Key Points
FVIII-specific IgG antibodies modulate FVIII half-life in patients with hemophilia A, independent of VWF antigen levels and age.
Screening for FVIII-specific IgG may be beneficial in tailoring FVIII prophylactic regimens for hemophilia A patients.
Abstract
The substantial variability in pharmacokinetic parameters in hemophilia patients A poses a challenge for optimal treatment with factor VIII (FVIII) products. We investigated the effect of FVIII-specific immunoglobulin G (IgG) on FVIII half-life in a cohort of 42 adult patients with severe and moderate hemophilia A without inhibitors. Fifteen (35.7%) of 42 patients tested positive for FVIII-binding IgG with titers ≥1:20 in the initial antibody screen, 9 of these 15 patients had FVIII-specific antibodies with titers ≥1:40, mostly low-to-moderate-affinity IgG1 and IgG3, and 1 had high-affinity IgG4 and later developed low-titer FVIII inhibitors. His brother with low-to-moderate-affinity IgG1 and IgG3 also later developed low-titer FVIII inhibitors. The presence of FVIII-specific IgG subclass titer ≥1:40 antibodies was significantly associated with shorter FVIII half-life (median, 7.8 hours [interquartile range, 6.6-9.2 hours]) vs 10.4 hours [interquartile range, 8.9-13.8 hours]); the regression coefficient adjusted for log age and log von Willebrand factor (VWF) antigen was −0.32 (P = .004), accounting for 16.9% of the observed variability of FVIII half-life in our cohort. Our data indicate a significant contribution of non-neutralizing FVIII-specific IgG to FVIII half-life reduction in hemophilia A patients. Thus, screening for FVIII-specific IgG could be beneficial in tailoring FVIII prophylactic regimens.
Introduction
Prophylactic treatment of hemophilia A patients with factor VIII (FVIII) products is presently state of the art.1 FVIII pharmacokinetics differ significantly, which poses a challenge for optimal treatment design. It is generally accepted that the von Willebrand factor (VWF) level significantly influences pharmacokinetic (PK) parameters such as FVIII half-life.2-5 Different VWF levels only partially explain the variability in FVIII half-life, which leaves the question of which other parameters are accountable. This study was conducted to evaluate the effect of non-neutralizing, FVIII-specific immunoglobulin G (IgG) antibodies on FVIII half-life.
Study design
Plasma samples from a cohort of 42 patients with hemophilia A (recently described by Kepa et al2 ) were screened for FVIII-binding IgG antibodies. Antibodies were analyzed by using fully validated semiquantitative, direct-binding enzyme-linked immunosorbent assays (ELISAs) as described in Whelan et al.6 Details of the validation procedure as well as details of the cutoff determination and the sensitivity of the ELISAs are provided in Whelan et al6 and Hofbauer et al.7
Initially, all samples were screened in a 1:20 dilution for FVIII-binding IgG antibodies. Samples that tested positive were characterized for titers of FVIII-binding IgG subclasses and apparent affinities. The specificity of FVIII-binding antibodies was tested in all samples with antibody titers ≥1:40 by using an affinity ELISA.7 Antibodies without conclusive apparent affinity were considered unspecific. Antibodies with titers <1:40 (eg, 1:20) were too low to be confirmable for specificity.
The prevalence of FVIII-binding IgG was evaluated for an association with FVIII half-life independent of the patient’s VWF antigen level and age. Statistical methods are detailed in the supplemental Data, available at the Blood Web site. The study was approved by the Ethics Committee of the Medical University of Vienna. All patients gave written informed consent for participation in the study.
Results and discussion
FVIII half-life for study patients was 6.2 to 20.7 hours. Key cohort characteristics are provided in supplemental Table 1.
Fifteen (35.7%) of 42 patients tested positive for FVIII-binding IgG with titers ≥1:20. Nine of these 15 patients had FVIII-specific antibodies with titers ≥1:40, and 5 had antibody titers of 1:20, which were too low to be confirmable for specificity or for assessment of apparent affinity. One patient had antibodies with a titer of 1:40 which were not specific for FVIII. Supplemental Table 2 summarizes all antibody titers for IgG subclasses and the respective apparent affinities detected in the samples that tested positive for FVIII-specific IgG with titers ≥1:40. FVIII-specific IgG1 and IgG3 were the predominant subclasses detected, consistent with data published for non-neutralizing FVIII-binding antibodies in hemophilia A patients.6 Apparent affinity clusters were in a range similar to that reported previously.7
One patient (patient 2) had apparent high-affinity FVIII-specific IgG4 with a titer of 1:40. This patient developed a low-titer FVIII inhibitor 12 months after the PK assessment. Between PK assessment and first positive inhibitor detection, he had 8 negative inhibitor tests. These data confirm our previous observation, that high-affinity FVIII-specific IgG4 might be a predictor for an evolving FVIII inhibitor response.7 FVIII inhibitors disappeared after a few months on intensified prophylaxis (35 units per kilogram of body weight every other day). Patient 3 is a brother of patient 2. He had FVIII-specific subclass IgG1 (titer 1:40) and IgG3 (titer 1:40) antibodies at the time of PK assessment. The IgG1 antibodies included 2 affinity clusters, one with low affinity and one with medium affinity. The IgG3 antibodies had low affinity. Patient 3 developed a low-titer inhibitor 7 months after the PK assessment. This patient had several negative inhibitor determinations between PK analysis and first inhibitor detection. We do not know whether his medium-affinity FVIII-specific IgG1, which was detectable at the time of PK assessment, underwent further affinity maturation into a high-affinity IgG1 or whether it became a high-affinity IgG4 in the course of inhibitor development.
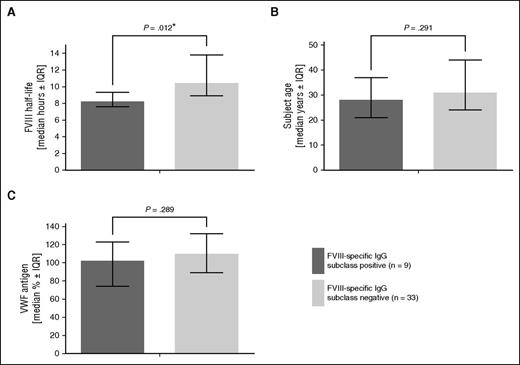
When we compared the FVIII half-life of all 15 patients who tested positive in the initial antibody screen with those who tested negative, we did not see any significant difference (P = .112; supplemental Table 3A). However, if we included only patients who had FVIII-specific antibodies (titer ≥1:40 and confirmed specificity), we found a significant difference in FVIII half-life (P = .012), indicating that those FVIII-specific non-neutralizing antibodies had a significantly shorter half-life (Figure 1 and supplemental Table 3B). No significant difference in VWF levels and age was observed between patients with and without FVIII-specific antibodies.
FVIII half-life, age, and VWF antigen levels in hemophilia A subjects that tested positive or negative for FVIII-specific IgG subclasses. (A) FVIII half-life, (B) patient age, and (C) VWF antigen levels. Statistical significance (*) was calculated by Student t test. Data are given as median and interquartile range.
FVIII half-life, age, and VWF antigen levels in hemophilia A subjects that tested positive or negative for FVIII-specific IgG subclasses. (A) FVIII half-life, (B) patient age, and (C) VWF antigen levels. Statistical significance (*) was calculated by Student t test. Data are given as median and interquartile range.
To evaluate whether the observed influence of FVIII-specific IgG on FVIII half-life was confounded by age and VWF antigen levels, simple and multiple regression analysis was performed (Table 1). Both models confirmed the association of FVIII-specific IgG and VWF antigen with FVIII half-life. Differences in marginal and partial results for the effect of age were primarily a result of nonlinear distribution of FVIII-specific IgG over age. The R2adj value for a model containing the variables log age and log VWF antigen increased from 25.9% to 35.8% when FVIII-specific IgG subclasses were added. This result indicated that FVIII-specific IgG is an independent, explanatory parameter for log FVIII half-life variability in this cohort.
Results of marginal and partial regression analyses for FVIII half-life
| Variable . | Marginal results . | Partial results (R2adj = 35.8%) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Regression coefficient . | 95% CI . | P . | R2adj (%) . | Regression coefficient . | 95% CI . | P . | R2adj (%) . | |
| Log age | 0.316 | 0.048 to 0.584 | .022 | 10.2 | 0.157 | –0.084 to 0.397 | .195 | 1.2 |
| Log VWF antigen | 0.465 | 0.210 to 0.721 | <.001 | 23.3 | 0.361 | 0.112 to 0.610 | .006 | 12.6 |
| FVIII-specific IgG subclasses | −0.32 | –0.532 to −0.11 | .004 | 16.9 | −0.249 | –0.439 to −0.058 | .012 | 9.9 |
| Variable . | Marginal results . | Partial results (R2adj = 35.8%) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Regression coefficient . | 95% CI . | P . | R2adj (%) . | Regression coefficient . | 95% CI . | P . | R2adj (%) . | |
| Log age | 0.316 | 0.048 to 0.584 | .022 | 10.2 | 0.157 | –0.084 to 0.397 | .195 | 1.2 |
| Log VWF antigen | 0.465 | 0.210 to 0.721 | <.001 | 23.3 | 0.361 | 0.112 to 0.610 | .006 | 12.6 |
| FVIII-specific IgG subclasses | −0.32 | –0.532 to −0.11 | .004 | 16.9 | −0.249 | –0.439 to −0.058 | .012 | 9.9 |
Marginal results for log age and log VWF antigen are not adjusted for FVIII-specific IgG subclasses. Partial results are adjusted for all other factors.
There were 4 pairs of brothers in our cohort; one pair had antibodies against FVIII and developed the inhibitors; none of the brothers in the 3 other pairs had antibodies against FVIII. After exclusion of one individual of each brother pair (always the younger one), the presence of FVIII-specific IgG subclass remained significantly associated with FVIII half-life (P = .019) and the R2adj value for log age and log VWF antigen increased from 22.6% to 31.7% when FVIII-specific IgG subclasses were added.
Neutralizing FVIII-specific IgG in FVIII inhibitor patients is well described.6-10 FVIII inhibitors mainly consist of apparent high-affinity polyclonal FVIII-specific IgG1 and IgG4 antibodies, and titers correlate with neutralizing capacity.6,7 Otherwise, non-neutralizing FVIII-specific IgG in hemophilia A patients without inhibitors is described to be of low to moderate apparent affinity, with a predominance of FVIII-binding IgG1 and IgG3.6,7
The clinical relevance of non-neutralizing FVIII-binding antibodies on FVIII PK in hemophilia A patients has been a subject of controversy for almost 20 years. Dazzi and colleagues11 suggested a possible interrelation, but the experimental evidence was limited. No influence of FVIII-binding antibodies on FVIII in vivo recovery (IVR) was found by using an FVIII-specific IgG by immunoprecipitation assay and ELISA-based FVIII-specific IgG detection,12,13 whereas a lower IVR was detected by a Luminex-based assay.3 We did not find an association between IVR and FVIII-binding antibodies. The median IVR was 2.3% (interquartile range [IQR] 1.7-3.1) U/kg in the 9 patients with and 2.4 (IQR 2.1-3.1) in those without FVIII-antibodies (P = .318).
Our study confirms the observed effect of FVIII-specific IgG on FVIII half-life. However, our data indicate that only FVIII-binding antibodies with titers ≥1:40 and confirmed specificity correlated with a shorter FVIII half-life, indicating that confirmation of FVIII specificity and the semiquantitative titer determination are important parameters.
Discrepant conclusions in previous studies might be explained by the lack of sufficient titers of antibodies together with the confirmation of FVIII specificity and by low statistical power.
Follow-up samples from all 9 patients who tested positive for FVIII-specific IgG subclasses with titer ≥1:40 and from 20 of 28 patients who tested negative were re-collected after a median time of 21 months (range, 6-43 months). The five patients with the highest FVIII-specific IgG titers did not undergo intensified FVIII treatment and remained positive for antibodies against FVIII, indicating that high titer of FVIII-specific IgG antibodies was persistent, whereas none of the 20 initially negative patients developed antibodies.
Patients 2 and 3 were re-analyzed after their inhibitors had been eradicated by either intensified prophylaxis or immune tolerance induction therapy. Both patients lost their FVIII-specific antibodies. The additional PK assessment after loss of FVIII-specific antibodies revealed an FVIII half-life of 10.8 hours (initially, 6.6 hours) in patient 2 and an FVIII half-life of 8.6 hours (initially, 6.6 hours) in patient 3. There was an extended FVIII half-life in these patients after the disappearance of FVIII-specific antibodies, which strengthens our hypothesis that non-neutralizing FVIII-specific antibodies have an influence on FVIII half-life.
In conclusion, our data demonstrate that non-neutralizing, FVIII-specific IgG with a titer ≥1:40 is an additional factor in determining FVIII half-life, independent of VWF antigen level. If these data could be confirmed in an independent clinical study, the screening for FVIII-specific IgG may be beneficial for tailoring FVIII prophylactic regimens.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Fatima Al-Awadi for excellent technical assistance and Karima Benamara for editing the manuscript. We also thank the patients for participating in this research.
This work was supported by Baxalta and by an unrestricted grant from the Austrian Haemophilia Society within the framework of the Austrian Haemophilia Registry.
Authorship
Contribution: C.J.H., B.M.R., and I.P. designed the research; S.K. and S.R.-P. collected clinical information, informed consent, and patient material; C.J.H., B.M.R., P.Q., S.K., C.M., and I.P. analyzed and interpreted data; M.S. performed statistical modeling; C.J.H., B.M.R., and I.P. wrote the paper; and all authors approved the final version.
Conflict-of-interest disclosure: C.J.H. and B.M.R. are employees of Baxalta. The remaining authors declare no competing financial interests.
Correspondence: Ingrid Pabinger, Medical University of Vienna, Department of Medicine I, Clinical Division of Haematology and Haemostaseology, Waehringer Guertel 18-20, A-1090 Vienna, Austria; e-mail: ingrid.pabinger@meduniwien.ac.at.
References
Author notes
C.J.H. and S.K. contributed equally to this manuscript.
B.M.R. and I.P. share senior authorship.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal