Key Points
ALPS DNT cells and their putative precursors reveal high proliferative activity in vivo, which is associated with hyperactive mTOR signaling.
Rapamycin therapy controls mitotic activity and abnormal differentiation of ALPS DNT cells and reduces CD4+ or CD8+ precursor DNT cells.
Abstract
Autoimmune lymphoproliferative syndrome (ALPS) is a human disorder characterized by defective Fas signaling, resulting in chronic benign lymphoproliferation and accumulation of TCRαβ+ CD4− CD8− double-negative T (DNT) cells. Although their phenotype resembles that of terminally differentiated or exhausted T cells, lack of KLRG1, high eomesodermin, and marginal T-bet expression point instead to a long-lived memory state with potent proliferative capacity. Here we show that despite their terminally differentiated phenotype, human ALPS DNT cells exhibit substantial mitotic activity in vivo. Notably, hyperproliferation of ALPS DNT cells is associated with increased basal and activation-induced phosphorylation of serine-threonine kinases Akt and mechanistic target of rapamycin (mTOR). The mTOR inhibitor rapamycin abrogated survival and proliferation of ALPS DNT cells, but not of CD4+ or CD8+ T cells in vitro. In vivo, mTOR inhibition reduced proliferation and abnormal differentiation by DNT cells. Importantly, increased mitotic activity and hyperactive mTOR signaling was also observed in recently defined CD4+ or CD8+ precursor DNT cells, and mTOR inhibition specifically reduced these cells in vivo, indicating abnormal programming of Fas-deficient T cells before the DNT stage. Thus, our results identify the mTOR pathway as a major regulator of lymphoproliferation and aberrant differentiation in ALPS.
Introduction
The autoimmune lymphoproliferative syndrome (ALPS) is a human disorder of dysregulated lymphocyte homeostasis resulting from defects in the Fas signaling cascade. The majority of patients with genetically defined ALPS harbor heterozygous germline or somatic FAS mutations or a combination of both.1-3 Defective Fas signaling results in chronic benign lymphoproliferation with often massive splenomegaly and lymphadenopathy, autoimmune manifestations, and an increased risk for lymphoma.2,4,5 Although absolute numbers of total T and B cells are increased in most patients with ALPS, the pronounced lymphoproliferation has mainly been attributed to the pathognomonic accumulation of TCRαβ+ CD4−/CD8− double-negative T (DNT) cells. DNT cells have for a long time been assumed to arise from chronically activated CD8+ T cells accumulating as a result of defective Fas-mediated elimination. Indeed, DNT cells exhibit some features of terminally differentiated effector-memory cells reexpressing CD45RA (TEMRA), a subset of end-stage or senescent T cells with poor proliferative capacity, low telomerase activity, and short telomeres. Moreover, DNT cells express inhibitory receptors and fail to respond to mitogen or TCR stimulation in vitro similar to senescent T cells.6-8
However, several findings are not compatible with this concept of DNT cell ontogeny and accumulation. Early immunohistochemical studies revealed that many cells in the lymph node paracortex, which is heavily infiltrated by DNT cells in patients with ALPS, stain positive for Ki67.8 Although the lineage and phenotype of these Ki67+ cells were not analyzed in detail, this was a first indication that DNT cells are not merely senescent cells, which cannot die, but that they actively proliferate in vivo. In addition, we and others have found that ALPS DNT cells have a unique differentiation pattern differing from terminally differentiated CD4+ or CD8+ T cells.7,9 Although their CCR7−/CD45RA+/CD127−/CD57+ phenotype suggests terminal differentiation, lack of killer-like receptor G1 (KLRG1) and expression of costimulatory receptors CD27 and CD28, as well as the high eomesodermin (Eomes) to T-bet expression ratio, are characteristics of long-lived memory T cells with potent proliferative capacity.9,10 Of interest, subsets of CD4+ and CD8+ T cells also show this unusual differentiation pattern and most likely represent direct precursors of DNT cells.9 Thus, DNT cells can presumably arise from CD4+ or CD8 T+ cells, and their abnormal differentiation occurs before the DNT stage.
Although some patients with ALPS do not require treatment, others need medical support, especially with regard to autoimmune cytopenia. First-line treatment of ALPS includes high-dose corticosteroids and intravenous immunoglobulins. However, as a result of the extensive adverse effects of long-term steroid treatment, other immunosuppressive drugs such as mycophenolate mofetil (MMF) and rapamycin are successfully used as second-line therapies.11 MMF is metabolized to the active compound mycophenolic acid, which inhibits lymphocyte proliferation by blocking purine synthesis. Rapamycin inactivates the serine/threonine protein kinase mechanistic target of rapamycin (mTOR), thereby inhibiting cell growth and differentiation. Interestingly, clinical studies have shown that rapamycin but not MMF treatment of patients with ALPS efficiently reduced lymphoproliferation and numbers of circulating DNT cells.11-13 Although it is not clear whether rapamycin affects expansion or induces apoptosis of DNT cells or both,14 its therapeutic efficiency suggests specific signaling requirements of DNT cells.
In this study, we sought to analyze the proliferative activity, stimulatory requirements, and underlying signaling pathways of DNT cells in patients with ALPS. We demonstrate that abnormally differentiated DNT cells are the predominant cell population undergoing cell division in patients with ALPS. Enhanced mitotic activity of ALPS DNT cells was associated with hyperactivation of the mTOR pathway and could be reverted by rapamycin treatment. Importantly, increased proliferation and mTOR signaling also occurred in CD4+ or CD8+ DNT putative precursor cells. Collectively, our data highlight the crucial role of the mTOR pathway in controlling lymphoproliferation and abnormal differentiation in human FAS deficiency.
Materials and methods
Patients
We analyzed 19 untreated patients with ALPS (n = 8 ALPS-FAS; n = 5 ALPS-sFAS; n = 6 ALPS-FAS-sLOH; mean age, 16 years [range, 2-62 years]), 11 patients with ALPS treated with rapamycin (n = 3 ALPS-FAS; n = 4 ALPS-sFAS; n = 4 ALPS-FAS-sLOH; mean age, 10 years [range, 2-20 years]), 3 patients with ALPS treated with MMF (ALPS-FAS), and 12 healthy volunteers (mean age, 23 years; range, 15-36 years). All procedures were based on standard of care, and established clinical guidelines were followed. Untreated patients had not received any medication for at least 4 weeks. The study was approved by the ethics committee of the University of Freiburg (protocol number 40/09) and the University Erlangen-Nuremberg (protocol number 4554-CH). Written informed consent was obtained from patients and controls. The study was conducted in accordance with the Declaration of Helsinki.
Flow cytometry
Peripheral blood mononuclear cells (PBMCs) were separated by density gradient centrifugation and stained according to the manufacturer’s recommendations, using fluorochrome-coupled antibodies (supplemental Table 1, available on the Blood Web site). Intracellular staining was performed using Fixation/Permeabilization Kit (BD Biosciences). Forward scatter/side scatter and single-cell gating were used to exclude dead cells from all analyses. For detection of phosphorylated proteins, blood samples were incubated with anti-CD3 and anti-CD28 monoclonal antibody (mAb; eBioscience) on ice, washed with ice-cold PBS, and cross-linked with goat anti-mouse mAb. Cells were stimulated at 37°C for 15 minutes and fixed and permeabilized with Perm Buffer 3 (BD Biosciences) according to the manufacturer’s recommendations. Cells were washed twice with PBS containing 2% fetal calf serum and incubated with fluorochrome-conjugated mAb for 30 minutes at room temperature. Baseline levels of phosphorylation were determined by fixation and permeabilization of untreated cells. Data acquisition was performed on a fluorescence-activated cell sorter Canto II and a fluorescence-activated cell sorter LSRFortessa (BD Biosciences). Y-axis of histogram overlays was normalized to mode. Data were analyzed with FlowJo, v10 (Tree Star, Inc.).
Cell culture and proliferation assays
CD4+, CD8+, and DNT cells were isolated from PBMC by cell sorting, using a MoFlo XP cell sorter (Beckmann Coulter). Allogeneic dendritic cells (DCs) were generated from human blood monocytes from healthy volunteers, as described previously.15 To determine cell proliferation, sorted CD4+, CD8+, and DNT cells were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Sigma-Aldrich) and washed twice with serum-containing medium. Cells (4 × 104/well) were stimulated with plate-bound anti-CD3 antibody (0.2 µg/well), phytohemagglutinin (PHA; 2.5 µg/mL, Sigma-Aldrich), allogeneic DC, or anti-CD3/CD28 coated beads (1 × 104/well; Life Technologies) in complete medium (RPMI 1640, 10% human AB serum) plus IL-2 (100 IU/mL; Novartis Pharma). After 6 days, cells were stained with indicated antibodies and analyzed by flow cytometry. The compound 7-amino-actinomycin D was used to determine cell viability. For rapamycin treatment, CFSE-labeled T cells were stimulated with anti-CD3/CD28-coated beads and IL-2. Rapamycin was added once at the beginning of cell culture in the presence of rapamycin (final concentration, 1 nM-10 µM). After 6 days, proliferation and viability of cells were determined by flow cytometry.
Quantitative PCR
Total RNA was isolated from indicated cell populations, using RNeasy Micro Kit (Qiagen). Reverse transcription was performed using SuperScript II Reverse Transcriptase (Life Technologies). cDNA was quantified by real-time PCR on a Rotor Gene Q platform, using QuantiTect SYBR Green (Qiagen). Relative gene expression was determined by normalizing target gene expression to β-2 microglobulin. The following primers and probes were used (Qiagen): Hs_B2M_1_SG QuantiTect Primer Assay (QT00088935), Hs_KLF2_1_SG QuantiTect Primer Assay (QT00204729), and Hs_IL10_1_SG QuantiTect Primer Assay (QT00041685).
Enzyme-linked immunosorbent assay
Serum levels of IL-10 were determined by enzyme-linked immunosorbent assay, according to manufacturer’s instructions (OptEIA; BD Biosciences).
Statistical analysis
Data were analyzed with Prism software (GraphPad). Populations were compared using Mann-Whitney U test; Spearman’s rank test was used for correlation analysis; P < .05 was considered significant.
Results
Abnormally differentiated DNT cells of patients with ALPS are highly proliferative in vivo
On the basis of the high proliferative activity of interfollicular cells observed in lymph node sections of patients with ALPS,8,16 we aimed to characterize the mitotic activity of circulating lymphocyte subsets in more detail. We studied a group of patients with ALPS with somatic or germline FAS mutations and variable DNT cell frequencies (supplemental Figure 1A). We first analyzed expression of the nuclear protein Ki67, which is present during active phases of the cell cycle (G1 to M). A proportion of circulating DNT cells and a small fraction of single positive CD4+ or CD8+ T cells from most patients with ALPS highly expressed Ki67, although it was absent in all 3 cell populations from healthy donors (Figure 1A). Moreover, we found a significant correlation of Ki67 expression and DNT cell frequency in patients with ALPS (supplemental Figure 1B). Reflecting their activated state, DNT cells highly expressed HLA-DR (supplemental Figure 1C). To confirm that Ki67 expression is associated with cell division, we determined the presence of proliferating cell nuclear antigen and cyclin A, which are expressed during S and G2 phase of the cell cycle.17,18 ALPS DNT cells expressed both cell cycle regulators, indicating these cells progress through cell cycle (Figure 1B). Phenotypic analysis of proliferating lymphocytes in patients with ALPS revealed that most Ki67+ cells are of the DNT cell phenotype, whereas γδ T cells, NK cells, NKT cells, and B cells displayed low mitotic activity (Figure 1C). The majority of ALPS DNT cells are CCR7−/CD45RO−/CD45RA+/CD57+, an expression pattern of terminally differentiated cells.7,9 Further analysis revealed a linear relationship between the percentage of DNT cells showing a CCR7−/CD45RA+ differentiation state and the fraction of DNT cells with mitotic activity (Figure 1D). More specifically, Ki67 expression was limited to DNT cells showing the unusual CD57+/CD27+/CD28+ differentiation state (Figure 1E) we have recently defined for ALPS DNT cells.9 Thus, in contrast to CCR7−/CD45RA+/CD57+ terminally differentiated T cells of healthy individuals, which are generally associated with poor proliferative potential and low Ki67 expression,19,20 ALPS DNT cells expressing the same markers are highly proliferative in vivo.
Abnormally differentiated ALPS DNT cells show high proliferative activity in vivo. (A) PBMC from patients with ALPS and healthy controls were gated for CD4+, CD8+, and DNT cells and analyzed for Ki67 expression. Histograms show a representative experiment, y-axis is normalized to mode. Cumulative data of all untreated patients with ALPS and healthy controls are graphed; each symbol represents an individual subject. ***P < .001 (Mann-Whitney U test). (B) Expression of proliferating cell nuclear antigen and cyclin A was determined in ALPS CD4+ (gray, filled), CD8+ (black, dotted), and DNT (black, solid) cells. Overlays show representative data from 1 of 5 independent experiments with similar results. (C) PBMC of patients with ALPS (n = 12) were gated for Ki67+ lymphocytes. Frequencies of B cells (CD3−/CD19+), NK cells (CD3−/CD56+), NKT cells (CD3+/CD56+ or TCRVα24−Jα18+), γδ T cells (CD3+/TCRγδ+), CD4+ T cells (CD3+/TCRαβ+/CD4+), CD8+ T cells (CD3+/TCRαβ+/CD8+), and DNT cells (CD3+/TCRαβ+/CD4−/CD8−) among all Ki67+ lymphocytes were determined by flow cytometry. Box plots depict the 75th percentile, median, and 25th percentile values; whiskers represent maximum and minimum values. (D) Correlation of percentage of Ki67+ DNT cells and frequency of CCR7−/CD45RA+ DN T cells among all ALPS DNT cells is shown; r = .7471; ***P < .001 (Spearman’s test). (E) Expression of CD27, CD28, CD57, and Ki67 were determined in ALPS DNT cells. Plots show representative data from 1 of 3 independent experiments with similar results.
Abnormally differentiated ALPS DNT cells show high proliferative activity in vivo. (A) PBMC from patients with ALPS and healthy controls were gated for CD4+, CD8+, and DNT cells and analyzed for Ki67 expression. Histograms show a representative experiment, y-axis is normalized to mode. Cumulative data of all untreated patients with ALPS and healthy controls are graphed; each symbol represents an individual subject. ***P < .001 (Mann-Whitney U test). (B) Expression of proliferating cell nuclear antigen and cyclin A was determined in ALPS CD4+ (gray, filled), CD8+ (black, dotted), and DNT (black, solid) cells. Overlays show representative data from 1 of 5 independent experiments with similar results. (C) PBMC of patients with ALPS (n = 12) were gated for Ki67+ lymphocytes. Frequencies of B cells (CD3−/CD19+), NK cells (CD3−/CD56+), NKT cells (CD3+/CD56+ or TCRVα24−Jα18+), γδ T cells (CD3+/TCRγδ+), CD4+ T cells (CD3+/TCRαβ+/CD4+), CD8+ T cells (CD3+/TCRαβ+/CD8+), and DNT cells (CD3+/TCRαβ+/CD4−/CD8−) among all Ki67+ lymphocytes were determined by flow cytometry. Box plots depict the 75th percentile, median, and 25th percentile values; whiskers represent maximum and minimum values. (D) Correlation of percentage of Ki67+ DNT cells and frequency of CCR7−/CD45RA+ DN T cells among all ALPS DNT cells is shown; r = .7471; ***P < .001 (Spearman’s test). (E) Expression of CD27, CD28, CD57, and Ki67 were determined in ALPS DNT cells. Plots show representative data from 1 of 3 independent experiments with similar results.
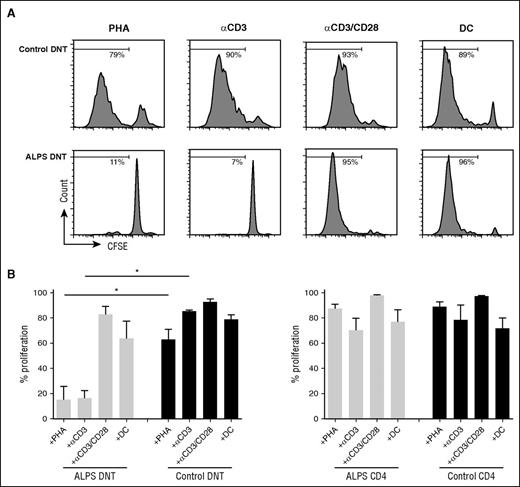
Proliferation of ALPS DNT cells can be induced by costimulatory signals in vitro
To address potential cellular signals controlling the mitotic activity of ALPS DNT cells, we isolated CD4+, CD8+, and DNT cells and cultured them in vitro in the presence of different stimuli. ALPS DNT cells are known to rapidly die ex vivo in the absence of stimulation.21 Addition of IL-2 or the homeostatic cytokines IL-7 or IL-15 did not enhance survival nor induce proliferation of ALPS DNT cells (data not shown). We next asked whether TCR ligation induces proliferation of ALPS DNT cells. ALPS DNT cells failed to respond to anti-CD3 stimulation or PHA (Figure 2A-B), whereas DNT cells from healthy donors proliferated vigorously. In contrast, proliferation of ALPS DNT cells could be induced by anti-CD3/CD28 stimulation or with allogeneic DC. CD4+ and CD8+ T cells from both healthy controls and patients with ALPS exhibited potent proliferation on stimulation with anti-CD3 or PHA, which cannot be enhanced by additional costimulation (Figure 2B; supplemental Figure 2; data not shown).
Proliferation of ALPS DNT cells can be induced by costimulatory signals in vitro. Sorted DNT cells (A, B) or CD4+ T cells (B) from patients with ALPS or healthy controls were labeled with CFSE and stimulated with PHA, anti-CD3, anti-CD3/CD28, and allogeneic DC in the presence of IL-2. Cell proliferation was determined on day 6, dead cells were excluded by 7AAD staining. (A) Histograms show 1 representative experiment, (B) graphs represent cumulative data (mean ± SD) of 5 patients with ALPS and healthy controls. *P < .05 (Mann-Whitney U test).
Proliferation of ALPS DNT cells can be induced by costimulatory signals in vitro. Sorted DNT cells (A, B) or CD4+ T cells (B) from patients with ALPS or healthy controls were labeled with CFSE and stimulated with PHA, anti-CD3, anti-CD3/CD28, and allogeneic DC in the presence of IL-2. Cell proliferation was determined on day 6, dead cells were excluded by 7AAD staining. (A) Histograms show 1 representative experiment, (B) graphs represent cumulative data (mean ± SD) of 5 patients with ALPS and healthy controls. *P < .05 (Mann-Whitney U test).
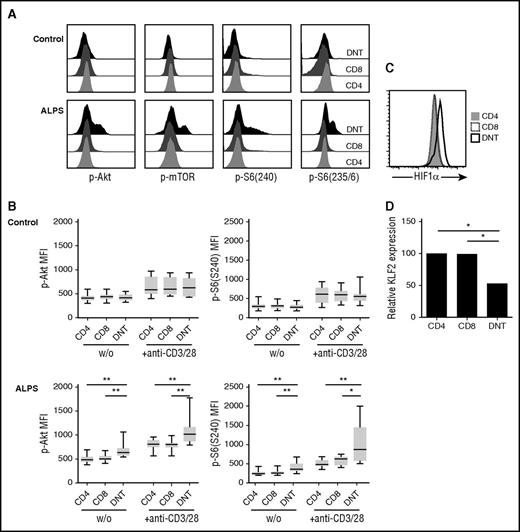
ALPS DNT cells show hyperactive mTOR signaling
As ALPS DNT cells vigorously proliferate in vivo, express costimulatory receptors CD27 and CD28, and require costimulation to proliferate in response to TCR stimulation in vitro, we considered that signal transduction downstream of costimulatory receptors might be crucial for accumulation of DNT cells in ALPS. An important molecule activated downstream of CD28 is mTOR, which plays a key role in regulating cell proliferation and effector differentiation.22 We therefore analyzed the phosphorylation status of protein kinase Akt at Ser473, mTOR at Ser2448, and its downstream target ribosomal protein S6 at both phosphorylation sites Ser235/6 and Ser240 in T cells from patients with ALPS ex vivo. Intriguingly, ALPS DNT cells showed enhanced basal and activation-induced phosphorylation of Akt, mTOR, and S6 compared with ALPS bulk CD4+ and CD8+ T cells or the respective cell populations from healthy controls (Figure 3A-B; supplemental Figure 3A-B). The percentage of Ki67+ lymphocytes positively correlated with phosphorylated Akt in ALPS DNT cells (data not shown). The main negative regulator of Akt-mTOR signaling is the lipid phosphatase PTEN (phosphatase and tensin homolog), which dephosphorylates phosphatidylinositol-3,4,5-triphosphate into phosphatidylinositol-4,5-bisphosphate, and thereby opposes PI3K activity.23,24 Of note, ALPS DNT cells showed increased expression of PTEN compared with CD4+ or CD8+ T cells (supplemental Figure 3C). Moreover, hyperphosphorylation of mTOR signaling molecules was lost after resting PBMC in PBS (data not shown), suggesting that external signals and/or nutrients are required for maintenance of this activated state.
ALPS DNT cells exhibit hyperactive mTOR signaling. (A) Phosphorylation of Akt at Ser473, mTOR at Ser2448, S6 at Ser235/236, and S6 at Ser240 was determined in T cells from patients with ALPS and healthy controls after stimulation by cross-linked anti-CD3/CD28 mAbs. Histograms depicting levels of phosphorylated proteins show 1 representative experiment, y-axis is normalized to mode. (B) Cells from patients with ALPS and healthy controls were left unstimulated (w/o) or stimulated with cross-linked anti-CD3/CD28 mAbs and analyzed for phosphorylation of indicated proteins. Graphs show mean fluorescence intensities of p-Akt(Ser473) and p-S6(Ser240) (n = 10). Box plots depict the 75th percentile, median and 25th percentile values; whiskers represent maximum and minimum values. *P < .05, **P < .01, ***P < .001 (Mann-Whitney U test). (C) Expression of HIF1α was determined in ALPS CD4+ (gray, filled), CD8+ (black, dotted), and DNT (black, solid) cells. Overlay shows representative data from 1 of 8 independent experiments with similar results. (D) Gene expression of KLF2 in the indicated purified cell populations relative to CD4+ T cells was determined by quantitative RT-PCR. Data represent relative KLF2 expression of 5 patients with ALPS. *P < .05 (Mann-Whitney U test).
ALPS DNT cells exhibit hyperactive mTOR signaling. (A) Phosphorylation of Akt at Ser473, mTOR at Ser2448, S6 at Ser235/236, and S6 at Ser240 was determined in T cells from patients with ALPS and healthy controls after stimulation by cross-linked anti-CD3/CD28 mAbs. Histograms depicting levels of phosphorylated proteins show 1 representative experiment, y-axis is normalized to mode. (B) Cells from patients with ALPS and healthy controls were left unstimulated (w/o) or stimulated with cross-linked anti-CD3/CD28 mAbs and analyzed for phosphorylation of indicated proteins. Graphs show mean fluorescence intensities of p-Akt(Ser473) and p-S6(Ser240) (n = 10). Box plots depict the 75th percentile, median and 25th percentile values; whiskers represent maximum and minimum values. *P < .05, **P < .01, ***P < .001 (Mann-Whitney U test). (C) Expression of HIF1α was determined in ALPS CD4+ (gray, filled), CD8+ (black, dotted), and DNT (black, solid) cells. Overlay shows representative data from 1 of 8 independent experiments with similar results. (D) Gene expression of KLF2 in the indicated purified cell populations relative to CD4+ T cells was determined by quantitative RT-PCR. Data represent relative KLF2 expression of 5 patients with ALPS. *P < .05 (Mann-Whitney U test).
To further confirm hyperactivity of the mTOR pathway in ALPS DNT cells, we determined expression of important transcription factors that are positively (hypoxia-inducible factor 1α [HIF1α]) or negatively (Kruppel-like factor 2 [KLF2]) regulated by mTOR. Indeed, we observed increased expression of HIF1α and reduced expression of KLF2 in ALPS DNT cells (Figure 3C-D). Together, these data support the finding that mTOR signaling is enhanced in DNT cells from patients with ALPS, but not from control donors, which likely contributes to proliferation and accumulation of these abnormally differentiated cells in vivo.
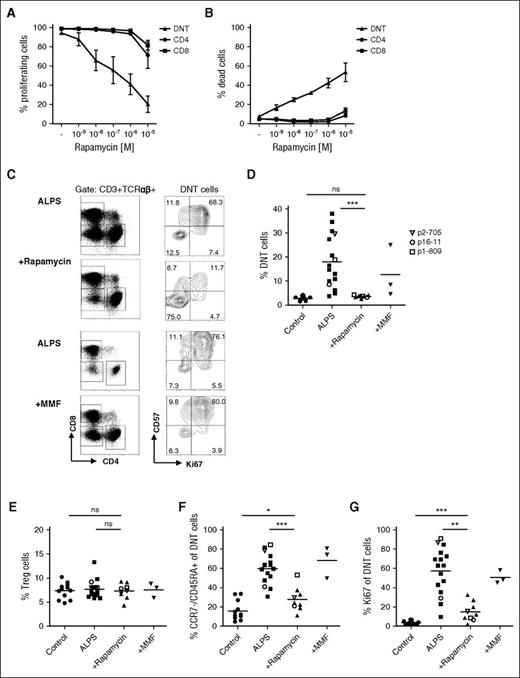
Rapamycin reduces abnormally differentiated DNT cells in vitro and in vivo
The mTOR inhibitor rapamycin has been successfully used to treat lymphoproliferation and autoimmune cytopenias in patients with ALPS.11-13 To further understand the effects of mTOR inhibition in this disease, we built on our above observations and addressed the question of whether the sensitivity to rapamycin is linked to the particular differentiation state of ALPS versus control DNT cells. We first investigated how rapamycin affects proliferation and survival of ALPS DNT cells in vitro. Notably, addition of rapamycin to anti-CD3/CD28 stimulated DNT cells reduced the mitotic activity of DNT cells from patients with ALPS, but not from healthy donors (Figure 4A; supplemental Figure 4A). In contrast, bulk CD4+ or CD8+ T cells from both groups were resistant to the antiproliferative effects of rapamycin across a large dose range. We also observed an increased rate of apoptosis in rapamycin-treated ALPS DNT cells, suggesting cell death contributes to the antiproliferative effect of rapamycin (Figure 4B).
mTOR inhibition reduces abnormally differentiated DNT cells. (A, B) CFSE-labeled ALPS CD4+ (circle), CD8+ (square), and DNT (triangle) cells were stimulated with anti-CD3/CD28 coated beads and IL-2 in the presence of 1 nM, 10 nM, 100 nM, 1 µM, and 10 µM rapamycin. Proliferation (A) and viability (B) were determined by flow cytometry. Data show mean of 3 independent experiments. (C) PBMC from patients with ALPS before and under rapamycin or MMF therapy were gated for CD3+/TCRαβ+ (%DNT cells) or CD3+/TCRαβ+/CD4−/CD8− (DNT cells). (D) Frequency of DNT cells among all T cells in healthy controls (circle), patients with ALPS (square), and patients with ALPS treated with rapamycin (up-pointing triangle) or MMF (down-pointing triangle) is shown. (E) Percentages of CD4+/CD25+/FoxP3+ Treg cells were determined in PBMC from healthy controls and untreated, rapamycin-treated, and MMF-treated patients with ALPS. (F, G) Percentages of CCR7−/CD45RA+ (F) and Ki67+ (G) cells among DNT cells of all studied healthy controls (n = 12) and untreated (n = 16), rapamycin-treated (n = 9), and MMF-treated (n = 3) patients with ALPS is graphed. Each symbol represents an individual subject; open symbols indicate patients with ALPS (p2-705, p16-11, p1-809) before and under therapy. ns, not significant; *P < .05; **P < .01; ***P < .001 (Mann-Whitney U test).
mTOR inhibition reduces abnormally differentiated DNT cells. (A, B) CFSE-labeled ALPS CD4+ (circle), CD8+ (square), and DNT (triangle) cells were stimulated with anti-CD3/CD28 coated beads and IL-2 in the presence of 1 nM, 10 nM, 100 nM, 1 µM, and 10 µM rapamycin. Proliferation (A) and viability (B) were determined by flow cytometry. Data show mean of 3 independent experiments. (C) PBMC from patients with ALPS before and under rapamycin or MMF therapy were gated for CD3+/TCRαβ+ (%DNT cells) or CD3+/TCRαβ+/CD4−/CD8− (DNT cells). (D) Frequency of DNT cells among all T cells in healthy controls (circle), patients with ALPS (square), and patients with ALPS treated with rapamycin (up-pointing triangle) or MMF (down-pointing triangle) is shown. (E) Percentages of CD4+/CD25+/FoxP3+ Treg cells were determined in PBMC from healthy controls and untreated, rapamycin-treated, and MMF-treated patients with ALPS. (F, G) Percentages of CCR7−/CD45RA+ (F) and Ki67+ (G) cells among DNT cells of all studied healthy controls (n = 12) and untreated (n = 16), rapamycin-treated (n = 9), and MMF-treated (n = 3) patients with ALPS is graphed. Each symbol represents an individual subject; open symbols indicate patients with ALPS (p2-705, p16-11, p1-809) before and under therapy. ns, not significant; *P < .05; **P < .01; ***P < .001 (Mann-Whitney U test).
We next monitored the effects of rapamycin on ALPS DNT cells in vivo. Consistent with previous studies,12,25 the percentage of DNT cells was dramatically reduced in patients with ALPS under rapamycin therapy (Figure 4C-D). Of interest, rapamycin treatment did not increase frequency of FoxP3+ regulatory T (Treg) cells, suggesting the effect of mTOR inhibition on disease phenotype is unlikely to be mediated by Treg cells (Figure 4E). A more detailed analysis revealed that rapamycin specifically affected ALPS DNT cells displaying the abnormal differentiation phenotype (Figure 4F). In addition, mitotic activity (Ki67, cyclin A, proliferating cell nuclear antigen) and expression of HLA-DR and Eomes was greatly diminished among ALPS DNT cells remaining after treatment with the mTOR inhibitor (Figure 4C, G; supplemental Figure 4B-E). We further analyzed the effect of MMF treatment on ALPS DNT cells. In contrast to rapamycin therapy, MMF did not affect differentiation, proliferative activity, or Eomes expression of ALPS DNT cells.
We then asked whether rapamycin therapy is able to control hyperactive mTOR signaling in vivo. DNT cells from rapamycin treated patients with ALPS demonstrated phosphorylation levels of Akt(Ser473) and S6(Ser240) similar to those of CD4+ and CD8+ T cells (Figure 5A). Moreover, HIF1α and KLF2 expression of DNT cells in rapamycin-treated patients with ALPS were similar to healthy controls (Figure 5B-C). These data suggest that the effect of rapamycin is linked neither to the absence of CD4 or CD8 coreceptor expression (as healthy donor DNT cells are not affected) nor to the impairment of FAS signaling (as normally differentiated DNT cells in patients with ALPS are not affected), but is closely linked to the abnormal differentiation pattern. To elucidate the effect of mTOR inhibition on DNT cell functionality, we analyzed expression of IL-10 in sorted T-cell populations of untreated and rapamycin-treated patients with ALPS. In line with previous studies,26,27 ALPS DNT cells highly expressed IL-10, whereas ALPS CD4+ and CD8+ T cells or DNT cells from healthy controls showed low IL-10 expression (Figure 5D). Notably, rapamycin therapy abolished expression of IL-10 in ALPS DNT cells, resulting in decreased serum IL-10 levels after therapy onset (Figure 5D-E). Together, our data indicate that mTOR inhibition in human ALPS specifically controls mitotic activity, aberrant differentiation, and dysregulated cytokine expression of DNT cells.
Rapamycin reduces mTOR hyperactivity of ALPS DNT cells in vivo. (A) Phosphorylation status of Akt(Ser473) and S6(Ser240) was determined in T cells of untreated (n = 10) and rapamycin-treated (n = 4) patients with ALPS after stimulation with cross-linked anti-CD3/CD28 mAbs. Graphs show mean fluorescence intensities, box plots depict the 75th percentile, median, and 25th percentile values; whiskers represent maximum and minimum values. (B) PBMC from healthy donors and untreated and rapamycin-treated patients with ALPS were analyzed for HIF1a expression. Graph shows HIF1a expression relative to CD4+ T cells. (C) Gene expression of KLF2 in the indicated purified cell populations relative to β-2 microglobulin was determined by quantitative RT-PCR. Data represent relative KLF2 expression of 4 rapamycin-treated ALPS. (D) Expression of IL-10 mRNA was assessed in CD4+, CD8+, and DNT cells from untreated (n = 7) and rapamycin-treated (n = 4) patients with ALPS. (E) Serum levels of IL-10 were determined in patients with ALPS before and during rapamycin therapy. Lines depict individual subjects. ns, not significant; *P < .05; **P < .01 (Mann-Whitney U test).
Rapamycin reduces mTOR hyperactivity of ALPS DNT cells in vivo. (A) Phosphorylation status of Akt(Ser473) and S6(Ser240) was determined in T cells of untreated (n = 10) and rapamycin-treated (n = 4) patients with ALPS after stimulation with cross-linked anti-CD3/CD28 mAbs. Graphs show mean fluorescence intensities, box plots depict the 75th percentile, median, and 25th percentile values; whiskers represent maximum and minimum values. (B) PBMC from healthy donors and untreated and rapamycin-treated patients with ALPS were analyzed for HIF1a expression. Graph shows HIF1a expression relative to CD4+ T cells. (C) Gene expression of KLF2 in the indicated purified cell populations relative to β-2 microglobulin was determined by quantitative RT-PCR. Data represent relative KLF2 expression of 4 rapamycin-treated ALPS. (D) Expression of IL-10 mRNA was assessed in CD4+, CD8+, and DNT cells from untreated (n = 7) and rapamycin-treated (n = 4) patients with ALPS. (E) Serum levels of IL-10 were determined in patients with ALPS before and during rapamycin therapy. Lines depict individual subjects. ns, not significant; *P < .05; **P < .01 (Mann-Whitney U test).
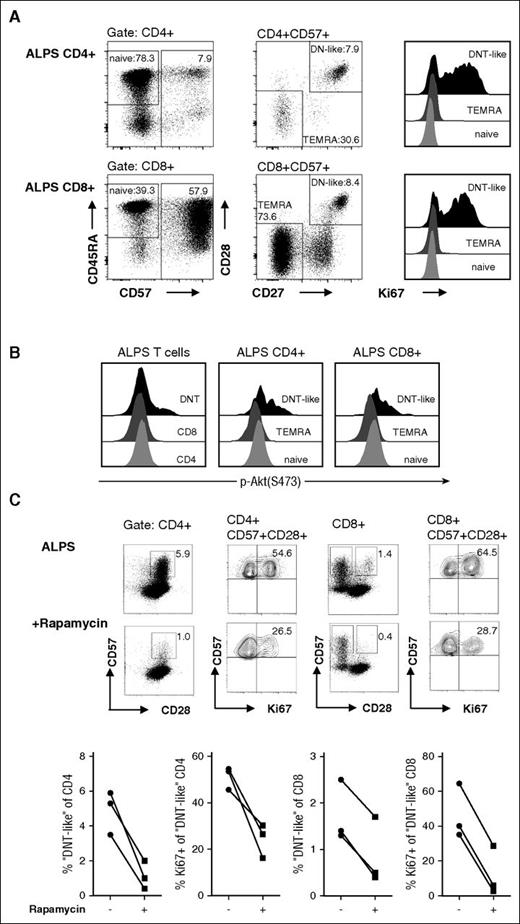
Aberrant CD4+ and CD8+ “DNT-like” putative precursors exhibit increased mitotic activity and mTOR signaling
We have previously shown that a small subset of ALPS CD4+ and CD8+ T cells exhibit the abnormal (CD45RA+/CD57+/CD127−/KLRG1−/CD27+/CD28+) differentiation pattern of DNT cells, and presumably represent their direct precursors.9 The above considerations would predict that this subset should also show hyperproliferation and hyperactive mTOR signaling. Therefore, we analyzed Ki67 expression in naive (CD45RA+/CD57−), terminal differentiated (TEMRA, CD57+/CD27−/CD28−), and “DNT-like” (CD57+/CD27+/CD28+) ALPS CD4+ and CD8+ T cells. Indeed, mitotic activity was markedly enhanced in CD4+ and CD8+ T cells with a “DNT-like” phenotype compared with conventional naive or TEMRA cells (Figure 6A).
ALPS “DNT-like” CD4+and CD8+ T cells show enhanced mitotic activity and mTOR signaling. CD4+ and CD8+ T cells of ALPS patients were gated for naive (CD45RA+/CD57−), TEMRA (CD57+/CD28−/CD27−), and “DNT-like” (CD57+/CD28+/CD27+) cells and analyzed for their Ki67 expression (A) or phosphorylation status after stimulation with anti-CD3/CD28 mAbs (B). Plots show representative data from 1 of 3 independent experiments with similar results. Y-axis in the histograms is normalized to mode. (C) PBMC from patients with ALPS before and under rapamycin therapy were stained with anti-CD3, anti-CD4, anti-CD8, anti-CD28, anti-CD57, and anti-Ki67 mAbs. Cells are gated for CD3+/CD4+ and CD3+/CD4+/CD57+ (left) or CD3+/CD8+ and CD3+/CD8+/CD57+/CD28− (right). Representative plots show data of a patient with ALPS before and during rapamycin therapy; graphs present frequencies of 3 patients.
ALPS “DNT-like” CD4+and CD8+ T cells show enhanced mitotic activity and mTOR signaling. CD4+ and CD8+ T cells of ALPS patients were gated for naive (CD45RA+/CD57−), TEMRA (CD57+/CD28−/CD27−), and “DNT-like” (CD57+/CD28+/CD27+) cells and analyzed for their Ki67 expression (A) or phosphorylation status after stimulation with anti-CD3/CD28 mAbs (B). Plots show representative data from 1 of 3 independent experiments with similar results. Y-axis in the histograms is normalized to mode. (C) PBMC from patients with ALPS before and under rapamycin therapy were stained with anti-CD3, anti-CD4, anti-CD8, anti-CD28, anti-CD57, and anti-Ki67 mAbs. Cells are gated for CD3+/CD4+ and CD3+/CD4+/CD57+ (left) or CD3+/CD8+ and CD3+/CD8+/CD57+/CD28− (right). Representative plots show data of a patient with ALPS before and during rapamycin therapy; graphs present frequencies of 3 patients.
We further examined the phosphorylation status of ALPS CD4+ and CD8+ T-cell subsets. Consistent with the increased proliferative potential, “DNT-like” CD4+ and CD8+ T cells showed enhanced phosphorylation of Akt (Figure 6B). To test the effect of rapamycin on this putative DNT cell precursor population, we aimed to determine putative precursors in patients with ALPS before and under rapamycin treatment. Notably, “DNT-like” CD4+ and CD8+ T cells clearly declined during therapy (Figure 6C). Moreover, remaining abnormal CD4+ and CD8+ T cells showed decreased mitotic activity, suggesting that mTOR inhibition is able to control not only DNT cells but also their putative progenitors. In summary, these data indicate that absence of Fas signaling leads to abnormal differentiation and high mitotic activity of T cell subsets resulting from hyperactive mTOR signaling, which can be pharmacologically reversed by rapamycin therapy (Figure 7).
Proposed model for the development of aberrant DNT cells in ALPS. Under normal conditions, CD4+ or CD8+ T cells can be eliminated by Fas/FasL interaction (black arrow). The majority of T cells in patients with ALPS-FAS show normal differentiation and normal mTOR activation, despite their defect in Fas signaling. Notably, a small fraction of CD4+ and CD8+ T cells show an abnormal differentiation and transcriptional program, enhanced mitotic activity, and hyperactive mTOR signaling (red arrow). Aberrant CD4+ and CD8+ T cells downregulate the coreceptor and convert to DNT cells, whereas the expression profile remains constant. Hyperactive mTOR pathway in Fas-deficient cells leads to expansion of pathognomonic DNT cells and chronic lymphoproliferation. Rapamycin therapy is able to control mTOR activity in both DNT cells and their precursors, resulting in reduced proliferation and increased apoptosis of aberrant cells.
Proposed model for the development of aberrant DNT cells in ALPS. Under normal conditions, CD4+ or CD8+ T cells can be eliminated by Fas/FasL interaction (black arrow). The majority of T cells in patients with ALPS-FAS show normal differentiation and normal mTOR activation, despite their defect in Fas signaling. Notably, a small fraction of CD4+ and CD8+ T cells show an abnormal differentiation and transcriptional program, enhanced mitotic activity, and hyperactive mTOR signaling (red arrow). Aberrant CD4+ and CD8+ T cells downregulate the coreceptor and convert to DNT cells, whereas the expression profile remains constant. Hyperactive mTOR pathway in Fas-deficient cells leads to expansion of pathognomonic DNT cells and chronic lymphoproliferation. Rapamycin therapy is able to control mTOR activity in both DNT cells and their precursors, resulting in reduced proliferation and increased apoptosis of aberrant cells.
Discussion
This study demonstrates that pathognomonic DNT cells, as well as a subset of CD4+ and CD8+ T cells in patients with ALPS are highly proliferative in vivo associated with a hyperactive mTOR pathway. In combination with our recent findings on their similar, unusual phenotype with expression of both memory (CD27/CD28/Eomes) and senescence (CD45RA/CD57/PD1) markers,9 these observations imply that abnormal signaling and differentiation occurs in a subset of T cells before they reach the DNT stage. Active proliferation of a subgroup of Fas-deficient T cells paradoxically showing signs of senescence, either caused or supported by hyperactive mTOR signaling, may thus be a key driver of the lymphoproliferative manifestations in patients with reduced Fas signaling.
On the basis of the role of Fas in the induction of apoptosis, the accumulation of DNT cells in patients with ALPS was initially considered to result from impaired death of senescent cells at the end of their life cycle. Indeed, lack of CCR7, CD62L, and CD127 and expression of CD45RA by ALPS DNT cells are features of a TEMRA phenotype.7,9 A senescent phenotype was further supported by high expression of CD57 and failure to respond to mitogen or TCR stimulation in vitro.6,8 In apparent contrast to these findings, a previous report has shown that many cells in the lymph node paracortex, which is heavily infiltrated by DNT cells in patients with ALPS, express the proliferation marker Ki67.8 Our analysis of Ki67 expression on a per cell basis demonstrates that the proliferative activity in vivo is restricted to DNT cells and their putative precursors and represents a key feature of these cells. Thus, there is a clear dissociation between a terminally differentiated, senescent phenotype and active proliferation, which has not been observed in other disease contexts so far.
What is the basis of this proliferative activity? Our data show that not only human ALPS DNT cells but also their putative precursors had increased phosphorylation of Akt, mTOR and ribosomal protein S6, indicating hyperactivity of both mTORC1 (which phosphorylates S6) and mTORC2 (which phosphorylates Akt on Ser473). The mTOR pathway has been well defined as a critical regulator of T-cell growth, survival, and differentiation,28,29 rendering a link between this pathway and proliferative activity plausible. Because of the effect of the mTOR inhibitor rapamycin on lymphoproliferation and DNT cell accumulation in both lpr mice and ALPS patients13,14 provides a strong argument for a causal association, we analyzed the effect of this treatment on the abnormally differentiated ALPS T cells in more detail.
We observed both reduced proliferation and increased apoptosis of ALPS DNT cells treated with rapamycin in vitro, suggesting that inhibition of proliferation contributes to the reduction of DNT cells and their putative precursors in vivo. Furthermore, mTOR inhibition abolished expression of IL-10 in ALPS DNT cells remaining after treatment, resulting in decreased serum IL-10 levels. Among these remaining DNT cells, we also found a reduction of abnormally differentiated cells and reduced mTOR activity. This could mean that in addition to blocking proliferation, rapamycin selectively induces apoptosis in abnormally differentiated DNT cells. In fact, there is evidence in the CBA-lprcg mouse model that rapamycin activates the proapoptotic Bcl-2 family member Bad, inducing apoptosis by the intrinsic mitochondrial pathway.14 A recent study showed increased expression of antiapoptotic molecules Bcl-2, Bcl-xl, and Mcl-1 in ALPS DNT cells.30 Interestingly, these molecules can be induced by the PI3K-Akt-mTOR signaling and attenuate intrinsic apoptosis pathway.31-33 Alternatively, mTOR inhibition may not only eliminate abnormally differentiated cells and reduce their proliferation but also to some extent revert their abnormal programming. In contrast, DNT cells from MMF-treated patients with ALPS retained the abnormal differentiation and mitotic activity, indicating that pharmacological regulation of the mTOR pathway might offer a superior therapeutic option than MMF therapy.
Various studies showed that rapamycin promotes generation, expansion, and functionality of FoxP3+ Treg cells, both in vitro and in vivo.34-36 Therefore, the benefit of rapamycin in ALPS might be enhanced by induction or expansion of Treg cells. However, we neither found a reduced Treg frequency in patients with ALPS nor an expansion of Treg cells after rapamycin therapy, suggesting efficacy of mTOR inhibition is attributed to reduction of DNT cells and not to Treg cell expansion.
Importantly, the role of mTOR in driving the proliferative activity of DNT cells and their putative precursors does not resolve the discrepancy between a TEMRA-like, senescent cellular phenotype and active proliferation of ALPS DNT cells. In normal T cells, sustained mTOR activation leads to terminal differentiation, as well as induction of T-bet and repression of Eomes expression.22,37,38 Such cells show reduced proliferative capacity and defective memory formation.19,39 The relevance of mTOR hyperactivation in terminal differentiation has been reinforced by the recent description of patients with PIK3CD gain-of-function mutations. CD8+ T cells in these patients are predominantly CD57+/KLRG1+ with pronounced effector gene expression but poor proliferative capacity both in vivo and in vitro.40 In patients with ALPS, hyperactive mTOR signaling in DNT cells and putative precursors is associated with a CCR7−/CD45RA+/CD57+/CD127−/CD62L-terminal differentiated phenotype, but paradoxically, cells do not express T-bet and KLRG1 and vigorously proliferate in vivo. Thus, mTOR signaling is required for DNT cell survival and expansion but appears at least partially uncoupled from transcriptional programming toward terminal effector cells.
Although patients with PIK3CD gain-of-function mutations show constitutive mTOR signaling, mTOR activation in ALPS DNT cells and their precursors is likely to be driven by upstream signals. Because CD28 is a potent activator of the PI3K-Akt-mTOR pathway and is expressed by DNT cells, despite their terminally differentiated phenotype, it may mediate relevant signals for DNT cell survival and expansion. The survival and proliferation of ALPS DNT cells on CD28 costimulation in vitro, as well as the enhanced phosphorylation of Akt, mTOR and S6 after anti-CD3/28 stimulation ex vivo, confirmed functionality of this pathway. However, studies in Fas and CD28 double-deficient mice question a crucial role for CD28 signaling in DNT cell accumulation. Although numbers of abnormal T cells were reduced in lymph nodes of CD28−/− lpr/lpr mice, splenomegaly was enhanced and associated with accumulation of DNT cells.41 Alternatively, mTOR signaling may be enhanced indirectly by alterations of signaling thresholds or intracellular modifiers of this pathway. Indeed, DNT cells of lpr mice exhibit elevated inositol phospholipid turnover, constitutive tyrosine phosphorylation, and aberrant expression of CD45.42,43 These alterations are expected to induce a state of T-cell activation likely associated with mTOR activation. Because KLRG1 inhibits mTOR signaling,44 lack of KLRG1 on ALPS DNT cells could further enhance this pathway. The hyperresponsive mTOR signaling may also be a result of downregulation of the lipid phosphatase PTEN, which could lower signaling thresholds. Indeed, targeted deletion of Pten induced hyperactive PI3K-Akt signaling, lymphoproliferation, impaired Fas-mediated apoptosis, and lethal autoimmunity.45 Paradoxically, we found enhanced PTEN expression in ALPS DNT cells, suggesting that mTOR signaling should even be suppressed in these cells. Both CD28 signaling and low TCR stimulation have been shown to induce PTEN expression in human T cells, and thereby to generate a negative feedback loop controlling PI3K activity.23,46 These data indicate that hyperactive mTOR pathway in ALPS is a result of a specific activation by costimulatory signals and/or TCR stimulation, rather than continuous signaling caused by absent regulatory proteins. Overall, further work is necessary to resolve whether and how upstream signals and their effects on pathways other than the mTOR pathway may contribute to the dissociation between cellular phenotype and proliferative activity.
One of the key unresolved questions is the link of the abnormal proliferative activity to impaired Fas signaling. In principle, impaired apoptosis of a small proportion of T cells in the absence of FAS could lead to survival of cells with enhanced mTOR activity, driving an atypical program of terminal differentiation and uncontrolled proliferation. Alternatively, or in addition, altered T-cell signaling in the absence of FAS could result in enhanced mTOR activity. Of interest, Fas costimulation of naive T cells has been shown to inhibit proliferation and cytokine production by excluding TCR-signaling proteins from lipid rafts without inducing apoptosis.47 Thus, lack of Fas signaling in ALPS might affect initial T-cell activation by increasing TCR-signaling strength, as well as inappropriate T-cell survival resulting from defective cell death induction.
Despite being a hallmark of disease, the functionality of ALPS DNT cells still remains unclear. In murine transplantation models, conventional DNT cells have been shown to suppress immune responses through Fas/FasL-mediated elimination of effector T cells48-53 Of interest, DNT cells from MRL/lpr mice, a murine model of ALPS, were shown to kill syngeneic wild-type, but not Fas-deficient, lpr CD4+ or CD8+ T cells, indicating that the regulatory function of DNT cells in lpr mice is retained.48 We demonstrated recently that activation of the mTOR pathway abrogates the immunoregulatory function of DNT cells from healthy volunteers by reversing their anergic phenotype while promoting proliferation.54 Because ALPS DNT cells show enhanced mTOR signaling and increased proliferative activity, it can be argued that a possible regulatory function of ALPS DNT cells is impaired. However, future work will need to address the potential suppressive properties of DNT cells in patients with ALPS.
In summary, the pathophysiological basis of lymphoproliferation in ALPS appears to be much more complex than an impairment of cell death by impaired Fas signaling. Also, the activated mTOR pathway in DNT cells and their putative precursors in combination with the effect of rapamycin elaborated in this study does not provide the full answer. Nevertheless, a step-by-step dissection of the molecular regulation of T-cell differentiation and proliferation will help us understand the basis of human lymphoproliferative diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge our patients, their families, and referring physicians who made this study possible. The authors also thank Ursula Warthorst (University Medical Center Freiburg), Dorothea Gebhardt (University Hospital Erlangen), and the technicians of the Advanced Diagnostic Unit of the Center for Chronic Immunodeficiency Freiburg for excellent technical assistance and Uwe Appelt and Florentine Koppitz (Core Unit Cell Sorting and Immunomonitoring Erlangen) for cell sorting and fluorescence-activated cell sorter analysis.
This work was supported by the SFB 1181 (project B04) from the German Research Foundation, the Interdisciplinary Center for Clinical Research Erlangen (IZKF, project A58), and the German Federal Ministry of Education and Research (Bundesministerium für Bildung und Forschung [BMBF] 01 EO 0803 grant to the Center of Chronic Immunodeficiency and BMBF 01GM1111B grant to the German Network on Primary Immunodeficiency Diseases [PID-NET] initiative).
Authorship
Contribution: S.V., A.R.-E., S.E., and A.M. designed the research; J.R., C.K., V.S., A.O.v.B., N.N.-B., E.G., K.S., M.N., R.K., P.D.A., M.M., K.-D.S., M.M., and C.S. repeatedly referred patients; I.F. carried out diagnostic tests; M.R.L. and K.S. performed genetic analysis; S.V., A.R.-E., A.A., E.S., P.D.A., M.M., K.-D.S., M.M. A.N.K., S.E., and A.M. collected data; S.V., A.R.-E., A.A., E.S., and A.N.K. performed experiments; S.V., A.R.-E., A.A., K.-D.S., M.M., A.N.K., S.E., and A.M. analyzed and interpreted data; S.V., A.R.-E., S.E., and A.M. wrote the manuscript; and all of the authors edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Simon Völkl, Department of Internal Medicine 5, University Hospital Erlangen, Ulmenweg 18, 91054 Erlangen, Germany; e-mail: simon.voelkl@uk-erlangen.de.
References
Author notes
S.V. and A.R.-E. contributed equally to this study.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal