Key Points
GCK signaling is activated in DLBCL, and this signaling is important to DLBCL proliferation and survival.
Therapeutic targeting of GCK is feasible and may advance efforts to cure DLBCL patients.
Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma, yet 40% to 50% of patients will eventually succumb to their disease, demonstrating a pressing need for novel therapeutic options. Gene expression profiling has identified messenger RNAs that lead to transformation, but critical events transforming cells are normally executed by kinases. Therefore, we hypothesized that previously unrecognized kinases may contribute to DLBCL pathogenesis. We performed the first comprehensive analysis of global kinase activity in DLBCL, to identify novel therapeutic targets, and discovered that germinal center kinase (GCK) was extensively activated. GCK RNA interference and small molecule inhibition induced cell-cycle arrest and apoptosis in DLBCL cell lines and primary tumors in vitro and decreased the tumor growth rate in vivo, resulting in a significantly extended lifespan of mice bearing DLBCL xenografts. GCK expression was also linked to adverse clinical outcome in a cohort of 151 primary DLBCL patients. These studies demonstrate, for the first time, that GCK is a molecular therapeutic target in DLBCL tumors and that inhibiting GCK may significantly extend DLBCL patient survival. Because the majority of DLBCL tumors (∼80%) exhibit activation of GCK, this therapy may be applicable to most patients.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is a genetically and clinically heterogeneous disease.1 The standard treatment includes the anti-CD20 antibody rituximab with cyclophosphamide, doxorubicin, vincristine, prednisone (R-CHOP), curing only about 50% of patients.2,3 Therefore, to improve the cure rate, novel molecular targets and therapeutic approaches are urgently needed. Achieving these goals will only be possible through mechanistic insights into DLBCL pathogenesis that will guide the development of targeted therapeutic agents.
The pathogenesis of DLBCL represents a multistep process that involves the accumulation of multiple genetic and molecular lesions.1 Marked advances in the understanding of DLBCL pathobiology have been made by the application of gene expression arrays, comparative genomic hybridization arrays, and “next” generation sequencing, leading to the identification of previously unrecognized germinal centerlike (GCB) and activated B-cell-like (ABC) DLBCL subtypes and subtype-specific deregulation of signaling pathways.4-9 Although markedly advancing our understanding of DLBCL pathogenesis, these approaches focused on genetic aberrations and mRNA expression profiles, whereas critical events transforming normal cells are executed by proteins. To broadly examine which kinases and signal transduction pathways may contribute to DLBCL pathogenesis and to identify novel therapeutic targets, we analyzed global kinase activity and expression in DLBCL.
Methods
Reagents
The following antibodies were used for western blots: JNK1 (FL), phospho-JNK (G-7), and GAPDH (glyceraldehyde-3-phosphate dehydrogenase; 0411) from Santa Cruz Biotechnology (Santa Cruz, CA), p38α (5F11) and phospho-p38α (28B10) from Cell Signaling (Boston, MA), and MAP4K2, p-MAP3K1, and p-MAP2K7 from Sigma-Aldrich (St. Louis, MO). Immunohistochemistry (IHC) antibodies are described in the supplemental Methods, available on the Blood Web site.
HG6-64-1 was synthesized in our laboratory as reported.10 Unless otherwise stated, all experiments in DLBCL cells were performed at a concentration of 400 nM. Doxorubicin was from Sigma-Aldrich.
Cell lines
The DLBCL cell lines SU-DHL-6, SU-DHL-8, VAL, Granta 452 (G452), OCI-LY-3, OCI-LY-8, OCI-LY-10, OCI-LY-19, and RIVA were grown in Iscove modified Dulbecco medium (Mediatech Inc, Manassas, VA) supplemented with 20% human plasma (Florida’s Blood Centers, Orlando, FL) and 50 μM 2-mercaptoethanol (Gibco, Grand Island, NY). Human Embryonic Kidney 293T and Chinese hamster ovary cells were grown in Dulbecco modified Eagle medium (Mediatech Inc). All media were supplemented with 2 nM glutamine (Gibco) and penicillin/streptomycin (Gibco).
Other methods are described in the supplemental Methods.
Results
The MAPK pathway is broadly activated in DLBCL cell lines
To broadly interrogate kinase signaling in DLBCL pathogenesis, we profiled functional kinase expression and activity in 9 DLBCL cell lines and a pool of B cells enriched from 2 normal tonsils using the chemical proteomics KiNativ Platform (ActivX Biosciences). In this assay, biotin-labeled adenosine triphosphate (ATP)- and adenosine 5′-diphosphate–mimetic acyl-phosphate probes bind covalently with conserved lysine residues in accessible kinase ATP-binding pockets, allowing quantitative assessment of affinity/occupancy across the majority of kinases present in cellular lysates using liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS)11 (supplemental Figure 1). The probe binds only those kinases with mutable ATP-binding pockets, which are in the active configuration, as well as all kinases that have static ATP-binding pockets. This methodology enables reproducible quantitative measurement of the activity and expression of ∼80% of the kinome.
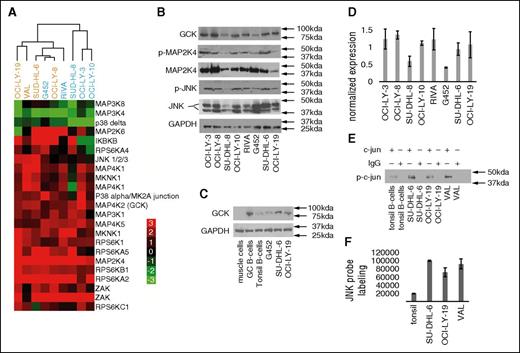
The KiNativ platform detected 153 kinases that were measurable in at least one of the analyzed samples. Of those, several kinases exhibited significantly higher or lower probe labeling in DLBCL cell lines compared with the pool of normal B cells. Kinases with at least a 2-fold change in probe labeling in DLBCL, relative to normal B cells, were selected, mapped to gene symbols, and annotated by comparison with curated gene sets corresponding to canonical molecular pathways (Broad Institute Molecular Signatures Database). The complete data set is available in the supplemental materials spreadsheet entitled, “Raw Kinase Data.” As anticipated from the current knowledge of DLBCL pathogenesis,12-14 there was statistically significant enrichment (P = 2.89 × 10−14 by hypergeometric test) in the components of the B-cell receptor (BCR) signaling pathway in DLBCL cell lines when compared with normal B cells (supplemental Table 1 and supplemental Figure 2). Additional pathways and kinases known to be activated in DLBCL (eg, MYD88) were also statistically significantly enriched. A summary of the functional annotation results is listed in supplemental Table 1. Interestingly, components of the MAPK signaling pathway were even more prominently enriched (P = 1.38 × 10−25) than the BCR pathway. A total of 19 MAPK pathway proteins demonstrated a 2-fold or greater increase in KiNativ probe labeling in DLBCL cell lines compared with normal B cells, whereas 3 MAPK pathway signaling proteins demonstrated lower probe labeling (Figure 1A).15
The MAPK family of proteins is upregulated in DLBCL cell lines. (A) An unsupervised heat map comprising the MAPK pathway proteins exhibiting up- or downregulation in 9 DLBCL cell lines, when compared with normal B cells from reactive tonsils. The displayed matrix represents the log2 ratio of LC-MS/MS detected activity/expression of the kinases from each experimental DLBCL cell line relative to normal B lymphocytes, as depicted by the corresponding color scale. Each row represents a separate probe binding site for the indicated kinase, identified and quantified by LC-MS/MS, and each column represents a separate DLBCL cellular lysate. KiNativ data were clustered with Cluster software and visualized with Treeview.15 ABC-like DLBCLs are depicted in blue, and GCB-like DLBCLs are depicted in orange. Similar results were obtained in one additional KiNativ experiment. (B) Immunoblotting of GCK, p-MAP2K4, MAP2K4, p-JNK, and JNK total proteins in unmanipulated DLBCL cell lines. Similar results were obtained in 3 sets of independent blots. (C) Immunoblotting of GCK total protein in muscle cells, human germinal center (GC) B cells, tonsillar B cells, and unmanipulated DLBCL cell lines. Similar results were obtained in 3 sets of independent blots. (D) Normalized expression reflecting average densitometry readings of 3 independent experiments in which total GCK protein levels were quantified and normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) level for protein concentration. Quantification was performed using Image J software (NIH). (E) JNK kinase assay. JNK was immunoprecipitated from the indicated cell lines and tonsils and used to phosphorylate its target c-jun in the presence of ATP. p-c-jun is detected by immunobloting. (F) LC-MS/MS signal for JNK after pull-down with the KiNativ ATP-mimetic probe in B cells purified from human tonsils, and in DLBCL cell lines. KiNativ data (A) is representative of 2 independent experiments. Results in (B-C,E-F) are representative of 3 independent experiments. Results in (D) are the normalized average ± standard error of the mean of 3 independent experiments.
The MAPK family of proteins is upregulated in DLBCL cell lines. (A) An unsupervised heat map comprising the MAPK pathway proteins exhibiting up- or downregulation in 9 DLBCL cell lines, when compared with normal B cells from reactive tonsils. The displayed matrix represents the log2 ratio of LC-MS/MS detected activity/expression of the kinases from each experimental DLBCL cell line relative to normal B lymphocytes, as depicted by the corresponding color scale. Each row represents a separate probe binding site for the indicated kinase, identified and quantified by LC-MS/MS, and each column represents a separate DLBCL cellular lysate. KiNativ data were clustered with Cluster software and visualized with Treeview.15 ABC-like DLBCLs are depicted in blue, and GCB-like DLBCLs are depicted in orange. Similar results were obtained in one additional KiNativ experiment. (B) Immunoblotting of GCK, p-MAP2K4, MAP2K4, p-JNK, and JNK total proteins in unmanipulated DLBCL cell lines. Similar results were obtained in 3 sets of independent blots. (C) Immunoblotting of GCK total protein in muscle cells, human germinal center (GC) B cells, tonsillar B cells, and unmanipulated DLBCL cell lines. Similar results were obtained in 3 sets of independent blots. (D) Normalized expression reflecting average densitometry readings of 3 independent experiments in which total GCK protein levels were quantified and normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) level for protein concentration. Quantification was performed using Image J software (NIH). (E) JNK kinase assay. JNK was immunoprecipitated from the indicated cell lines and tonsils and used to phosphorylate its target c-jun in the presence of ATP. p-c-jun is detected by immunobloting. (F) LC-MS/MS signal for JNK after pull-down with the KiNativ ATP-mimetic probe in B cells purified from human tonsils, and in DLBCL cell lines. KiNativ data (A) is representative of 2 independent experiments. Results in (B-C,E-F) are representative of 3 independent experiments. Results in (D) are the normalized average ± standard error of the mean of 3 independent experiments.
To better delineate the specific contributors to MAPK cascade activation in DLBCL, we searched for activated kinases whose downstream signaling components were also activated. This analysis highlighted the activation of MAP4K2 (germinal center kinase, GCK), a member of the MAP4K family.16,17 To assert the specificity of the platform, we analyzed several non-DLBCL cell lines. We found heterogeneous probe labeling of GCK in non-DLBCL cell lines using this methodology (supplemental Table 2). This result further suggests that GCK activation is not an inherent property of all cancer cell lines. GCK is a constitutively active kinase regulated by ubiquitination and proteosomal degradation.18 Its activation is induced by extracellular signaling via activation of TRAF6. Concordantly, there was statistically significant enrichment (P = 1.30 × 10−13 by hypergeometric test) in the components of the TRAF6 signaling pathway in DLBCL cell lines when compared with normal B cells (supplemental Table 1). GCK signals through many downstream kinases, including MAP3K, MAP2K, and MAPK proteins. At the MAP2K level, MKK4 (MAP2K4) is extensively activated in our samples (Figure 1A), which is reported to lead to activation of the JNK pathway.19 In the analyzed DLBCL cell lines, JNK family probe labeling was amplified. Overall, we demonstrated the enrichment of multiple MAPKs that have never before been implicated in the pathogenesis of DLBCL.
To independently confirm the KiNativ results, we immunoblotted for members of the MAPK cascade. Immunoblotting revealed expression of GCK and suggested relatively greater activation of p-MAP2K4 and p-JNK in those cell lines with higher levels of total GCK protein (Figure 1B). To explore the apparent relationship between total GCK, p-MAP2K4, and p-JNK expression levels, we used densitometry to quantitate the relationship between GCK protein level and its reported downstream signaling components. We discovered a general tendency for levels of p-JNK and p-MAP2K4 to be higher in those cells lines that also had higher GCK (supplemental Figure 3), in good concordance with the KiNativ results. We further compared GCK levels in select DLBCL cell lines to germinal center B cells (purified as described20 ) and tonsillar B cells, as well as to muscle cells, which are known to be GCK negative. As expected from KiNativ results, tonsillar B cells as a whole expressed lower levels of GCK than DLBCL cells lines. Isolated germinal center B cells also had a higher GCK expression level than tonsillar B cells (Figure 1C).
Importantly, the kinase activity of GCK is reported to be regulated at the protein level: GCK is primarily active when dimerized, and GCK phosphorylation has been shown to be irrelevant to its kinase activity.18 Although GCK total protein level varies across the tested DLBCL cell lines, it was apparent that the lowest expression of GCK was observed in the G452 cell line, as can be seen from densitometry of several independently run experiments (Figure 1D). Notably, the DLBCL cell line G452 expresses very low GCK levels using both KiNativ and Western methodologies. The kinase array data were further corroborated with classical immunoprecipitation-based JNK and p38 kinase assays in selected cell lines (Figure 1E-F, and supplemental Figure 4).
The reason for GCK expression in DLBCL tumors is unknown and may reflect their origin from germinal center B cells that also express GCK. Analysis of the publicly available data failed to identify reported mutations or gene locus amplifications in DLBCL tumors. However, in the course of our study, we observed that GCK protein levels in the cells vary according to the degree at which their media have been preconditioned. Spinning down the cells, washing them, and replacing their media lead to a rapid degradation of GCK protein levels for up to 24 hours. However, reintroduction of the same conditioned media restores GCK levels within 2 hours (supplemental Figure 5, left panel). These observations suggest the presence of an autocrine factor that may stimulate GCK expression. It is possible that, in addition to autocrine factors in vivo, GCK may be regulated by paracrine factors. For example, CD40 engagement is a known mechanism of GCK stimulation.18 Accordingly, CD40L stimulation in DLBCL cell lines induced GCK expression in DLBCL cell lines (supplemental Figure 5, middle and right panels). These observations suggest that autocrine and paracrine signaling regulates GCK protein expression in DLBCL cell lines and primary tumors. Therefore, all the experiments reported herein were performed while strictly controlling growth conditions and monitoring of GCK levels in cell lines.
The GCK pathway is activated in primary DLBCL tumors
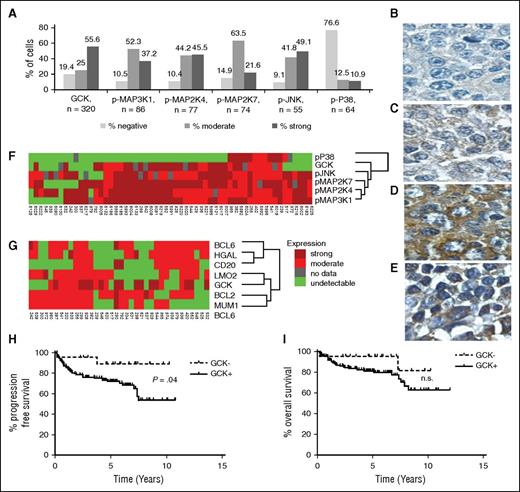
To demonstrate that GCK signaling pathway activation was not restricted to established DLBCL cell lines, we evaluated the expression of GCK, and several of its reported downstream signaling components, including p-MAP3K1, p-MAP2K4, p-MAP2K7, and p-JNK, by IHC in up to 320 de novo untreated primary DLBCL tumors. One hundred seventy-eight of 320 tumors (55.6%) exhibited strong positive cytoplasmic staining for GCK, and 80 tumors (25.0%) had moderate cytoplasmic GCK staining, accounting for a total of 80.6% GCK-positive cases (Figure 2A). This quantity corresponds well to the KiNativ data in which 8 of 9 DLBCL cell lines (88.9%) were GCK positive. Representative staining for GCK in primary DLBCL tumors is shown in Figure 2B-D. It is important to note that noncancerous lymphoid tissue may also be GCK positive (Figure 2E), corresponding to western blot findings in normal B cells (Figure 1C) and suggesting that GCK expression per se is not the result of transformation. Many of GCK’s reported downstream signaling components16 were activated in >80% of the analyzed primary DLBCL samples (Figure 2A) and demonstrated excellent concordance with GCK expression by coclustering (Figure 2F). In contrast, only 23.4% of primary DLBCL tumors were positive for p38 expression, which clustered on a separate dendrogram branch. To determine whether GCK expression was DLBCL subtype specific, we performed a cluster analysis using germinal center markers (CD10, HGAL, BCL6, and LMO2) and nongerminal center markers (BCL2 and MUM1).4,21-23 GCK clustered with germinal center marker LMO2, as well as ABC-like markers BCL2 and MUM1, suggesting that it is not specifically expressed in either the GCB-like or the non-GCB-like DLBCLs (Figure 2G). Furthermore, classification of the DLBCL cases by Han’s algorithm confirmed that GCK is expressed in both GCB and non-GCB DLBCL (not shown). Overall, these findings demonstrate activation of GCK in the majority of DLBCL tumors irrespective of their cell of origin.
GCK and its downstream effectors are upregulated in de novo primary DLBCL, in a subtype-independent manner. (A) Summary of IHC results from primary DLBCL tumors. Representative staining for GCK negative (B), moderate (C), and strong (D) expressing de novo primary DLBCL tumors. (E) A noncancerous lymph node stained for GCK expression. (F) Fifty-seven primary DLBCLs were analyzed by IHC for GCK, pMAP3K1, pMAP2K7, pMAP2K4, pJNK, and pP38, and (G) 36 primary DLBCLs were stained for GCK, the germinal center markers BCL6, CD10, HGAL, and LMO2, and the ABC-like markers BCL2 and MUM1. Tumor samples were clustered with Cluster software and visualized with Treeview. Kaplan–Meier plots of (H) PFS (P = .04) and (I) overall survival of 151 DLBCL patients treated with R-CHOP grouped on the basis of IHC staining for GCK. The positive cases including GCK medium (n = 33) and high expressing cases (n = 98) were analyzed together. n.s., results that are not statistically significant.
GCK and its downstream effectors are upregulated in de novo primary DLBCL, in a subtype-independent manner. (A) Summary of IHC results from primary DLBCL tumors. Representative staining for GCK negative (B), moderate (C), and strong (D) expressing de novo primary DLBCL tumors. (E) A noncancerous lymph node stained for GCK expression. (F) Fifty-seven primary DLBCLs were analyzed by IHC for GCK, pMAP3K1, pMAP2K7, pMAP2K4, pJNK, and pP38, and (G) 36 primary DLBCLs were stained for GCK, the germinal center markers BCL6, CD10, HGAL, and LMO2, and the ABC-like markers BCL2 and MUM1. Tumor samples were clustered with Cluster software and visualized with Treeview. Kaplan–Meier plots of (H) PFS (P = .04) and (I) overall survival of 151 DLBCL patients treated with R-CHOP grouped on the basis of IHC staining for GCK. The positive cases including GCK medium (n = 33) and high expressing cases (n = 98) were analyzed together. n.s., results that are not statistically significant.
To determine whether patients with GCK expressing or nonexpressing tumors may exhibit different outcomes, we analyzed the survival of these subgroups in a total of 151 primary diagnostic DLBCL specimens from patients with a median age of 62 years (range 24-86) that were treated uniformly with R-CHOP chemotherapy. Notably, DLBCL patients with no GCK expression exhibited significantly longer progression-free survival (PFS) compared with DLBCL patients whose tumors expressed GCK (P = .04; Figure 2H), with an estimated PFS of 89% at 10 years of follow-up (95% confidence interval 0.76 to 1). Although there was a similar trend in overall survival (OS) (Figure 2I), it did not reach statistical significance, which may be due to the relatively small number of DLBCL cases not expressing GCK and the potential rescue of these patients with second-line treatments. The analyzed GCK-positive DLBCL cases comprised 33 tumors with medium GCK expression and 98 with high GCK expression. There was no difference in PFS and OS between cases with medium and high GCK expression (not shown). In multivariate analyses that included International Prognostic Index (IPI) scores or IPI individual components and GCK protein expression with OS or PFS as the dependent variable(s), GCK expression did not reach independent predictive significance, likely due to the small number of patients with GCK-negative DLBCL tumors (only 20 cases). We did not detect a correlation between GCK expression and the IPI. Overall, these observations suggest that DLBCL tumors expressing GCK may exhibit more aggressive biological behavior associated with chemotherapy resistance and more frequent relapses.
The GCK pathway contributes to DLBCL proliferation and survival
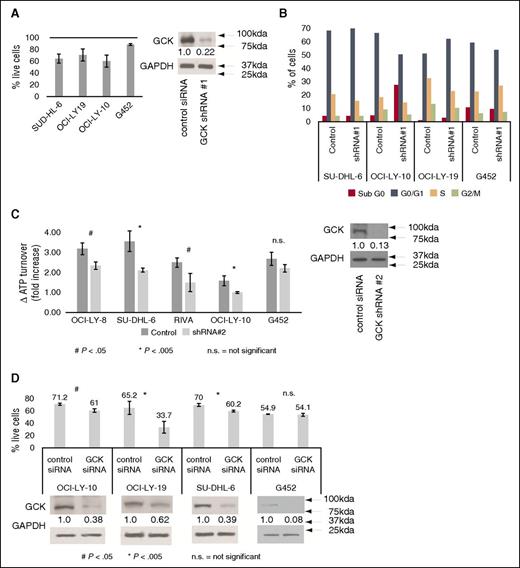
To delineate the function of GCK in DLBCL, we infected cell lines with a lentivirus-based vector containing GFP and a short hairpin RNA (shRNA; #1) against GCK, or a control nontargeting shRNA. We examined cell survival by flow cytometry by gating for GFP-positive cells, to confirm vector expression, and using the viability stain SYTOX Blue. GCK knockdown led to a reduction in the number and percentage of live cells in SU-DHL-6, OCI-LY-19, and OCI-LY-10 cell lines. The effect was most pronounced 72 hours postinfection (Figure 3A). Supplemental Figure 6 depicts a representative time course of the same experiments. This effect was muted in G452, which minimally expresses GCK, and these effects were associated with a partial G0/G1 arrest in sensitive cell lines (Figure 3B).
GCK RNA interference leads to cell death and cell-cycle arrest of DLBCL cell lines. (A) DLBCL cells were infected with lentiviral vectors expressing both GFP and either shRNA#1 directed against GCK or a nontargeting (control) vector. The percentage of live cells in the GCK shRNA vector expressing cells at 72 hours postinfection are presented as a percentage of the control cells. The efficacy of these shRNAs is demonstrated by immunoblotting for V5-tagged GCK in 293T cells. (B) Cell-cycle analysis of GCK shRNA#1 vector or control infected cells, representative of results in 3 independent experiments. (C) DLBCL cells were infected with lentiviral vectors with a puromycin resistance cassette and either shRNA#2 directed against GCK or a nontargeting (control) vector. ATP turnover was measured using the ATPlite System, as described in “Methods,” to simultaneously assess growth and viability. A western blot showing representative knockdown in SU-DHL-6 cells is to the right. This experiment was performed 3 times. (D) ON-TARGETplus siRNA was transfected using AMAXA electroporation to knock down GCK expression in OCI-LY-10, OCI-LY-19, SU-DHL-6, or G452 cells. Survival of cells at 72 hours posttransfection was analyzed by flow cytometry. Western blots showing representative knockdown in the same cell line are beneath each survival graph. All experiments were performed at least 3 times. Data in (A,C-D) are represented as mean ± standard error of the mean.
GCK RNA interference leads to cell death and cell-cycle arrest of DLBCL cell lines. (A) DLBCL cells were infected with lentiviral vectors expressing both GFP and either shRNA#1 directed against GCK or a nontargeting (control) vector. The percentage of live cells in the GCK shRNA vector expressing cells at 72 hours postinfection are presented as a percentage of the control cells. The efficacy of these shRNAs is demonstrated by immunoblotting for V5-tagged GCK in 293T cells. (B) Cell-cycle analysis of GCK shRNA#1 vector or control infected cells, representative of results in 3 independent experiments. (C) DLBCL cells were infected with lentiviral vectors with a puromycin resistance cassette and either shRNA#2 directed against GCK or a nontargeting (control) vector. ATP turnover was measured using the ATPlite System, as described in “Methods,” to simultaneously assess growth and viability. A western blot showing representative knockdown in SU-DHL-6 cells is to the right. This experiment was performed 3 times. (D) ON-TARGETplus siRNA was transfected using AMAXA electroporation to knock down GCK expression in OCI-LY-10, OCI-LY-19, SU-DHL-6, or G452 cells. Survival of cells at 72 hours posttransfection was analyzed by flow cytometry. Western blots showing representative knockdown in the same cell line are beneath each survival graph. All experiments were performed at least 3 times. Data in (A,C-D) are represented as mean ± standard error of the mean.
To confirm these observations, we next infected a panel of DLBCL cell lines with a second lentivirus-based vector containing a puromycin resistance cassette and a different shRNA (shRNA#2) against GCK, or a control nontargeting shRNA. One week after infection (on day 3 after puromycin selection), cells were immunoblotted to ensure GCK knockdown and simultaneously analyzed for proliferation and cytotoxicity by evaluating ATP turnover (Figure 3C). We found that shRNA#2 reduced ATP turnover in OCI-LY-8, SU-DHL-6, RIVA, and OCI-LY-10 relative to the control shRNA, and the effect was muted in G452. These experiments were also performed with an additional shRNA with similar results (supplemental Figure 7).
Finally, we transfected small interfering RNA (siRNA) against GCK, or a control nontargeting siRNA, into the cell lines OCI-LY-10, OCI-LY-19, SU-DHL-6, and G452. Transfection of GCK siRNA also reduced the viability of all analyzed cell lines except G452 (Figure 3D).
Overall, our results obtained by application of different shRNAs and siRNA implicate GCK in the regulation of proliferation and survival of DLBCL, suggesting that it may serve as a potential therapeutic target.
Chemical targeting of GCK signaling
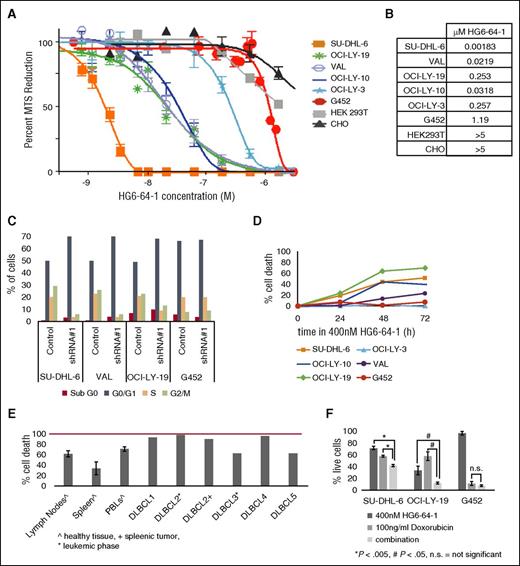
To evaluate the effects of inhibiting this pathway in DLBCL cell lines, we initially examined the effects of inhibiting GCK using a recently developed GCK inhibitor, HG6-64-1.10 In DLBCL cell lines, the 50% effective concentration (EC50) of HG6-64-1 ranged from 1.83 to 257 nM, with the exception of the low GCK-expressing cell line G452, which had an EC50 of 1.19 µM (Figure 4A-B). The molecular mechanism that leads to a relatively wide range of sensitivities to HG6-64-1 within DLBCL cell lines is not known, but may relate to variable dependence on this particular pathway. Such variable sensitivity to drugs within a disease model is fairly common, although the specific reason for this type of response is currently not well understood. Of note, HEK293T and Chinese hamster ovary cells were minimally affected by HG6-64-1 at concentrations up to 5 µM. A commercially available assay measuring the kinase activity/direct targets of GCK was not available, so we looked at its reported downstream effector, JNK. As predicted, HG6-64-1 (400 nM) impeded the ability of JNK to phosphorylate c-jun in western-based kinase assay in all 5 tested cell lines (supplemental Figure 8A). Notably, HG6-64-1 acts by directly inhibiting GCK but not its downstream effector JNK. All HG6-64-1-treated cell lines exhibited reductions of 35% to 75% in p-c-jun when compared with DMSO-treated controls. We also blotted for p-JNK in several cell lines, after HG6-64-1 treatment. We found that p-JNK levels were reduced in a time-dependent manner in SU-DHL-6 cells following treatment with HG6-64-1 (supplemental Figure 8B). This reduction was reproducible in other DLBCL cell lines (supplemental Figure 8C).
HG6-64-1, a chemical GCK inhibitor. (A) Six DLBCL cell lines and 2 control, non-DLBCL cell lines, were treated with serial dilutions of HG6-64-1. 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium (MTS) readings were taken just prior to and after drug addition and compared with control, DMSO-treated cells. Cellular proliferation was calculated as the change in MTS reading for each experimental condition, divided by the increase in DMSO-treated cells. (B) The calculated EC50 of HG6-64-1 for each listed cell line. (C) Asynchronously growing DLBCL cell lines were subject to media change followed by treatment with HG6-64-1 or the vehicle, DMSO. After treatment, cells were ethanol permeabilized and saturated with PI in PBS. Cell cycle was analyzed by flow cytometry, and results are depicted in a bar graph. (D) Time-dependent measurement of cellular death by flow cytometry in DLBCL cell lines. (E) Flow cytometry measurement of cellular death at 48 hours of tumor B cells isolated from fresh biopsies of DLBCL primary tumors, or normal B cells isolated from healthy tissue, and treated with HG6-64-1 or DMSO. In one primary sample, cells were derived from the leukemic phase (DLBCL2A) and from a spleenic tumor (DLBCL2B) of the same patient. (F) Measurement of cellular death, by flow cytometry, of DLBCL cells lines treated with HG6-64-1, doxorubicin, or both to measure potential additive effects of combination treatment. All cell-line experiments were performed at least 3 times.
HG6-64-1, a chemical GCK inhibitor. (A) Six DLBCL cell lines and 2 control, non-DLBCL cell lines, were treated with serial dilutions of HG6-64-1. 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl-2-(4-sulfophenyl)-2H-tetrazolium (MTS) readings were taken just prior to and after drug addition and compared with control, DMSO-treated cells. Cellular proliferation was calculated as the change in MTS reading for each experimental condition, divided by the increase in DMSO-treated cells. (B) The calculated EC50 of HG6-64-1 for each listed cell line. (C) Asynchronously growing DLBCL cell lines were subject to media change followed by treatment with HG6-64-1 or the vehicle, DMSO. After treatment, cells were ethanol permeabilized and saturated with PI in PBS. Cell cycle was analyzed by flow cytometry, and results are depicted in a bar graph. (D) Time-dependent measurement of cellular death by flow cytometry in DLBCL cell lines. (E) Flow cytometry measurement of cellular death at 48 hours of tumor B cells isolated from fresh biopsies of DLBCL primary tumors, or normal B cells isolated from healthy tissue, and treated with HG6-64-1 or DMSO. In one primary sample, cells were derived from the leukemic phase (DLBCL2A) and from a spleenic tumor (DLBCL2B) of the same patient. (F) Measurement of cellular death, by flow cytometry, of DLBCL cells lines treated with HG6-64-1, doxorubicin, or both to measure potential additive effects of combination treatment. All cell-line experiments were performed at least 3 times.
Treatment of cells with HG6-64-1 induced a pronounced G0/G1 cell-cycle arrest (Figure 4C) as measured by flow cytometry analysis of propidium iodide (PI) intercalation. HG6-64-1 also induced a time-dependent increase in cell death in the majority of the analyzed DLBCL cell lines, with the exception of G452 and OCI-LY-3 (Figure 4D), as measured by flow cytometry analysis of YO-PRO/PI staining. The decreased sensitivity of G452 (Figure 4A-D) suggests that GCK inhibition is critical to HG6-64-1’s function, because this DLBCL cell line expresses low levels of GCK. The reason that OCI-LY-3 does not respond is unknown. However, the lack of response in G452 and OCI-LY-3 suggests that HG6-64-1 is not a generally cytotoxic compound. This data suggest that GCK inhibition affects cell viability by inducing apoptosis and limits tumor growth by inhibiting the cell cycle.
To demonstrate that HG6-64-1-induced cytotoxicity was not restricted to established DLBCL cell lines, we evaluated HG6-64-1’s effects on 5 fresh de novo untreated primary DLBCL tumors and on several fresh biopsies from control patients. HG6-64-1 treatment induced marked apoptosis and cell death in all examined primary DLBCL tumors (Figure 4E) as measured by flow cytometry analysis of YO-PRO/PI staining. The extent of HG6-64-1-induced cell death in DLBCL tumors was greater than, or approximately equal to, killing in normal B lymphocytes extracted from biopsies of noncancerous lymph nodes (n = 19), which also express GCK (Figure 2E). Furthermore, treatment of DLBCL cell lines with a combination of HG6-64-1 and doxorubicin exhibited additive cytotoxicity in SU-DHL-6 and OCI-LY-19 cell lines as measured by flow cytometry analysis of YO-PRO/PI staining. Predictably, G452 cells were not affected by the HG6-64-1 (Figure 4F). The siRNA and shRNA interference with GCK led to a 10% to 40% induction in cell death (Figure 3A,C-D), and the GCK inhibitor HG6-64-1 led to slightly more pronounced death of 20% to 60% in DLBCL cell lines and over 90% in primary DLBCL tumors (Figure 4C-F).
Overall, this biochemical, IHC, and small molecule inhibitor data suggest that GCK may be a viable target for DLBCL therapeutics.
Specificity of HG6-64-1
In addition to GCK, HG6-64-1 may potentially target a small number of additional kinases at the concentration (400 nM) that was used in this study (supplemental Figure 9 and supplemental Tables 3-4). In order to determine the relative contribution of these targets in DLBCL, we performed independent chemical inhibition, western blotting, and siRNA studies (supplemental Table 5, supplemental Figure 10). The majority of tested targets were excluded, with respect to their potential to affect DLBCL cell proliferation and survival, based on these studies.
BCR signaling is functionally active and contributes to the survival of ABC-like and BCR-type DLBCLs.12,13,24 Lyn, a critical upstream component of this pathway,25 is a potential target of HG6-64-1, as shown by KinomeScan and KiNativ analyses (supplemental Tables 3-4). Consequently, we have examined the effects of HG6-64-1 on BCR signaling in DLBCL cell lines. HG6-64-1 decreased BCR signaling, as demonstrated by decreased phosphorylation of Syk, BTK, and PLCγ2, leading to decreased activation of the NFκB and NFAT reporters, Ca++ influx, and phosphorylation of the ERK and its targets (supplemental Figure 11A-D). In contrast, knockdown of the GCK did not affect activation of the BCR, as demonstrated by no effect on Syk phosphorylation (supplemental Figure 11E), suggesting that the observed effects on the BCR signaling are GCK independent.
To examine the potential contribution of Lyn inhibition by HG6-64-1 on DLBCL survival and cell death, we used the pan-SRC inhibitor AZD053026 or knocked down Lyn expression by specific siRNAs. Treatment with AZD530 did not affect the proliferation or survival of tested DLBCL cell lines (supplemental Figure 10). However, a modest 10% decrease in DLBCL cell viability was observed following siRNA-mediated Lyn knockdown (supplemental Figure 11F). Overall, our studies suggest the GCK inhibitory activity of HG6-64-1 is primarily responsible for the effects it exerts on DLBCL cells with a potentially minor contribution due to Lyn inhibition.
In vivo activity of HG6-64-1
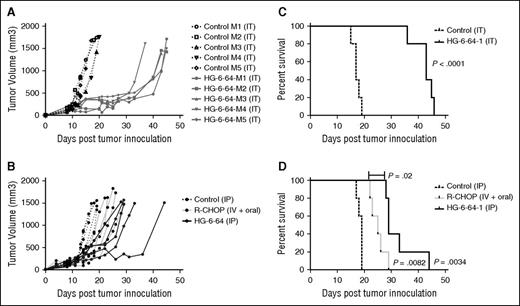
The proapoptotic activity of GCK inhibition in DLBCL cell lines and primary tumors suggests that specific inhibitors of this enzyme could be useful for the treatment of patients. To evaluate the effects of GCK inhibition in vivo, groups of nonobese diabetic/severe combined immunodeficient mice were inoculated subcutaneously with GCK-expressing non-BCR-type double-hit DLBCL cell line OCI-LY-19.24 After tumor development, HG6-64-1 or phosphate-buffered saline (PBS) was injected intratumorally (50 mg/kg) or intraperitoneally (37.5 mg/kg) daily for 14 consecutive days. HG6-64-1 treatment resulted in marked delays in tumor growth (Figure 5A-B). Although mice treated with HG6-64-1 did eventually succumb to tumors, the single 14-day round of HG6-64-1 treatment significantly prolonged animal survival compared with controls (Figure 5C-D). Administration of HG6-64-1 in vivo was well tolerated and led to significantly prolonged survival of mice harboring human DLBCL xenografts.
HG6-64-1 inhibits the growth of DLBCL xenograft tumors and prolongs the survival of tumor-bearing mice. Mice bearing subcutaneous OCI-LY-19 xenograft tumors were treated with injections of HG6-64-1, R-CHOP, or PBS. Tumor volume (area in cubic millimeters) is depicted in mice treated with intratumoral injections of HG-6-64-1 (A) or intraperitoneal injections of HG6-64-1, intravenous R-CHOP with orally administered prednisone as described,26 or PBS (B). Overall survival of the mice is shown in (C-D). Similar results to those shown in (A-D) were observed in one additional independent experiment. The P value for survival of mice given R-CHOP treatment compared with control was P = .0082; HG6-64-1 compared with control was P = .0034, and HG6-64-1 compared with R-CHOP was P = .02. IT, intratumoral administration; IP, intraperitoneal administration; IV + oral, intravenous administration alongside oral prednisone.
HG6-64-1 inhibits the growth of DLBCL xenograft tumors and prolongs the survival of tumor-bearing mice. Mice bearing subcutaneous OCI-LY-19 xenograft tumors were treated with injections of HG6-64-1, R-CHOP, or PBS. Tumor volume (area in cubic millimeters) is depicted in mice treated with intratumoral injections of HG-6-64-1 (A) or intraperitoneal injections of HG6-64-1, intravenous R-CHOP with orally administered prednisone as described,26 or PBS (B). Overall survival of the mice is shown in (C-D). Similar results to those shown in (A-D) were observed in one additional independent experiment. The P value for survival of mice given R-CHOP treatment compared with control was P = .0082; HG6-64-1 compared with control was P = .0034, and HG6-64-1 compared with R-CHOP was P = .02. IT, intratumoral administration; IP, intraperitoneal administration; IV + oral, intravenous administration alongside oral prednisone.
To relate HG6-64-1 treatment to the current standard of care, we treated mice with CHOP as described27 with the addition of rituximab to that protocol (Figure 5B,D). The current standard of care, R-CHOP, led to an expected statistically significant (P = .0082) increase in survival for this xenograft model. However, this was a less significant rescue than with the GCK inhibitor HG-6-64-1 (P = .0034), and the difference between the treatment with R-CHOP and HG6-64-1 was also statistically significant (P = .02).
Discussion
We have performed the first comprehensive analysis of the human kinome in DLBCL and discovered the activation of MAPK signaling pathway proteins that were not previously implicated in the pathogenesis of DLBCL. We did this using KiNativ technology, which we have, herein, demonstrated can identify activated pathways in disease models. We confirmed activations of known signaling pathways in DLBCL, such as the BCR pathway and MYD88, lending validity to the MAPK findings and suggesting that this technology can be applied to other lymphomas and disease models.
There are many reported functions of the MAPK family of proteins, including induction of cellular proliferation, transformation, differentiation, and promoting cellular death.16,28,29 Prior to this study, aberrations in MAPK signaling have been linked to a variety of cancers but not to the pathogenesis of DLBCL tumors.16,30-32 Our specific findings include previously unknown activation of the GCK signaling pathway in DLBCL. We further demonstrate that GCK knockdown in DLBCL leads to decreased proliferation and cell death. Only 2 studies have implicated GCK in cancer (melanoma and colon carcinoma),33,34 and no prior studies have examined the potential role of GCK in lymphoma. The mechanism underlying GCK activation in DLBCL is currently unknown, and previous deep sequencing studies did not identify mutations in this gene.35,36 Similar to other MAPK pathways, it is likely that unknown autocrine or paracrine stimuli lead to GCK activation. We demonstrated that CD40L can induce GCK expression in our cell lines and also found evidence of the activation of TRAF6 signaling in these cells (supplemental Table 1). These are both reported GCK activators and may provide insight to the mechanism of GCK activation in DLBCL. Further studies will be needed to identify additional autocrine and paracrine pathway upregulating GCK in DLBCL.
We further demonstrate that GCK may be targetable by a previously described small molecule inhibitor HG6-64-1,10 which induces marked DLBCL cell cytotoxicity in vitro, in vivo, and in primary DLBCL tumors. Of note, marked activity was also observed in double-hit DLBCL cell lines (OCILY-19, SUDHL-6, and VAL), a subtype of DLBCL with very poor response to current therapies, representing a subgroup with unmet medical need for novel therapeutic approaches. Our studies showed that HG6-64-1 may also target the SRC-family kinase Lyn, thereby functioning as a dual GCK/BCR inhibitor. Although we have demonstrated that HG6-64-1-induced DLBCL cytotoxicity may be observed in cells nondependent on Lyn function, such as double-hit OCI-LY-19, we believe the dual inhibition may be advantageous for the treatment of DLBCL and may augment the effects of GCK signaling interference in DLBCL for the following reasons. First, there was a 10% to 20% increase in DLBCL cell line killing with HG-6-64-1 when compared with GCK siRNA or shRNA alone, corresponding to extent of killing induced by shRNA directed to Lyn. Second, the GCK pathway activation is observed in more than 80% of DLBCL tumors irrespective of their cell of origin, suggesting the possibility of wide use of this or a similar therapeutic compound. Third, BCR activation plays a major role in the pathogenesis of ABC-like DLBCL,13 and some DLBCL tumors, irrespective of their cell of origin, exhibit tonic BCR signaling; the latter is necessary for their survival.37 Finally, simultaneous targeting of independent canonical pathways may lead to better efficacy and reduce the development of drug resistance. Multiple kinase targeting is reported for several kinase inhibitors commonly used in clinical practice (eg, ibrutinib,38 imatinib,39 and sunitinib40 ).
JNK is a downstream target of the GCK signaling. Although direct JNK inhibitors are available and were evaluated in clinical trials,30 JNKs are activated by multiple signaling pathways.29 It is possible that regulating the oncogenic proteins upstream of JNKs, to leave some of their normal functions intact, is beneficial. Consequently, the residual low levels of p-JNK observed after treatment with HG6-64-1 may lead to a better therapeutic window compared with direct JNK inhibitors. However, Jun-regulated genes activation was recently reported to promote lymphoma growth and dissemination to extranodal sites in DLBCL,41 and the JNK pathway was reported as a therapeutic target in ABC-like DLBCL42; so it is possible that direct JNK inhibition should be revisited in future studies.
In summary, we show that the GCK pathway is activated in DLBCL and promotes the proliferation and survival of these tumors. We demonstrate that both RNA interference-mediated depletion and pharmacological inhibition of GCK inhibit DLBCL cell proliferation and induce cell-cycle arrest, apoptosis, and cell death. Therefore, therapeutic targeting of this signaling pathway is feasible and may advance efforts to cure DLBCL patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
I.S.L. is supported by the Dwoskin Family, Recio Family, Anthony Rizzo Family, and Greg Olsen Foundations. N.S.G., S.B., and L.T. were supported by National Institutes of Health National Cancer Institute grant CA173469-01 and the Linde Family chemical biology funds.
This work was funded by Lymphoma Research Foundation (I.S.L. and N.S.G.).
M.P.P. was previously and T.K.N. is currently an employee at ActivX Biosciences.
Authorship
Contribution: J.M.M. conceptualized and designed the study, performed the experiments, analyzed the data, and wrote the paper; S.B., M.P.P., T.K.N., X.J., E.M., and R.V. performed the experiments and analyzed data; Y.N. and D.G. provided specimens, performed the experiments and analyzed the data; A.J.G., R.S., S.C., and R.T. analyzed the data; D.Z., L.T., J.Z., and H.G.C. generated reagents; J.R.C. and R.A. provided specimens; E.C. performed the experiments; S.J.B. and N.S.G. designed the study and analyzed the data; and I.S.L. conceptualized and designed the study, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Izidore S. Lossos, University of Miami, Sylvester Comprehensive Cancer Center, Division of Hematology and Oncology, 1475 NW 12th Ave (D8-4), Miami, FL 33136; e-mail: ilossos@med.miami.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal