difference between the 2 veins in the number of clots formed, despite undetectable levels of circulating FIX at the time of the injury of the first vein. This indicates that it is not residual circulating FIX that is responsible for clotting, but instead extravascular FIX.
Hemophilia B mice, that had been infused with 150 IU/kg BeneFIX 7 days previously, were subjected to the saphenous vein injury. After the vein ceased clotting, the contralateral saphenous vein was transected. The data show that there is no significant difference between the 2 veins in the number of clots formed, despite undetectable levels of circulating FIX at the time of the injury of the first vein. This indicates that it is not residual circulating FIX that is responsible for clotting, but instead extravascular FIX.
Immunohistological evidence for extravascular FIX
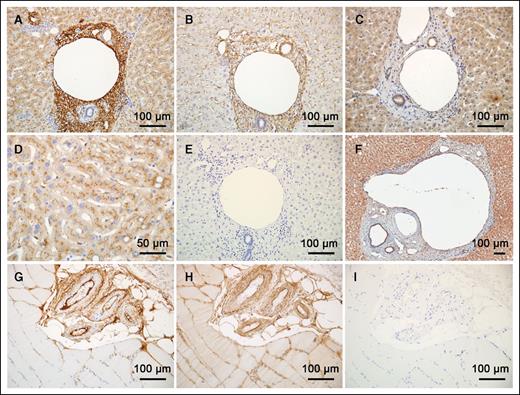
Because of the evidence for extravascular FIX, and the observation that it binds to collagen IV, we used immunohistochemistry to determine the location of FIX in human tissues. We examined human tissues because we have previously shown that mice infused with human FIX exhibit FIX beneath their arterial endothelium, and periportal human FIX has been reported in mouse livers of mice expressing human FIX.16,20 Initially, we examined normal human liver tissue; because it is highly vascularized and rich in connective tissue, and because FIX is synthesized in the liver, it serves as a positive control. Figure 3A,D shows that hepatocytes, which synthesize FIX, stain positive for FIX; their most intense FIX staining appears to be intracellular.
Histology of extravascular FIX distribution. Antibodies to FIX (A,D), Collagen IV (B), FX (C), and FVII (F) were used to stain human liver sections with 3,3′-diaminobenzidine. (E) No primary antibody; all no primary antibody controls lacked background noise. Notice that collagen IV in the sinusoids (B) is not stained with FIX, but that collagen IV and FIX staining generally coincide. The tissues stained with antibodies to FX and FVII differ noticeably from those stained for FIX. (G) A cluster of arteries sectioned in the human skeletal muscle stained for FIX. (H) An adjacent section stained for collagen IV. (I) No primary antibody control. Staining was visualized using a Vectastain ABC Elite Kit (Vector Labs PK-6100), followed by 3,3′-diaminobenzidine. The images were captured with a Nikon Optiphot-2 with a plan-apo lenses and an Olympus DP-70 camera.
Histology of extravascular FIX distribution. Antibodies to FIX (A,D), Collagen IV (B), FX (C), and FVII (F) were used to stain human liver sections with 3,3′-diaminobenzidine. (E) No primary antibody; all no primary antibody controls lacked background noise. Notice that collagen IV in the sinusoids (B) is not stained with FIX, but that collagen IV and FIX staining generally coincide. The tissues stained with antibodies to FX and FVII differ noticeably from those stained for FIX. (G) A cluster of arteries sectioned in the human skeletal muscle stained for FIX. (H) An adjacent section stained for collagen IV. (I) No primary antibody control. Staining was visualized using a Vectastain ABC Elite Kit (Vector Labs PK-6100), followed by 3,3′-diaminobenzidine. The images were captured with a Nikon Optiphot-2 with a plan-apo lenses and an Olympus DP-70 camera.
Interestingly, both the basement membrane of the portal vein and the stroma surrounding the portal triad are densely stained with the FIX antibody. An adjacent histological section (Figure 3B) shows collagen IV staining that overlaps the same region of the portal triad that is stained by FIX antibody. However, an area positive for collagen IV, around the liver sinusoids, did not stain for FIX. Likewise, the cuboidal epithelial cells, comprising the bile ducts, stain well for collagen IV but are negative for FIX. Therefore, FIX stains some but not all regions containing collagen IV, demonstrating a selectivity of unknown molecular basis. Immunodetection of FX and FVII (Figure 3C,F, respectively) demonstrates the specificity of FIX detection around the portal triad, because these 2 proteins prominently stain the hepatocytes but not the area of FIX concentration around the portal triad.
FIX's extravascular presence is not unique to hepatic tissue, because staining of skeletal muscles reveals the presence of FIX around the arteries. Figures 3G,H show staining with antibody to FIX or collagen IV, respectively, while Figures 3E,I are negative controls for human liver and skeletal muscle, respectively, stained in the absence of any primary antibody. Again, FIX codistributes in many, but not all, areas where collagen IV is observed. The basement membrane and outer adventitia layers of these artery walls stain well for FIX, but FIX staining is largely absent from the tunica media.
Potential thrombogenicity
We employed an electrolytic model of venous thrombosis14 to examine the thrombogenicity of a high dose of FIX. Hemophilia B mice were infused with 250 IU/kg of BeneFIX, and, 6 hours later, the accumulation of either fibrin (Figure 4A) or platelets (Figure 4B) was monitored at the site of vessel injury. The in situ levels of fibrin formation or platelet aggregation were not greater than that observed in control WT mice. Thus, 250 IU/kg BeneFIX did not increase thrombogenicity.
Venous thrombosis model at 250 IU/kg. The intravital thrombosis assay: an electrolytic injury was delivered to the surface of a mouse femoral vein for 30 seconds (1.5 V anodal current) after the injection of fluorophore labels for fibrin (A) and platelets (B) captured with time-lapse fluorescence imaging from 1 to 60 minutes later. Data are quantitated and normalized every video frame showing comparative levels for WT (blue), FIX knockout mice without treatment (red), or FIX knockout mice with 250 IU/kg BeneFIX injected 6 hours prior to thrombus induction (green lines), using a group of 5 to 6 mice per genotype and treatment. Data are means; errors bars are standard error of the mean. There are no statistical differences between the WT and HemB mice with BeneFIX treatment, whereas the untreated HemB mice had lower levels of accumulation for both thrombotic parameters (P < .001 at 10 or more minutes; analysis of variance).
Venous thrombosis model at 250 IU/kg. The intravital thrombosis assay: an electrolytic injury was delivered to the surface of a mouse femoral vein for 30 seconds (1.5 V anodal current) after the injection of fluorophore labels for fibrin (A) and platelets (B) captured with time-lapse fluorescence imaging from 1 to 60 minutes later. Data are quantitated and normalized every video frame showing comparative levels for WT (blue), FIX knockout mice without treatment (red), or FIX knockout mice with 250 IU/kg BeneFIX injected 6 hours prior to thrombus induction (green lines), using a group of 5 to 6 mice per genotype and treatment. Data are means; errors bars are standard error of the mean. There are no statistical differences between the WT and HemB mice with BeneFIX treatment, whereas the untreated HemB mice had lower levels of accumulation for both thrombotic parameters (P < .001 at 10 or more minutes; analysis of variance).
Discussion
In this study, we compared the efficacy of 2 recombinant commercial FIX products (a WT recombinant FIX and a longer-circulating FIX-Fc fusion protein) at 7 days postinfusion using a saphenous vein bleeding model in hemophilia B mice. FIX binds tightly (5 nM) to collagen IV, which is abundant in the subendothelial matrix. We hypothesized that this interaction might result in large stores of extravascular FIX that could provide hemostatic protection days after circulating levels of injected FIX were undetectable. Measured at 7 days postinfusion of 150 IU/kg, the plasma concentration of Alprolix was still at 2% of normal and BeneFIX was undetectable, yet their clotting efficacy was essentially identical. It is puzzling that both molecules appear to fill an extravascular compartment at the same dose of 150 IU/kg. Given Alprolix's ∼4-fold lower molar-specific activity than BeneFIX,21 and given that the binding of these molecules to collagen IV involves FIX’s Gla domain,22 one would expect that saturation of the extravascular compartment with a given number of FIX molecules of either type should yield less clotting activity (∼3-fold less) in those animals receiving Alprolix than in those receiving BeneFIX. Of course, this assumes that these 2 molecules have the same affinities for collagen IV, the same abilities to access the extravascular collagen IV binding sites, and the same abilities to bind to that collagen. We currently do not know why similar clotting efficacies are achieved 7 days after injecting equal doses (IU/kg) of BeneFIX and Alprolix.
We concentrated on the more clinically relevant human tissue for these experiments; this is because we had previously shown that human FIX, when infused into mice, can be observed in the subendothelium of mouse arteries,16 and there are observations of periportal FIX in hemophilia mice in gene therapy correction trials.18,20 Our observation, that collagen IV lining the normal human liver sinusoids is not heavily decorated with FIX, suggests that these collagen IV molecules are different from those surrounding other vessels. Otherwise, given the KD of FIX for collagen IV and FIX’s normal plasma concentration (∼90 nM), any available collagen IV should be in complex with FIX. Collagen IV, like other collagens, is a triple helix formed from 3 monomers. However, collagen IV molecules are assembled from a combination of 6 different monomers, α1 through α6. Robertson et al23 recently demonstrated that about 12% of collagen IV in the bovine aorta is α121–α565 rather than the predominant α121–α121 form. These findings are consistent with reports that type IV collagen α565 is present in the basement membranes of tubular structures exposed to constant mechanical stress.24 Therefore, a different monomer composition may explain the absence of FIX binding to the sinusoidal collagen IV. Another possible explanation for variable FIX–collagen IV binding is posttranslational modification of collagen IV. A recent report demonstrates that posttranslational modification of collagen IV by a specific prolyl hydroxylase is required to prevent lethal platelet aggregation via the platelet receptor G6BG6B.25 A similar posttranslational modification could render the collagen IV lining the sinusoids incapable of binding with FIX.
The first question that arises when discussing this data with clinicians is: “What about thrombosis at higher doses?” This anxiety arises for several reasons. Historically, when prothrombin complex concentrates were used to treat hemophilia B, frequent thrombotic events were observed. Presumably, this was caused by variable amounts of activated factors, including FVIIa. Two papers that correlate thrombosis and plasma FIX levels are also cited.26,27 These publications report that a 1.3- or 1.5-fold increase in plasma FIX concentrations (ie, greater than the 90th percentile measured in control subjects) correlates with an ∼2-fold increase in thrombosis risk. This risk is modest when compared with the 4-fold increased thrombotic risk of widely used oral contraceptives.28 Even at a dose of 150 IU/kg, plasma BeneFIX concentrations are only transiently elevated above the normal plasma concentration of FIX (D.W.S. and Tong Gui, unpublished data); the normal level is 90 nM or 5 μg/mL (Anthony Hubbard, National Institute of Biological Standards and Control, UK, e-mail, 2016). The convention for pooled normal human plasma, 1 IU/mL, equates to ∼40 IU/kg, assuming 40 mL of plasma per kilogram. In addition to these abstract risk assessments, there is considerable clinical evidence that treating with higher doses of FIX is not thrombogenic. When purified FIX was first introduced, several patients were infused with up to 161 IU/kg and had no observed thromboses.29,30 Moreover, 1000 IU/kg BeneFIX failed to induce thrombosis in the Wessler Stasis model in rabbits.31,32 Also, strong additional clinical evidence is provided by a patient mutation: FIX Padua (FIXR338L). This FIX variant has a specific activity that is about 8-fold greater than that of FIXWT.33 The affected males, who have 8 times the normal level of FIX, developed thromboses at 16 to 20 years of age; their mother, however, who has endogenous expression of 3.5- fold higher-than-normal FIX activity, has had no reported thrombosis. Despite the significant increased risk for thrombosis during her pregnancy (6-fold overall and 60-fold during the 3 months after delivery34 ), this mother had 3 successful pregnancies with no thrombotic complications. FIX Padua patients are chronically exposed to high-activity FIX, whereas hemophilia B patients are only transiently exposed to higher doses of circulating FIX. The normal dose of FIX, 40 to 50 IU/kg, would correspond to normal levels of FIX (90 nM) if all of the infused FIX were retained within the circulation (with an assumed plasma volume of 40 mL/kg). Because a normal individual has about 3 times more extravascular FIX than FIX in circulation, an infusion of 150 IU/kg therapeutic FIX should, after recovery in plasma, result in less circulating FIX than that found in the circulation of a normal patient.10 Thus, 150 IU/kg of infused FIX is less than the total amount of FIX in the combined circulating and extravascular compartments of a normal individual. For example, when hemophilia B mice are infused with 200 IU/kg of BeneFIX, plasma FIX falls to normal levels (∼90 nM, 5 µg/mL) ∼7 minutes postinfusion (D.W.S. and B.C., unpublished observations). The optimal dose for patients whose FIX is defective but nevertheless binds normally to collagen IV is an issue yet to be explored. If the infused FIX must compete with the patient’s defective FIX for extravascular binding sites, these patients may require even higher doses of FIX for prophylactic efficacy.
We also present here experimental evidence that, in mice, 250 IU/kg is not thrombogenic in a specific electrolytic ferric chloride model of injury. Although these experiments were done in mice and do not necessarily extrapolate to human studies, the clinical data discussed above overwhelmingly suggest that 150 IU/kg will not be thrombogenic.
Our hypothesis is that normal coagulation occurs using the extracellular matrix as a scaffold. This hypothesis is reasonable because clotting has evolved primarily to protect from trauma and is required when a vessel is breached, exposing the underlying matrix. Conversely, intravascular clotting is considered pathological. Both tissue factor and FVII are found surrounding vessels.35,36 Because FIX binds to collagen IV in the extracellular matrix, we have most of the important initiators of coagulation already present. However, how does FVIII reach the extravascular scaffold? One possibility is that von Willebrand factor (VWF) binds to collagen IV and brings FVIII to the waiting FIX. This theory is supported by a recent paper demonstrating that VWF binds specifically to collagen IV through its A1 domain. Moreover, several VWF mutations that affect collagen IV binding are associated with bleeding diatheses in patients, which suggests that VWF’s binding to collagen IV is physiologically important.37
In summary, in hemophilia B mice, Alprolix and BeneFIX both protect from bleeding at 7 days postinfusion with essentially identical efficacy. FIX localizes to extravascular tissues surrounding blood vessels in regions that also stain for collagen IV. However, not all apparently available collagen IV binds to FIX. An extravascular compartment—putatively collagen IV—appears to fill at about 150 IU/kg of FIX. We suggest that future research should compare products claiming longer-lasting effects with plasma-derived or recombinant FIXWT, focusing on clinical outcomes rather than plasma serum levels. We also suggest that higher doses of FIX be considered for better prophylactic outcomes. This recommendation, although tempered by the obvious fact that our experimental animals were mice, is strengthened by the recent paper by Kavakli et al,38 which found that patients administered 100 IU/kg of BeneFIX once per week had a bleeding rate only slightly higher than that reported for Alprolix.5
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jane Shealy, Heath Sledge, Katherine A. Stafford, Lynn Rehm, and Sheue-Mei Wu for their help in editing and suggestions to the final version of the paper. The authors also acknowledge the expert animal management provided by Stacie VanLeeuwen. This work was accomplished with a grant from Pfizer Corporation.
Authorship
Contribution: B.C. performed all of the mouse work. A.E. performed the histology work. W.F. interpreted the histology work. D.M. did several important assays and contributed ideas. P.E.M. oversaw animal husbandry of hemophilia B mouse strains and contributed ideas and discussions. F.-C.L. analyzed statistics. D.W.S. wrote the first versions of the paper. D.M.M. helped with several important writing revisions and provided many ideas and contributions regarding experimental design and interpretation.
Conflict-of-interest disclosure: D.W.S. has a research grant from Pfizer and is an occasional consultant. P.E.M. receives research support through the University of North Carolina from Asklepios BioPharmaceutical and Novo Nordisk; he has received research support in the past from Baxter Healthcare, Novo Nordisk, Pfizer, and Prolor. He holds patents licensed to Asklepios, for which he receives royalties. He has received payment for consultation, services, and speaking for Asklepios, Chatham LLC, Baxter Healthcare, and Pfizer and has additionally consulted for Bayer, Novo Nordisk, and Biogen. The remaining authors declare no competing financial interests.
Correspondence: Darrel W. Stafford, Department of Biology, University of North Carolina–Chapel Hill, Chapel Hill, NC 27599-3280; e-mail: dws@e-mail.unc.edu.