Key Points
CCL5 increases MK ploidy and subsequent proplatelet formation in a CCR5-dependent manner.
CCL5 may act to increase platelet counts during physiological stress.
Abstract
In times of physiological stress, platelet count can transiently rise. What initiates this reactive thrombocytosis is poorly understood. Intriguingly, we found that treating megakaryocytes (MKs) with the releasate from activated platelets increased proplatelet production by 47%. Platelets store inflammatory cytokines, including the chemokine ligand 5 (CCL5, RANTES); after TRAP activation, platelets release over 25 ng/mL CCL5. We hypothesized that CCL5 could regulate platelet production by binding to its receptor, CCR5, on MKs. Maraviroc (CCR5 antagonist) or CCL5 immunodepletion diminished 95% and 70% of the effect of platelet releasate, respectively, suggesting CCL5 derived from platelets is sufficient to drive increased platelet production through MK CCR5. MKs cultured with recombinant CCL5 increased proplatelet production by 50% and had significantly higher ploidy. Pretreating the MK cultures with maraviroc prior to exposure to CCL5 reversed the augmented proplatelet formation and ploidy, suggesting that CCL5 increases MK ploidy and proplatelet formation in a CCR5-dependent manner. Interrogation of the Akt signaling pathway suggested that CCL5/CCR5 may influence proplatelet production by suppressing apoptosis. In an in vivo murine acute colitis model, platelet count significantly correlated with inflammation whereas maraviroc treatment abolished this correlation. We propose that CCL5 signaling through CCR5 may increase platelet counts during physiological stress.
Introduction
Circulating blood platelets are specialized cells that function to minimize bleeding and blood vessel injury. As such, platelets play a critical role in both normal and disease physiology. Large progenitor cells in the bone marrow called megakaryocytes (MKs) release platelets by extending long processes, designated proplatelets, into sinusoidal blood vessels.1 Despite the importance of platelets in thrombosis and hemostasis, the mechanism by which MKs complete differentiation and release platelets is poorly understood. Specifically, very little is known about what triggers mature, resting MKs to form proplatelets. Platelet counts rise transiently in the setting of physiological stress, such as myocardial infarction, infection, inflammation, and malignancy.2-4 What initiates this upregulation is not well understood and has largely been attributed to an inflammatory response and increased cytokine release.5-7 One cytokine that is highly expressed in inflammatory states is CCL5 (RANTES).8 CCL5, which is abundant in human platelets, signals predominantly through CCR5, a 7-transmembrane G-protein–coupled receptor that mediates diverse signaling cascades.9
Methods
Platelet purification and activation
Blood collection was performed with institutional review board/institutional animal care and use committee approval and in accordance with the Declaration of Helsinki. Platelets were isolated from healthy volunteers or mice as described previously.10 Platelets were activated for 10 minutes at 37°C and CCL5 measured by enzyme-linked immunosorbent assay (R&D Systems).
Megakaryocyte cultures
Microscopy
Flow cytometry
MK and platelet CCR5 expression and MK number were determined by flow cytometry (BD FACSCanto II) using anti-CCR5 (R&D Systems) and anti-CD41/61 (Emfret), respectively. Ploidy was determined by DNA binding via propidium iodide. Data were analyzed with BD FACSDiva 6.1.3 software.
Murine colitis model
Dextran sulfate sodium (5% wt/vol in drinking water) was used to induce acute colitis in C57/BL/6 mice. Maraviroc (100 mg/kg) or saline vehicle was injected intraperitoneally daily. After 7 days, mice were euthanized and blood was collected.
Results and discussion
Releasate from activated platelets increases proplatelet production
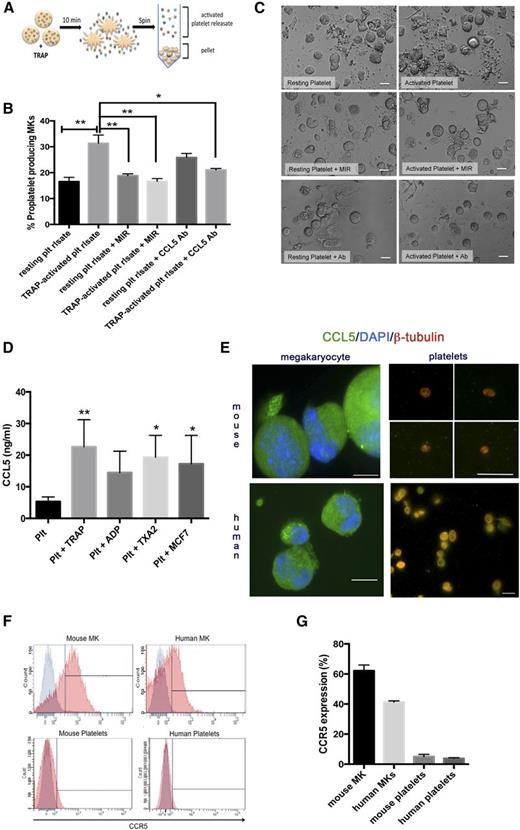
Platelets contain proteins such as platelet factor 4 that act on MKs to negatively regulate platelet production.13 We hypothesized that platelets also contain positive regulators of megakaryopoiesis. We therefore tested the effect of total platelet releasate on MK proplatelet production. Releasate derived from TRAP (thrombin receptor activator peptide)-activated platelets was added to MK cultures (Figure 1A). Intriguingly, platelet releasate increased MK proplatelet production 47% (Figure 1B-C).14-16 This novel and unexpected finding prompted further exploration. Previously, we observed that platelets release agonist-dependent factors and cytokines including abundant amounts of CCL5.10 We hypothesized that CCL5 may be the component of platelet releasate causing increased proplatelet formation. We pretreated MKs with maraviroc, an antagonist specific for the CCL5 receptor CCR5, prior to addition of platelet releasate or immunodepleted CCL5 from the platelet releasate using a neutralizing antibody. Maraviroc and CCL5 neutralization diminished the effect of platelet releasate on proplatelet production by 95% and 70%, respectively, suggesting that platelet-derived CCL5 significantly increased MK proplatelet production through CCR5.
Platelet-derived CCL5 enhances proplatelet production. (A) Generation of activated platelet releasate. Platelet number was counted using a fluorescence-activated cell sorter and adjusted to 2 × 108/mL. The resting state of platelets was confirmed by P-selectin antibody (BD Biosciences) labeling by flow cytometry. Platelets were either activated with 25 μM TRAP (Thrombin Receptor Activator Peptide, Sigma-Aldrich) or incubated with vehicle control for 10 minutes at 37°C. The resulting supernatant, or “releasate,” was separated from the cell pellet by centrifugation and used in subsequent experiments. (B-C) MKs from fetal liver cultures on day 4 of maturation were resuspended in 300 μL TRAP-activated or unactivated platelet releasate (with or without addition of anti-CCL5 antibody). 100 nM maraviroc (MIR) was added to indicated cultures 30 minutes prior to resuspension in platelet releasate. Proplatelet production from MKs was manually quantified after 6 hours based on images generated from a Nikon TE-2000-E Microscope (Nikon) equipped with a 20× (0.3 numerical aperture) Plan-Fluoro objective, using a Hamamatsu charged-coupled device camera, as previously described.14-16 Briefly, for each replicate, at least 100 cells per condition were counted and scored as either “round” or “proplatelet-producing.” n = 3-6; *P < .05, **P < .01, with data plotted as mean and standard error of the mean and statistical analysis done by 1-way ANOVA with Tukey’s multiple comparisons test. Representative images of proplatelet formation are shown in panel C, indicating enhanced, long proplatelet strings with the addition of TRAP-activated platelet releasate. (D) Platelets were prepared as above and activated with 25 μM TRAP, 25 μM ADP (Biodata), 100 μM Thromboxane A2 (Caymen), or 3 × 106/mL MCF-7 breast tumor cells (ATCC). CCL5 in releasate was measured using the Quantikine human CCL5 enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions (R&D Systems). n = 3; *P < .05, **P < .01, with data plotted as mean and standard error of the mean and statistical analysis done by 1-way ANOVA showing differences compared with resting platelet control. (E) Platelets and mouse MKs were isolated and prepared as previously described. Human MKs were isolated from umbilical cord blood collected with institutional review board approval from healthy full-term neonates (38-42 weeks gestation) at Brigham and Women’s Hospital Labor and Delivery. Briefly, CD34+ cells were then isolated using a positive magnetic selection system (Miltenyi Biotec) and plated in 24-well plates at 1 × 105 cells/mL and cultured in serum-free medium with rTPO (50 ng/mL, PeproTech), with twice-weekly medium changes for 14 days. Live-cell number was quantified twice weekly by staining with 0.4% Trypan blue. For immunofluorescence, samples were fixed in 4% formaldehyde and centrifuged onto poly-l-lysine (1 μg/mL)-coated coverslips, permeabilized with 0.5% Triton-X-100, and blocked in blocking buffer.18 Samples were examined with a Zeiss Axiovert 200 (Carl Zeiss, Thornwood, NY) equipped with a 63× or 100× (1.4 numerical aperture) Plan-ApoChromat oil-immersion objective, and images were obtained and analyzed using Metamorph software (Molecular Devices, Sunnyvale, CA) and ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/). Scale bars represent 2 μm (platelets) and 20 μm (MKs); green indicates CCL5. In platelet samples only, red indicates β-tubulin. In MK samples only, blue indicates Hoechst (nucleus). (F-G) Surface expression of CCR5 was determined on mouse and human MKs and platelets using a phycoerythrin-conjugated anti-human/mouse CCR5 antibody (R&D Systems) compared with an isotype control. (G) Representative histograms are shown with CCR5-positive staining (red) overlayed onto isotype control (gray). (H) CCR5 expression was quantified. n = 3. Ab, antibody; plt, platelet; rlsate, releasate.
Platelet-derived CCL5 enhances proplatelet production. (A) Generation of activated platelet releasate. Platelet number was counted using a fluorescence-activated cell sorter and adjusted to 2 × 108/mL. The resting state of platelets was confirmed by P-selectin antibody (BD Biosciences) labeling by flow cytometry. Platelets were either activated with 25 μM TRAP (Thrombin Receptor Activator Peptide, Sigma-Aldrich) or incubated with vehicle control for 10 minutes at 37°C. The resulting supernatant, or “releasate,” was separated from the cell pellet by centrifugation and used in subsequent experiments. (B-C) MKs from fetal liver cultures on day 4 of maturation were resuspended in 300 μL TRAP-activated or unactivated platelet releasate (with or without addition of anti-CCL5 antibody). 100 nM maraviroc (MIR) was added to indicated cultures 30 minutes prior to resuspension in platelet releasate. Proplatelet production from MKs was manually quantified after 6 hours based on images generated from a Nikon TE-2000-E Microscope (Nikon) equipped with a 20× (0.3 numerical aperture) Plan-Fluoro objective, using a Hamamatsu charged-coupled device camera, as previously described.14-16 Briefly, for each replicate, at least 100 cells per condition were counted and scored as either “round” or “proplatelet-producing.” n = 3-6; *P < .05, **P < .01, with data plotted as mean and standard error of the mean and statistical analysis done by 1-way ANOVA with Tukey’s multiple comparisons test. Representative images of proplatelet formation are shown in panel C, indicating enhanced, long proplatelet strings with the addition of TRAP-activated platelet releasate. (D) Platelets were prepared as above and activated with 25 μM TRAP, 25 μM ADP (Biodata), 100 μM Thromboxane A2 (Caymen), or 3 × 106/mL MCF-7 breast tumor cells (ATCC). CCL5 in releasate was measured using the Quantikine human CCL5 enzyme-linked immunosorbent assay kit according to the manufacturer’s instructions (R&D Systems). n = 3; *P < .05, **P < .01, with data plotted as mean and standard error of the mean and statistical analysis done by 1-way ANOVA showing differences compared with resting platelet control. (E) Platelets and mouse MKs were isolated and prepared as previously described. Human MKs were isolated from umbilical cord blood collected with institutional review board approval from healthy full-term neonates (38-42 weeks gestation) at Brigham and Women’s Hospital Labor and Delivery. Briefly, CD34+ cells were then isolated using a positive magnetic selection system (Miltenyi Biotec) and plated in 24-well plates at 1 × 105 cells/mL and cultured in serum-free medium with rTPO (50 ng/mL, PeproTech), with twice-weekly medium changes for 14 days. Live-cell number was quantified twice weekly by staining with 0.4% Trypan blue. For immunofluorescence, samples were fixed in 4% formaldehyde and centrifuged onto poly-l-lysine (1 μg/mL)-coated coverslips, permeabilized with 0.5% Triton-X-100, and blocked in blocking buffer.18 Samples were examined with a Zeiss Axiovert 200 (Carl Zeiss, Thornwood, NY) equipped with a 63× or 100× (1.4 numerical aperture) Plan-ApoChromat oil-immersion objective, and images were obtained and analyzed using Metamorph software (Molecular Devices, Sunnyvale, CA) and ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/). Scale bars represent 2 μm (platelets) and 20 μm (MKs); green indicates CCL5. In platelet samples only, red indicates β-tubulin. In MK samples only, blue indicates Hoechst (nucleus). (F-G) Surface expression of CCR5 was determined on mouse and human MKs and platelets using a phycoerythrin-conjugated anti-human/mouse CCR5 antibody (R&D Systems) compared with an isotype control. (G) Representative histograms are shown with CCR5-positive staining (red) overlayed onto isotype control (gray). (H) CCR5 expression was quantified. n = 3. Ab, antibody; plt, platelet; rlsate, releasate.
Platelets release CCL5
CCL5 is present in MKs and platelets, but CCR5 is restricted to MKs
Using immunofluorescence, we confirmed that platelets and MKs contain CCL5 (Figure 1E).18 We measured CCR5 surface expression on human and mouse platelets and MKs utilizing flow cytometry (Figure 1F-G), finding that MKs expressed CCR5 on their surface whereas platelets did not, suggesting that platelet-released CCL5 can signal through MK-localized CCR5. Although lack of CCR5 on platelets has been demonstrated,19 it is rare for a receptor to be present on MKs but not platelets, indicating that MK CCR5 is specifically excluded or degraded from the platelet surface.
Recombinant CCL5 recapitulates platelet releasate
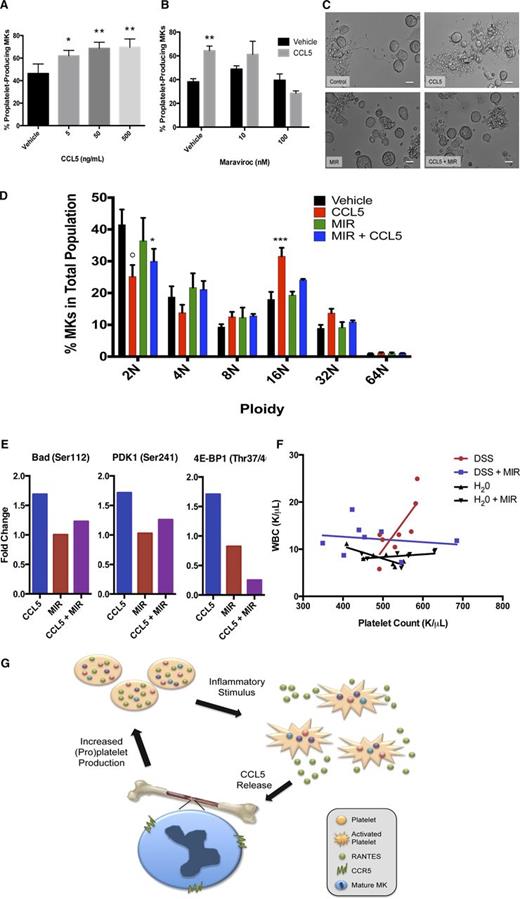
We next tested whether recombinant CCL5 could recapitulate the increased proplatelet production seen with platelet releasate. Indeed, CCL5 significantly increased proplatelet production by 50% (Figure 2A), and Maraviroc significantly and dose-dependently reversed the effect of CCL5 up to 97% (Figure 2B-C).
The CCL5/CCR5 axis enhances megakaryocyte maturation and proplatelet formation through apoptosis suppression. (A) Recombinant CCL5 (5, 50, or 500 ng/mL) or vehicle control was added to MK cultures on day 4 of maturation. Proplatelet production from MKs was manually quantified after 6 hours as described in Figure 1 and previously.14-16 n = 6; *P < .05, **P < .01, with statistical analysis done by Student t test compared with vehicle control. (B) Maraviroc (10 or 100 nM) was added to indicated cultures and allowed to incubate for 30 minutes. 50 ng/mL CCL5 or vehicle control was then added to MK cultures on day 4 of maturation. Proplatelet production from MKs was manually quantified after 6 hours as described elsewhere.14-16 n = 3; **P < .01, with statistical analysis done by Student t test compared with vehicle control. (C) Representative images of proplatelet production in MKs treated with CCL5 ± maraviroc (MIR). Images were obtained with a Zeiss Axiovert 200 (Carl Zeiss) equipped with a 20× objective, and images obtained and analyzed using Metamorph software (Molecular Devices) and ImageJ. Scale bars represent 20 μm. (D) On day 1 of MK maturation, maraviroc (100 nM) was added to indicated cultures and allowed to incubate for 30 minutes. 50 ng/mL CCL5 or vehicle control was then added to MK cultures. On day 4, MK ploidy was determined using a fluorescence-activated cell sorter (30 000 events per sample) by gating on fluorescence intensity based on DNA binding via propidium iodide (Sigma Aldrich). Statistical analysis was done using 2-way ANOVA with α = 0.05, comparisons made between vehicle control and various treatment groups, as indicated. n = 6; *P < .05, **P < .01, ***P < .005, and ○P < .0001, with data plotted as mean and standard error of the mean and statistical analysis done by 2-way ANOVA with Dunnett’s multiple comparisons test. (E) To interrogate the Akt signaling pathway, maraviroc (100 nM) was added to indicated MK cultures on day 4 of maturation and allowed to incubate for 30 minutes prior to the additional of 50 ng/mL CCL5 or vehicle control. Lysates were generated 15 minutes after the addition of CCL5 and analyzed using the PathScan Akt Signaling Antibody Array (Cell Signaling Technology), which detects phosphorylation levels of 18 proteins in the Akt signaling pathway. The array was performed according to the manufactures instructions, imaged using a G:Box Imaging System (Syngene) and analyzed with ImageJ software. Data were normalized to untreated day 4 MK lysate, and fold changes of 1.5 or greater are shown. (F) Mice were exposed to DSS (5% wt/vol in drinking water) to induce colitis or given untreated drinking water and treated with maraviroc (10mg/kg intraperitoneally, daily) or saline vehicle. After 7 days, mice were euthanized and blood was collected to determine platelet and WBC count (HemaVet, Drew Scientific). Platelet count was correlated with WBC (a marker of inflammation) in each of the four treatment groups using GraphPad Prism 6 software. Pearson’s correlation, P = .018. (G) Proposed model of CCL5/CCR5-induced thrombocytosis. In a state of physiological stress, platelet activation by agonists leads to release of platelet CCL5. CCL5 binds to MK CCR5, causing increased MK maturation and proplatelet formation and a subsequent increase in circulating platelet levels. Thus, a positive feedback loop is established.
The CCL5/CCR5 axis enhances megakaryocyte maturation and proplatelet formation through apoptosis suppression. (A) Recombinant CCL5 (5, 50, or 500 ng/mL) or vehicle control was added to MK cultures on day 4 of maturation. Proplatelet production from MKs was manually quantified after 6 hours as described in Figure 1 and previously.14-16 n = 6; *P < .05, **P < .01, with statistical analysis done by Student t test compared with vehicle control. (B) Maraviroc (10 or 100 nM) was added to indicated cultures and allowed to incubate for 30 minutes. 50 ng/mL CCL5 or vehicle control was then added to MK cultures on day 4 of maturation. Proplatelet production from MKs was manually quantified after 6 hours as described elsewhere.14-16 n = 3; **P < .01, with statistical analysis done by Student t test compared with vehicle control. (C) Representative images of proplatelet production in MKs treated with CCL5 ± maraviroc (MIR). Images were obtained with a Zeiss Axiovert 200 (Carl Zeiss) equipped with a 20× objective, and images obtained and analyzed using Metamorph software (Molecular Devices) and ImageJ. Scale bars represent 20 μm. (D) On day 1 of MK maturation, maraviroc (100 nM) was added to indicated cultures and allowed to incubate for 30 minutes. 50 ng/mL CCL5 or vehicle control was then added to MK cultures. On day 4, MK ploidy was determined using a fluorescence-activated cell sorter (30 000 events per sample) by gating on fluorescence intensity based on DNA binding via propidium iodide (Sigma Aldrich). Statistical analysis was done using 2-way ANOVA with α = 0.05, comparisons made between vehicle control and various treatment groups, as indicated. n = 6; *P < .05, **P < .01, ***P < .005, and ○P < .0001, with data plotted as mean and standard error of the mean and statistical analysis done by 2-way ANOVA with Dunnett’s multiple comparisons test. (E) To interrogate the Akt signaling pathway, maraviroc (100 nM) was added to indicated MK cultures on day 4 of maturation and allowed to incubate for 30 minutes prior to the additional of 50 ng/mL CCL5 or vehicle control. Lysates were generated 15 minutes after the addition of CCL5 and analyzed using the PathScan Akt Signaling Antibody Array (Cell Signaling Technology), which detects phosphorylation levels of 18 proteins in the Akt signaling pathway. The array was performed according to the manufactures instructions, imaged using a G:Box Imaging System (Syngene) and analyzed with ImageJ software. Data were normalized to untreated day 4 MK lysate, and fold changes of 1.5 or greater are shown. (F) Mice were exposed to DSS (5% wt/vol in drinking water) to induce colitis or given untreated drinking water and treated with maraviroc (10mg/kg intraperitoneally, daily) or saline vehicle. After 7 days, mice were euthanized and blood was collected to determine platelet and WBC count (HemaVet, Drew Scientific). Platelet count was correlated with WBC (a marker of inflammation) in each of the four treatment groups using GraphPad Prism 6 software. Pearson’s correlation, P = .018. (G) Proposed model of CCL5/CCR5-induced thrombocytosis. In a state of physiological stress, platelet activation by agonists leads to release of platelet CCL5. CCL5 binds to MK CCR5, causing increased MK maturation and proplatelet formation and a subsequent increase in circulating platelet levels. Thus, a positive feedback loop is established.
Although the relationship between ploidy and proplatelet formation is complex, elevated ploidy correlates with increased proplatelet formation.20 Therefore, we probed CCL5’s impact on MK endomitosis (repeated DNA replication without cell division).21 Interestingly, CCL5 treatment caused significantly higher ploidy; 65% fewer MKs were 2N, whereas 76% more were 16N (Figure 2D). Maraviroc pretreatment inhibited the CCL5 augmented ploidy, substantiating the idea that the increased ploidy was mediated though CCR5.20
The mechanism of CCL5/CCR5’s effect on MKs may be through apoptosis suppression
We next examined the mechanism by which the CCL5/CCR5 axis enhanced MK maturation and proplatelet formation. We examined the Akt pathway because it is downstream of CCR5 and has a role in megakaryocyte maturation.22 We performed an antibody-based bioarray probing AKT pathway signaling (Cell Signaling Technology). The results from the array revealed a >1.5-fold increase in BAD, 4E-BP1, and PDK1 phosphorylation, proteins crucial for apoptosis suppression (Figure 2E).23-25 Maraviroc alone had no affect on BAD, 4E-BP1, and PDK1 phosphorylation, indicating that maraviroc does not have off-target effects. Additionally, the increase observed with CCL5 administration was reversed when megakaryocytes were pretreated with maraviroc, confirming the specificity of CCL5 for CCR5 (Figure 2E). In megakaryocytes, the role of apoptosis in proplatelet formation is an area of active investigation, and recent studies have revealed that the intrinsic apoptosis pathway must be restrained in order for cells to undergo proplatelet formation.26,27 Therefore, these results suggest that CCL5/CCR5 may support platelet production by facilitating apoptosis inhibition.
Maraviroc inhibits the correlation between inflammation and platelet count in vivo
Finally, we employed an in vivo murine colitis model in which CCL5 has been linked with inflammation. Mice were given dextran sodium sulfate (DSS) with or without maraviroc to examine the role of the CCL5/CCR5 axis in platelet production during acute inflammation. The platelet counts of DSS-treated mice positively correlated with the white blood cell (WBC) count (Figure 2F; Pearson’s correlation, P = .018). This correlation was not present in sham-treated mice (water or maraviroc alone). Importantly, maraviroc eliminated the correlation between platelet and WBC counts (Figure 2F), suggesting that the component of the inflammatory response that regulates platelet count is mediated through CCR5.
Our data demonstrate that in vitro, CCL5 increased MK ploidy and subsequent proplatelet formation in a CCR5-dependent manner. We propose that CCL5 may increase platelet counts during acute inflammation (Figure 2G, schematic); this is supported by our in vivo data. CCR5 knockout mice do not demonstrate thrombocytopenia, suggesting that CCL5/CCR5 functions in times of stress and not hemostatic platelet production. Although a prior mechanistic link between platelet production and CCL5 has not been identified, clinical data support this association; patients with thrombocytopenia due to idiopathic thrombocytopenic purpura or aplastic anemia have a direct correlation between CCL5 level and platelet count.28 Our data (1) provide a possible explanation for the elevated platelet counts observed in reactive thrombocytosis and (2) suggest that CCR5 may be a therapeutic target for treating thrombocytopenia. Although CCL5 itself may not be a good therapeutic candidate owing to its role in the body’s immunogenic response, small molecules could work to stimulate CCR5 directly. This would provide a therapeutic intervention to stimulate platelet release from existing bone marrow MKs, resulting in an immediate increase in platelet count, representing a significant advantage over current TPO-based therapies, which take 5 to 12 days to be effective.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Nancy Berliner, Dr Robert Flaumenhaft, and Dr Marsha Moses for their insightful feedback and unyielding support.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants F32HL118865 (K.R.M.), T32HL116324 (K.E.J.), and R01HL68130 (J.E.I.).
Authorship
Contribution: K.R.M. and K.E.J. designed and carried out experiments, analyzed data, and wrote the manuscript; S.W., J.A.F., M.D.T., T.S.S., R.K., S.H.E.-H., and S.K.W. carried out experiments and analyzed data; R.S.W. and J.E.I. contributed to the manuscript; and E.M.B. supervised all research, contributed to design of experiments, analyzed data, and wrote manuscript.
Conflict-of-interest disclosure: J.E.I. has financial interest in and is a founder of Platelet BioGenesis, a company that aims to produce donor-independent human platelets from human-induced pluripotent stem cells at scale. J.E.I. is an inventor on this patent. The interests of J.E.I. were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies. The remaining authors declare no competing financial interests.
Correspondence: Elisabeth M. Battinelli, Brigham and Women’s Hospital, 1 Blackfan Circle, Karp 5, Boston, MA 02115; e-mail: ebattinelli@partners.org.
References
Author notes
K.R.M. and K.E.J. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal