In this issue of Blood, Machlus et al report a novel potential positive feedback mechanism whereby the platelet-borne inflammatory cytokine chemokine ligand 5 (CCL5; also known as regulated on activation, normal T cell expressed and secreted [RANTES]) can stimulate megakaryocytes to produce platelets.1
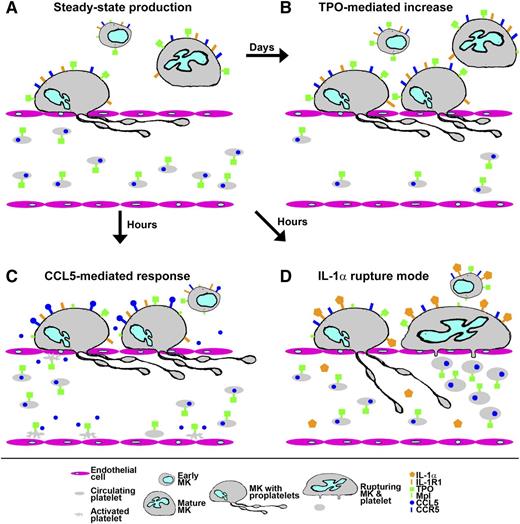
Response of platelet production to stresses. (A) Under steady-state conditions, early MK precursors develop over several days in the bone marrow into mature MKs that move to sinusoids and extend proplatelets through the endothelium into the bloodstream to shed platelets. Progression of early MKs is determined by the levels of TPO coming from blood, where most is bound by receptors (Mpl) on circulating platelets. (B) When circulating platelet numbers drop, the resulting rise in TPO reaching early MKs stimulates increased numbers to complete development and produce platelets, restoring steady-state levels after several days. (C) In a response proposed by Machlus et al, physiologic stress stimulates activated platelets and other cells to release CCL5/RANTES, which can directly stimulate mature MKs to rapidly increase platelet production, creating increased levels associated with reactive thrombocytosis. (D) Acute systemic inflammation and/or immune-modulated platelet loss produces increased levels of IL-1α, which, according to Nishimura et al,9 can directly stimulate mature MKs to undergo a rupture mode that allows them to rapidly shed large numbers of platelets that are somewhat larger than normal but otherwise functional. Illustration by Fred G. Pluthero.
Response of platelet production to stresses. (A) Under steady-state conditions, early MK precursors develop over several days in the bone marrow into mature MKs that move to sinusoids and extend proplatelets through the endothelium into the bloodstream to shed platelets. Progression of early MKs is determined by the levels of TPO coming from blood, where most is bound by receptors (Mpl) on circulating platelets. (B) When circulating platelet numbers drop, the resulting rise in TPO reaching early MKs stimulates increased numbers to complete development and produce platelets, restoring steady-state levels after several days. (C) In a response proposed by Machlus et al, physiologic stress stimulates activated platelets and other cells to release CCL5/RANTES, which can directly stimulate mature MKs to rapidly increase platelet production, creating increased levels associated with reactive thrombocytosis. (D) Acute systemic inflammation and/or immune-modulated platelet loss produces increased levels of IL-1α, which, according to Nishimura et al,9 can directly stimulate mature MKs to undergo a rupture mode that allows them to rapidly shed large numbers of platelets that are somewhat larger than normal but otherwise functional. Illustration by Fred G. Pluthero.
Platelets are the cellular effectors of hemostasis in mammals. They also play important roles in physiologic processes such as wound healing and inflammatory/immune responses, and in pathological developments including atherosclerosis and tumor metastasis. Postnatal platelet production is centered on the bone marrow, where mature megakaryocytes (MKs) move to sinusoids and extend long processes (proplatelets) that shed platelets into the bloodstream,2 where they can circulate for several days. The regulation of platelet production, or thrombopoiesis, has recently been reviewed in Blood,3 and although recent findings have added new complexities, the process as it proceeds under normal circumstances can still be depicted in a fairly simple manner.
Like most blood cell production lines, thrombopoiesis is regulated by a specific hematopoietic factor, in this case thrombopoietin (TPO).4 TPO is released at a constant rate by the liver into the bloodstream, where much of it is bound by circulating platelets and thus prevented from entering the marrow. TPO that does make it into the hematopoietic compartment can be bound by cells at all stages of MK development, but as was recently shown by Ng et al,5 in mice the only cells that absolutely require TPO to continue down the MK developmental pathway are the early progenitors. Thus the end products of thrombopoiesis (ie, platelets), together with mature MKs and most of the cells leading up to them, buffer the amount of TPO available to the stem cells that feed into the process (see figure, panel A). In situations where increased consumption or other losses lead to a drop in circulating platelet numbers, the resulting rise in levels of TPO reaching the marrow allows a higher proportion of progenitors to commit to and/or complete MK development (see figure, panel B). The result, after a lag determined by the time it takes for MKs to mature (up to 12 days in humans), is a rise in platelet production that first overshoots normal levels and then restores them. Thus under normal physiologic circumstances, thrombopoiesis is controlled by a regulatory balance between cells at the end and beginning of the process, which maintains steady-state levels of circulating platelets that can vary considerably among individuals (eg, normal adults have 150-450 platelets × 109/L blood) and species (eg, mice have ∼1000 platelets × 109/L blood).
It has long been known, however, that this elegant balance can be influenced by a variety of factors. For example infections and inflammation can trigger a rise in platelet count (ie, reactive thrombocytosis), which has been linked to increased TPO production, and possibly to factors that can independently stimulate platelet production. In their current paper, Machlus et al investigate such a role for CCL5, also known as RANTES, a member of the interleukin (IL)-8 cytokine superfamily that is systemically elevated in inflammatory states. CCL5 is abundant in platelets and released on activation,6 and it is a ligand for CCR5, a G-coupled protein receptor expressed by leukocytes and MKs. CCL5 is also a cellular coreceptor (with CD4) for HIV, and the expression of CCR5 by MKs has been linked to HIV-induced thrombocytopenia.7 Among the recently approved drugs developed to combat HIV infection is the CCR5 antagonist Maraviroc.8
Machlus et al observed that when exposed to releasates recovered from stimulated platelets, MKs cultured from murine fetal liver showed a rapid increase in the proportion of cells producing proplatelets. This effect was inhibited in a dose-dependent manner by Maraviroc or by immunodepletion of CCL5 from platelet releasates. Similar MK-stimulatory effects were also obtained with recombinant CCL5. An interrogation of signaling pathways downstream of CCR5 indicated that its activation by CCL5 may exert a positive effect on the maturation of MKs by stimulating AKT pathway components that inhibit apoptosis. They also observed that Maraviroc treatment prevented reactive thrombocytosis in a mouse model of acute inflammation. Thus for the first time, Machlus et al demonstrate that a platelet-derived cytokine, CCL5, may have a direct stimulatory effect on platelet production by bone marrow MKs that induces a more rapid response than steady-state regulation via TPO (see figure, panel C).
This observation comes close on the heels of a recent report by Nishimura et al9 describing the potent thrombopoietic effects of another inflammatory/immune modulator. Using in vivo 2-photon imaging of murine MKs, they characterized normal platelet production via shedding from proplatelets and then examined the effects of treatments that induced acute immune-mediated platelet loss (injection with anti-CD42b antibody) or acute inflammation (thioglycolate injection). Both treatments caused a rapid and dramatic increase in platelet production, largely attributed to the inducement of some MKs to undergo a previously unobserved mode of development dubbed “MK rupture.” This process produces platelets that are larger than normal but otherwise fully functional (see figure, panel D). Via a series of in vitro and in vivo experiments, Nishimura et al demonstrated that MK rupture can be triggered by high circulating levels of IL-1α, which is released by many cell types (but is much less abundant than CCL5 in platelets) in response to damage and infective/inflammatory stress.9
The recent discoveries reported by Machlus et al and Nishimura et al provide novel insights into the potential of the thrombopoietic system to rapidly increase platelet production in response to physiologic stress. These newly revealed regulatory and developmental aspects contribute to a complex emerging picture of platelet production, and they also point to previously uncharacterized links to pathologic processes such as inflammation and infection that are likely to be highly relevant to understanding and treating platelet count fluctuations.
Conflict-of-interest disclosure: The authors declare no competing financial interests.