Key Points
Enrichment of atypical MPL mutations in essential thrombocythemia.
MPLS204P and MPLY591N mutants are weak gain-of-function mutants.
Abstract
Mutations in signaling molecules of the cytokine receptor axis play a central role in myeloproliferative neoplasm (MPN) pathogenesis. Polycythemia vera is mainly related to JAK2 mutations, whereas a wider mutational spectrum is detected in essential thrombocythemia (ET) with mutations in JAK2, the thrombopoietin (TPO) receptor (MPL), and the calreticulin (CALR) genes. Here, we studied the mutational profile of 17 ET patients negative for JAK2V617F, MPLW515K/L, and CALR mutations, using whole-exome sequencing and next-generation sequencing (NGS) targeted on JAK2 and MPL. We found several signaling mutations including JAK2V617F at very low allele frequency, 1 homozygous SH2B3 mutation, 1 MPLS505N, 1 MPLW515R, and 2 MPLS204P mutations. In the remaining patients, 4 presented a clonal and 7 a polyclonal hematopoiesis, suggesting that certain triple-negative ETs are not MPNs. NGS on 26 additional triple-negative ETs detected only 1 MPLY591N mutation. Functional studies on MPLS204P and MPLY591N revealed that they are weak gain-of-function mutants increasing MPL signaling and conferring either TPO hypersensitivity or independence to expressing cells, but with a low efficiency. Further studies should be performed to precisely determine the frequency of MPLS204 and MPLY591 mutants in a bigger cohort of MPN.
Introduction
According to the World Health Organization (WHO) classification, essential thrombocythemia (ET), polycythemia vera, and primary myelofibrosis (PMF) belong to the classical non-BCR-ABL1 myeloproliferative neoplasms (MPNs).1,2 All of the non-BCR-ABL1 MPNs are associated with persistent and hyperactivation of the Janus kinase 2 (JAK2)/signal transducer and activator of transcription (STAT) signaling pathway, but ET and PMF are heterogeneous disorders. JAK2V617F is the predominant mutation found in 50% to 60% of the ET cases.3 Other signaling molecules are mutated in ET including myeloproliferative leukemia (MPL) and SH2B3 (LNK). The most prevalent MPL mutations in ET and PMF occur on tryptophan 515, an amino acid that maintains MPL in an inactive form in the absence of cytokine. The most frequent mutations are the substitution of W515 to L and K found in 3% to 5% of ETs and PMFs. Of note, it is the loss of tryptophan, but not the acquisition of a particular residue, that induces constitutive activation of MPL,4 leading to the MPN phenotype. Less frequently, the S505N mutation located in the exon 10 coding for the transmembrane domain induces a dimerization of the transmembrane helix in an active conformation.5 This last mutation has been initially found not only in some hereditary thrombocytosis,6 but it has also been detected in rare sporadic ETs and PMFs.7,8 In addition, other MPL mutants have already been described, more particularly in the extracellular domain of MPL in very rare ETs and PMFs, but without providing evidence for a gain-of-function.8-12 SH2B3 loss-of-function mutations are present in <1% of ET and PMF, but may not be sufficient to induce a MPN.13,14
Recurrent mutations in the exon 9 of calreticulin (CALR) have been recently found in around 25% of ETs and PMFs. They all induce a frameshift leading to a new C-terminus peptide composed of at least 35 aa with loss of the endoplasmic reticulum (ER) retention signal, KDEL.15,16 Whereas wild-type (WT) CALR is essentially involved in the quality control of proteins and in calcium storage in the ER,17 the CALR mutants are able to activate the JAK2/STAT pathway via an unknown mechanism like the other MPN driver mutations.15 The same signaling mutations are found in ETs and PMFs, underscoring they have a close pathogenesis requiring megakaryocyte hyperplasia although they are clinically different diseases.15,16
Presently, the ETs and PMFs, which are negative for JAK2V617F, CALR, and MPL mutations are called “triple-negative” MPNs.1 However, the entire sequence of MPL is not usually examined in patients. Depending on the particular clinical centers, there is a wide variety of MPL mutations that are screened (MPLW515K/L or all substitutions of W515 or mutations on exon 10 including MPLS505N). Usually, only MPLW515K/L are searched by allele-specific polymerase chain reaction (PCR). Whereas the triple-negative PMF have a poor prognosis,18,19 triple-negative ETs have a good prognosis. However, it is presently unknown whether these triple-negative MPNs are a homogeneous entity.
In this study, we have investigated, using whole-exome sequencing (WES), the mutational profile of 17 triple-negative ET patients previously identified as negative for JAK2, MPL, and CALR mutations. This group was heterogeneous with 7 cases that did not display any clonal mutations. In the other cases, there was enrichment in SH2B3 and atypical MPL mutations located in the exons 10 and 4 (MPLS204P). NGS targeted on MPL and JAK2 exons in 5 of these patients and 26 additional patients led to the discovery of a second MPLS204P mutation not seen in WES and another atypical MPL mutation (MPLY591N). Sensitive functional studies revealed that both were low gain-of-function mutants. Our results demonstrate that some clonal noncanonical MPL gain-of-function mutations are associated with triple-negative ETs.
Material and methods
Patient cohort
Blood samples were obtained from Saint Louis Hospital and Saint Antoine Hospital (Paris, France) and Brest Centre Hospitalier Universitaire (CHU) and approved by the local ethics committees. Written informed consent was in accordance with the Declaration of Helsinki. For most of the patients, the diagnosis was performed according to the WHO recommendation including a bone marrow biopsy.20,21 MPLW515K/L and JAK2V617F genotyping were performed by allele-specific quantitative PCR.22,23 The mutational status of CALR was determined using previously described high-resolution sizing of fluorescent dye-labeled PCR amplification of exon 9 and Sanger sequencing for validation.24 Plasma thrombopoietin (TPO) levels were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s instructions (R&D Systems).
CGH arrays
Comparative genomic hybridization (CGH) arrays from granulocytes were conducted on human CGH 2x400K (G4448A) by hybridization of sample vs normal-matched commercial reference and a hierarchical clustering was performed.25
Whole-exome sequencing
WES was performed on genomic DNA (1 μg) from paired samples (granulocytes vs T cells) The final libraries were indexed, pooled, and sequenced on an Illumina HiSeq-2000 sequencer. Only nonsynonymous and splice-site mutations were further pursued. A special analysis using BWA ALN for mapping and GATK HaplotyCaller allowed us to detect insertions and deletions. Candidate mutations observed in the exon sequencing were validated using Sanger sequencing.15,16,26
MPL and JAK2 gene sequencing
Ion AmpliSeq custom panel primer pools for MPL and JAK2 were used (10 ng of genomic DNA per primer pool) to perform multiplex PCR but the protocol to generate libraries was modified by adding paired-end adaptors (NEXTflex; Bio Scientific) to sequence in an Illumina MiSeq flow cell using the onboard cluster method, as paired-end sequencing (2 × 250 bp reads) (Illumina). The entire MPL and JAK2 exons were sequenced to reach a 1000× depth. Minimum variant frequency was set at 1%. Sanger sequencing of the CD3+ DNA assessed the somatic status of the mutation.
Cell lines and cell culture
The cytokine-dependent (granulocyte macrophage colony-stimulating factor [GM-CSF]) human UT7 cell line was cultured in α medium 10% fetal calf serum and 5 ng/mL GM-CSF. This UT7 cell line did not express MPL and did not respond to TPO. Interleukin-3 (IL-3)-dependent murine pro-B Ba/F3 cells were cultured in Dulbecco medium with 10% fetal calf serum and 5% WEHI3.
DNA manipulations, production of retroviruses
The 1531T>A (Y591N), 610T>C (S204P) point mutations were introduced into the MEGIX-human MPL-internal ribosomal entry site (IRES)-green fluorescent protein (GFP) plasmid by the site-directed mutagenesis method using the PfuUltra high-fidelity DNA polymerase (Stratagene; Agilent Technologies). Vesicular stomatitis virus glycoprotein pseudotyped viral particles were produced into 293EBNA cells as previously described.27
Flow cytometry
Cells were labeled with a monoclonal antihemagglutinin (HA) antibody conjugated to phycoerythrin (PE) or an isotype control. Allophycocyanin-Annexin V staining was performed according to the manufacturer’s recommendations (Becton Dickinson). For cell cycle analysis, cells were treated with lysis buffer (sodium acetate with Triton X-100), RNAse, and labeled with propidium iodide for 30 minutes. Cells were analyzed on a LSR2 flow cytometer (Becton Dickinson). Data were analyzed with Flow Jo software.
Confocal microscopy
Cells (100 000) were plated on polylysine slides, fixed with 4% paraformaldehyde for 10 minutes, permeabilized with 0.2% Triton. Primary monoclonal antibodies against GM130 and CALR (Abcam) and HA (Covance; Eurogenec) were incubated in bovine serum albumin 0.2% solution for 1 hour followed by secondary goat anti-mouse (Alexa 546) or anti-rabbit (Alexa 633) antibodies (Life Technologies). 4,6 Diamidino-2-phenylindole was used to label nuclei. Cells were analyzed using a confocal Leica microscope.
Western blotting
Signaling studies were performed on UT7 and Ba/F3 cell lines by western blot analysis of JAK2 (Tyr 1007/1008), STAT1 (Tyr 701), STAT3 (Tyr 705), STAT5 (Tyr 694), extracellular signal-regulated kinase 1/2 (ERK1/2; Thr 202/Tyr 204), and AKT (Thr 308) and of these different pan proteins (Cell Signaling Technology [Ozyme]). heat shock cognate protein HSC70 (Stressgen) or β-actin (Sigma) serves as loading control.
STAT5 reporter assay
γ2A JAK2-deficient cells28 were transfected with complementary DNA (cDNA) coding for MPL or MPL mutants, STAT5, JAK2, Spi-Luc (STAT5 transcriptional reporter), and pRL-TK.29,30 After 5 hours, the cells were stimulated with 10 ng/mL human TPO. Cells were lysed from 24 hours to 48 hours later and the luciferase activity was measured, as described.4
Cell proliferation
Thirty thousand cells per mL were seeded in a 96-well plate with TPO or GM-CSF at various concentrations. Cell proliferation was quantified 3 days later using a WST-1 assay (Takara [Ozyme]).31 Experiments were done in triplicate. Dose-response curves to TPO were expressed as a percentage of viability of the maximal response.
Colony formation assay
Twenty cells per well were sorted in a 96-well plate. For each condition, we filled 1 plate with GM-CSF at 5 ng/mL and 8 plates without cytokine. After 3 days, 5 ng/mL GM-CSF or 10 ng/mL TPO were added to perform rescue experiments in half of the plates grown without cytokine. Colony formation was counted at day 8. Each experiment was repeated 3 times, but the TPO addition was performed in only 1 experiment.
Results
WES and overview of genetic alterations
We studied 17 patients diagnosed as triple-negative ETs namely negative for any JAK2V617F, MPLW515K/L, and CALR exon 9 mutations. We performed WES on paired granulocyte and T-cell samples that were considered as the tumoral and control cells, respectively. The sequencing coverage mean was 110× and 75% of the target sequences could be analyzed with a coverage above 15×. All candidate mutations with a P < .05 generated by the bioinformatics analysis were verified by Sanger sequencing.
Sanger sequencing confirmed 22 missense, 4 nonsense and 2 splice mutations, and 3 frame-shifting indels, leading to a total of 31 mutations of 80 candidates. The mutation rate per sample was low (1.82), even in comparison with other myeloid malignancies (supplemental Table 1, see supplemental Data available on the Blood Web site). Furthermore, CGH arrays performed on 5 samples were normal.
In a first analysis, 3 patients displayed previously described MPL mutations, including 2 in exon 10 (MPLW515R and MPLS505N) and 1 in exon 4 (MPLS204P) (Figure 1B). A homozygous nonsense mutation in the SH2B3 gene was identified in 1 patient. The unexpected identification of known MPL mutations led us to perform deep resequencing of all MPL and JAK2 exons, resulting in the detection of JAK2V617F in 1 patient and again a MPLS204P mutation in another patient (Figure 1; supplemental Table 2). These mutations were not seen in WES because of low allelic burden for the JAK2 mutation (<5%) and low depth of coverage at this position for the MPLS204P.
Genomic analysis. (A) A schematic representation of the signaling mutations in the 17 patients and of the type of hematopoiesis in triple-negative ETs. We consider as polyclonal ETs the patients that exhibit no mutation (driver or passenger) in WES. (B) Sanger sequencing of MPLS204P. Nucleotides and amino acid numbers are indicated.
Genomic analysis. (A) A schematic representation of the signaling mutations in the 17 patients and of the type of hematopoiesis in triple-negative ETs. We consider as polyclonal ETs the patients that exhibit no mutation (driver or passenger) in WES. (B) Sanger sequencing of MPLS204P. Nucleotides and amino acid numbers are indicated.
No signaling mutations were identified in the remaining 11 ET patients (Figure 1A). We divided them into clonal and nonclonal disorders, as performed in several previous studies.32-35 Four of them showed a clonal hematopoiesis in granulocytes based on the presence of nonrecurrent mutations of unknown function. Seven patients had no detectable clonal mutations, suggesting the presence of a polyclonal hematopoiesis in granulocytes. Neither CALR exon 9 mutations nor JAK2V617F could be detected in their platelets (data not shown).
It is noteworthy that the 2 MPLS204P-positive samples were associated with other mutations: 1 displayed 3 other mutations, including another driver mutation ASXL1, and the second presented 5 mutations different from known MPN driver mutations, but with a mutation of the RNA helicase HELZ, which may play a role in oncogenesis (Table 1). The patient identified with an acquired MPLS505N mutation (absent in T cells) also exhibited a mutation in SRSF2 with a higher variant allele frequency (VAF), suggesting that MPLS505N was a secondary event (Table 1). Of interest, this case presented some myelodysplatic features detected by pathology associated with a slight increase in blast count in a bone marrow aspirate. This patient was reclassified as a myelodysplastic syndrome (MDS) with a thrombocytosis rather than as a true ET patient. Finally, there was a case exhibiting both homozygous nonsense SH2B3 and SF3B1 mutations (Table 1) without any noticeable increase in ring sideroblasts or sign of MDS at bone marrow aspiration, thus eliminating a refractory anemia with ring sideroblasts with thrombocytosis.
Mutational profile in granulocytes from 10 ET patients by exome sequencing
| Sample . | Mutations . | |||||
|---|---|---|---|---|---|---|
| 3 | SCNN1G (21.34) | |||||
| 12 | SHOC2 (22.22) | CASC5 (25.0) | ||||
| 14 | MPL S204P (19.26)* | ASXL1 (20.97)* | EDA (41.18) | |||
| 21 | KPNA1 (28.21) | VPS13D (39.29) | EEA1 (39.79) | SF3B1 (54.55)* | SH2B3 (98.33)* | |
| 24 | FUBP1 (23.88) | PPP1R37 (30.77) | COMP (36.14) | ADCY5 (36.67) | HELZ (37.25) | MPL S204P (47.74)* |
| 25 | ATXN7L1 (21.95) | |||||
| 32 | LYZL2 (30.77) | |||||
| 91 | MPL S505N (23.02)* | DDI1 (24.18) | IMPG2 (24.24) | PPP2R5A (43.75) | SRSF2 (44.35)* | APOL4 (86.52) |
| 92 | JAK2 V617F (11.77)* | ALDH3A2 (28.12) | ||||
| 98 | MPL W515R (23.94)* | |||||
| Sample . | Mutations . | |||||
|---|---|---|---|---|---|---|
| 3 | SCNN1G (21.34) | |||||
| 12 | SHOC2 (22.22) | CASC5 (25.0) | ||||
| 14 | MPL S204P (19.26)* | ASXL1 (20.97)* | EDA (41.18) | |||
| 21 | KPNA1 (28.21) | VPS13D (39.29) | EEA1 (39.79) | SF3B1 (54.55)* | SH2B3 (98.33)* | |
| 24 | FUBP1 (23.88) | PPP1R37 (30.77) | COMP (36.14) | ADCY5 (36.67) | HELZ (37.25) | MPL S204P (47.74)* |
| 25 | ATXN7L1 (21.95) | |||||
| 32 | LYZL2 (30.77) | |||||
| 91 | MPL S505N (23.02)* | DDI1 (24.18) | IMPG2 (24.24) | PPP2R5A (43.75) | SRSF2 (44.35)* | APOL4 (86.52) |
| 92 | JAK2 V617F (11.77)* | ALDH3A2 (28.12) | ||||
| 98 | MPL W515R (23.94)* | |||||
The percentage of VAF is indicated in parentheses.
Driver mutation.
Taken together, these data indicated that atypical MPL mutations seem to be recurrent in the so-called triple-negative ET. Next-generation sequencing (NGS) targeted on MPL and on JAK2 was performed on 31 patients including 5 ETs studied in WES and 26 additional less well-characterized ETs. Another mutation of MPL with a VAF of 9.28% was detected. This missense MPL mutation (c.1351T>A) leads to the substitution of a tyrosine by asparagine in the cytosolic domain (Y591N). We also detected a missense mutation in JAK2 (N1108S) with a VAF of 50% in both the granulocytes and the T cells. Sequencing of hair DNA confirmed it was a germ-line mutation (supplemental Figure 1). The c.3323A>G has been reported as a rare polymorphism (0.36%). However, the 3-dimensional modeling of JAK2N1108S predicts that it may provide a slight gain-of-function based on a higher propensity of formation of a tighter hydrogen bond between S1108 and N1085, which is linked to the active state of JAK2 kinase domain (supplemental Figure 2).
Functional analysis of the MPLS204P mutant
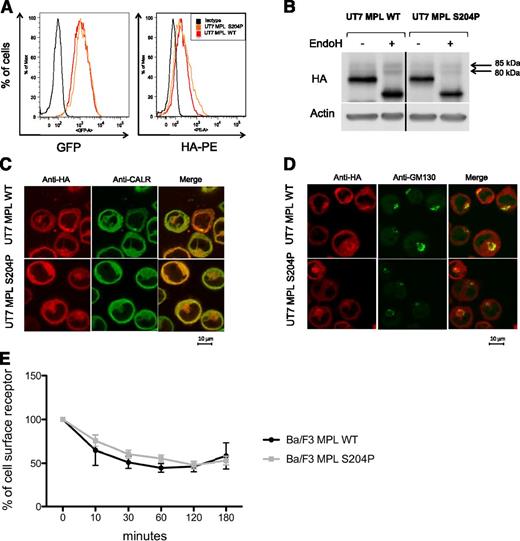
In 1 of the 2 MPLS204P-positive patients, we examined the TPO level that was in the normal range (12 pg/mL; normal, <30 pg/mL) and comparable to other ETs (range, 8.25-96.1 pg/mL). To study cell-surface localization of MPL, we transduced the human factor-dependent UT7 cell line with the HA-tagged MPLWT- and MPLS204P-expressing retrovirus (IRES-GFP). At similar GFP levels, the mean fluorescence intensity of HA labeling was comparable for both receptors using flow cytometry (Figure 2A). Expression of MPLS204P at the cell surface was further confirmed by western blotting. A 85-kDa endoglycosidase H (EndoH)-resistant band corresponding to the mature form of MPL was detected for MPLWT and MPLS204P (Figure 2B). The subcellular localization of the 2 receptors was examined by confocal microscopy. They had a similar localization in the ER (anti-CALR antibody) and Golgi (anti-GM130 antibody) compartments (Figure 2C-D). Finally, we studied the internalization of the receptors after TPO addition using the Ba/F3 cell line. No difference was observed between the MPLWT and MPLS204P (Figure 2E). Taken together, these results suggest that the thrombocytosis was not related to a defect in cell-surface localization of MPLS204P, which would have induced a loss of TPO clearance activity.
MPLS204P does not exhibit cellular trafficking defects. (A) MPL cell-surface expression analysis by flow cytometry. UT7 cells transduced with either the HA-tagged MPLWT or HA-tagged MPLS204P receptor were incubated with an anti-HA antibody coupled with PE. Histograms show equivalent GFP expression (left) monitoring the total MPL levels and HA-PE labeling (right) corresponding to similar cell-surface expression of MPLWT and MPLS204P. (B) Analysis of the different forms of MPL. UT7 MPLWT and MPLS204P cell extracts were treated with EndoH (500 U, overnight at 37°C) and western blot analysis was performed using anti-HA antibody. The different forms of MPL with the EndoH-resistant mature form corresponding to a 85-kDa band which is equivalent in both cell lines are shown. Samples were run on the same gel and vertical lines have been inserted to indicate a repositioned gel lane. (C) Localization of MPL in the ER and (D) in the Golgi apparatus. MPLWT and MPLS204P UT7 cells were analyzed by immunofluorescence using anti-CALR (ER marker), anti-GM130 (Golgi apparatus marker), and anti-HA (MPL detection) antibodies. (E) Internalization of the MPLWT and MPLS204P after TPO binding. These experiments were performed with Ba/F3 cells expressing either MPLWT or MPLS204P. MPL cell-surface expression was studied by flow cytometry after TPO addition. Results are means ± SD of 3 independent experiments. SD, standard deviation.
MPLS204P does not exhibit cellular trafficking defects. (A) MPL cell-surface expression analysis by flow cytometry. UT7 cells transduced with either the HA-tagged MPLWT or HA-tagged MPLS204P receptor were incubated with an anti-HA antibody coupled with PE. Histograms show equivalent GFP expression (left) monitoring the total MPL levels and HA-PE labeling (right) corresponding to similar cell-surface expression of MPLWT and MPLS204P. (B) Analysis of the different forms of MPL. UT7 MPLWT and MPLS204P cell extracts were treated with EndoH (500 U, overnight at 37°C) and western blot analysis was performed using anti-HA antibody. The different forms of MPL with the EndoH-resistant mature form corresponding to a 85-kDa band which is equivalent in both cell lines are shown. Samples were run on the same gel and vertical lines have been inserted to indicate a repositioned gel lane. (C) Localization of MPL in the ER and (D) in the Golgi apparatus. MPLWT and MPLS204P UT7 cells were analyzed by immunofluorescence using anti-CALR (ER marker), anti-GM130 (Golgi apparatus marker), and anti-HA (MPL detection) antibodies. (E) Internalization of the MPLWT and MPLS204P after TPO binding. These experiments were performed with Ba/F3 cells expressing either MPLWT or MPLS204P. MPL cell-surface expression was studied by flow cytometry after TPO addition. Results are means ± SD of 3 independent experiments. SD, standard deviation.
We studied the effects of MPLS204P on cell proliferation. MPLS204P did not induce cytokine-independent growth nor render cells hypersensitive to TPO or GM-CSF hypersensitivity (Figure 3A-B). In addition, we could not find any difference in apoptosis in the presence (Figure 3C) or absence (Figure 3D) of TPO or in cell cycle at different TPO doses (Figure 3E). No constitutive activation in STAT3, STAT5, and AKT was observed in UT7 cells expressing MPLS204P (Figure 4A). These results suggest that MPLS204P is a weak gain-of-function mutant. We further used a more sensitive technique to ask whether the MPLS204P was able to support TPO-independent JAK/STAT signaling like MPLW515K.4 We used a luciferase reporter assay for STAT5 activation induced via MPL in the JAK2-deficient γ2A cells.4 Expression of MPLS204P supported TPO-independent JAK2/STAT5 signaling, albeit to lower levels than MPLW515K (Figure 4B). Of interest, although MPLW515K-induced JAK2/STAT5 activation was evident at 24 to 30 hours after transfection, a longer period was necessary for MPLS204P (>40 hours). This indicates that the MPLS204P mutant signals are weaker than those induced by MPLW515K.
MPLS204P does not present proliferative advantage in bulky cultures. (A-B) Cell proliferation induced by TPO of MPLWT and MPLS204P UT7 cells. Cells were cultured in the presence of various concentrations of TPO (A) or GM-CSF (B) for 72 hours. Viable cells were quantified by WST-1 proliferation assay. Dose-response curves are means ± SEM (n = 3 in triplicate). (C-D) Apoptosis analysis in MPLWT and MPLS204P UT7 cells. (C) Cells were cultured for 2 days with various concentration of TPO (0.01, 0.05, and 10 ng/mL) and the percentage of apoptotic cells (Annexin V positive) was analyzed by flow cytometry using the Annexin V assay. (D) Cells were cultured for 24 hours with TPO (10 ng/mL) followed by overnight deprivation. The percentage of apoptotic cells (Annexin V-positive) was analyzed at different times after TPO removal (1, 2, 3, and 4 days). (E) Cell-cycle analysis of MPLWT and MPLS204P UT7 cells. Cells were cultured for 2 days with various concentrations of TPO (0.01, 0.05, and 10 ng/mL) and the percentage of cells in G1, S, and G2 phases were determined after propidium iodide labeling, by flow cytometry analysis. SEM, standard error of the mean.
MPLS204P does not present proliferative advantage in bulky cultures. (A-B) Cell proliferation induced by TPO of MPLWT and MPLS204P UT7 cells. Cells were cultured in the presence of various concentrations of TPO (A) or GM-CSF (B) for 72 hours. Viable cells were quantified by WST-1 proliferation assay. Dose-response curves are means ± SEM (n = 3 in triplicate). (C-D) Apoptosis analysis in MPLWT and MPLS204P UT7 cells. (C) Cells were cultured for 2 days with various concentration of TPO (0.01, 0.05, and 10 ng/mL) and the percentage of apoptotic cells (Annexin V positive) was analyzed by flow cytometry using the Annexin V assay. (D) Cells were cultured for 24 hours with TPO (10 ng/mL) followed by overnight deprivation. The percentage of apoptotic cells (Annexin V-positive) was analyzed at different times after TPO removal (1, 2, 3, and 4 days). (E) Cell-cycle analysis of MPLWT and MPLS204P UT7 cells. Cells were cultured for 2 days with various concentrations of TPO (0.01, 0.05, and 10 ng/mL) and the percentage of cells in G1, S, and G2 phases were determined after propidium iodide labeling, by flow cytometry analysis. SEM, standard error of the mean.
MPLS204P is a weak gain-of-function mutant. (A) Signaling studies induced by high TPO concentration in MPLWT and MPLS204P UT7 cells. After cytokine deprivation, cells were stimulated with TPO (100 ng/mL for 5 minutes, 30 minutes, or 2 hours). The phosphorylation status of STAT3, STAT5, AKT, and ERK1/2 was examined by western blotting and results were compared with cells stimulated by GM-CSF (5 ng/mL). (B) STAT5 transcriptional activity in γ2A JAK2-deficient cells expressing MPLWT or MPLS204P. Cells were transfected with cDNAs coding for MPLWT or MPLS204P, JAK2, STAT5, and with Firefly STAT5 luciferase reporter spi-Luc and pRL-TK vectors coding for renilla luciferase. Luminescence was measured after 48 hours’ transfection. Shown are average units ± SEM of 1 representative experiment performed in triplicate of 3. (C) Persistent signaling in UT7 MPLS204P. UT7 MPLWT and UT7 MPLS204P were stimulated by TPO (10 ng/mL) for 10 minutes and subsequently washed. A 3-hour time course was performed followed with western blotting, as in previous figures. MPLWT and MPLS204P samples were run on the same gel. P-STAT5/STAT5 or P-ERK/ERK ratios are indicated after quantification by image J software.
MPLS204P is a weak gain-of-function mutant. (A) Signaling studies induced by high TPO concentration in MPLWT and MPLS204P UT7 cells. After cytokine deprivation, cells were stimulated with TPO (100 ng/mL for 5 minutes, 30 minutes, or 2 hours). The phosphorylation status of STAT3, STAT5, AKT, and ERK1/2 was examined by western blotting and results were compared with cells stimulated by GM-CSF (5 ng/mL). (B) STAT5 transcriptional activity in γ2A JAK2-deficient cells expressing MPLWT or MPLS204P. Cells were transfected with cDNAs coding for MPLWT or MPLS204P, JAK2, STAT5, and with Firefly STAT5 luciferase reporter spi-Luc and pRL-TK vectors coding for renilla luciferase. Luminescence was measured after 48 hours’ transfection. Shown are average units ± SEM of 1 representative experiment performed in triplicate of 3. (C) Persistent signaling in UT7 MPLS204P. UT7 MPLWT and UT7 MPLS204P were stimulated by TPO (10 ng/mL) for 10 minutes and subsequently washed. A 3-hour time course was performed followed with western blotting, as in previous figures. MPLWT and MPLS204P samples were run on the same gel. P-STAT5/STAT5 or P-ERK/ERK ratios are indicated after quantification by image J software.
Finally, we tested whether MPLS204P could induce prolonged signaling in UT7 cells. Cells were stimulated with 10 ng/mL TPO, subsequently washed, and then cultured without cytokine for 3 hours. A sustained phosphorylation of STAT5 and AKT was observed for the mutant cells (Figure 4C). To demonstrate that this weak constitutive activity exerts some biological consequence, a limiting dilution of MPLWT and MPLS204P UT7 cells (20 cells per well in a 96-well plate) were grown without cytokine for 1 week (Figure 5A). Autonomous growth numbered as colonies was observed with UT7 MPLS204P (5%-10%), but not with UT7 MPLWT, except in rare wells, suggesting that around 0.25% of UT7 cells became cytokine-independent due to MPLS204P. Furthermore, after 3 days of culture without cytokines, we added GM-CSF or TPO for rescue, and observed a twofold to fourfold increased cloning efficiency for UT7 MPLS204P compared with MPLWT (Figure 5B-C).
UT7 MPL S204P cells are less cytokine-dependent than UT7 MPL WT. (A) Growth of UT7 MPLS204P and UT7 MPLWT at limiting dilution without cytokines. Cells (20 per well) were cultured without cytokines in a 96-well plate. Colony growth was counted 1 week later. (B) Rescue experiments by GM-CSF. Cells (20 per well) were cultured without cytokines in a 96-well plate. After 3 days, GM-CSF (5 ng/mL) was added and colonies were counted 1 week later. (C) Rescue experiments by TPO. Cells (20 per well) were cultured without cytokines in a 96-well plate. After 3 days, TPO (10 ng/mL) was added and colonies were counted 1 week later. Results represent the means ± SD of the number of colonies/96-well plate. Each point corresponds to the number of clone in 1 plate.
UT7 MPL S204P cells are less cytokine-dependent than UT7 MPL WT. (A) Growth of UT7 MPLS204P and UT7 MPLWT at limiting dilution without cytokines. Cells (20 per well) were cultured without cytokines in a 96-well plate. Colony growth was counted 1 week later. (B) Rescue experiments by GM-CSF. Cells (20 per well) were cultured without cytokines in a 96-well plate. After 3 days, GM-CSF (5 ng/mL) was added and colonies were counted 1 week later. (C) Rescue experiments by TPO. Cells (20 per well) were cultured without cytokines in a 96-well plate. After 3 days, TPO (10 ng/mL) was added and colonies were counted 1 week later. Results represent the means ± SD of the number of colonies/96-well plate. Each point corresponds to the number of clone in 1 plate.
Altogether, these results demonstrate that MPLS204P is a gain-of-function mutant.
Functional analysis of the MPLY591N mutant
We conducted the functional analysis of another MPL mutant identified by NGS using Ba/F3 cells lines. Flow cytometry analysis showed that MPLY591N was addressed more efficiently (1.63- ± 0.07-fold) to the cell-surface membrane compared with MPLWT at similar GFP levels (Figure 6A), indicating either an increased trafficking of MPL mutant to cell surface or a decreased internalization. We measured the impact of this mutant on cellular proliferation in response to TPO. MPLY591N conferred a fourfold to fivefold hypersensitivity to TPO compared with MPLWT (50% inhibitory concentration of 0.10 ng/mL for WT and 0.02 ng/mL for Y591N), but without spontaneous growth in the absence of cytokine (Figure 6B).
Analysis of the MPLY591N mutant. (A) MPL cell-surface localization in Ba/F3 cells. Ba/F3 cells expressing the bicistronic retroviral pMEGIX-IRES-GFP vector encoding HA-tagged MPLWT or MPLY591N and GFP were maintained in IL3-supplemented medium. Flow cytometry analysis was assessed and histograms show equivalent GFP expression (left) monitoring the total MPL levels and HA-PE labeling (right) corresponding to cell-surface expression of MPLWT and MPLY591N. (B) Sensitivity to TPO of Ba/F3 cells. Ba/F3-MPLWT or MPLY591N cells were cultured for 72 hours either in absence of cytokine (black arrow, x-axis) or in presence of increasing doses of TPO (0.01, 0.1, 1, and 10 ng/mL). Viable cells were quantified by WST-1 proliferation assay. Dose-response curves are means expressed in percentages of maximum growth value ± SEM (n = 3 in triplicate). Two-tailed t test; *< .05; **P < .01. (C) Signaling studies in Ba/F3-MPL cells. Ba/F3-MPLWT or MPLY591N cells were serum- and cytokine-starved for 6 hours prior to a 15-minute stimulation without or with TPO (0.01, 0.1, 1, and 10 ng/mL) as indicated. Cells were lysed and the phosphorylation status of STAT1, STAT3, STAT5, AKT, and ERK1/2 was examined by western blotting with the respective antiphospho-specific antibodies, as indicated. MPL expression was also verified. Expression of β-actin in the samples was used as loading control and was consistent with expression of total AKT, ERK1/2, and the individual STAT isoforms. Blots shown were reproduced in 3 independent experiments.
Analysis of the MPLY591N mutant. (A) MPL cell-surface localization in Ba/F3 cells. Ba/F3 cells expressing the bicistronic retroviral pMEGIX-IRES-GFP vector encoding HA-tagged MPLWT or MPLY591N and GFP were maintained in IL3-supplemented medium. Flow cytometry analysis was assessed and histograms show equivalent GFP expression (left) monitoring the total MPL levels and HA-PE labeling (right) corresponding to cell-surface expression of MPLWT and MPLY591N. (B) Sensitivity to TPO of Ba/F3 cells. Ba/F3-MPLWT or MPLY591N cells were cultured for 72 hours either in absence of cytokine (black arrow, x-axis) or in presence of increasing doses of TPO (0.01, 0.1, 1, and 10 ng/mL). Viable cells were quantified by WST-1 proliferation assay. Dose-response curves are means expressed in percentages of maximum growth value ± SEM (n = 3 in triplicate). Two-tailed t test; *< .05; **P < .01. (C) Signaling studies in Ba/F3-MPL cells. Ba/F3-MPLWT or MPLY591N cells were serum- and cytokine-starved for 6 hours prior to a 15-minute stimulation without or with TPO (0.01, 0.1, 1, and 10 ng/mL) as indicated. Cells were lysed and the phosphorylation status of STAT1, STAT3, STAT5, AKT, and ERK1/2 was examined by western blotting with the respective antiphospho-specific antibodies, as indicated. MPL expression was also verified. Expression of β-actin in the samples was used as loading control and was consistent with expression of total AKT, ERK1/2, and the individual STAT isoforms. Blots shown were reproduced in 3 independent experiments.
We next verified the effect of the MPLY591N mutant on signaling in Ba/F3 cells by western blot analysis. Noticeably, these cells expressed similar levels of exogenous MPL. Interestingly, a constitutive phosphorylation of STAT3, STAT5, and ERK was observed in MPLY591N mutant cells compared with MPLWT cells. The TPO dose-dependent increase in the phosphorylation of these molecules was higher in presence of MPLY591N than MPLWT. No difference was observed on AKT or STAT1 phosphorylation (Figure 6C).
Altogether, these results show that MPLY591N is also a weak gain-of-function mutation that leads to TPO hypersensitivity without independent growth and to a partial spontaneous activation of signaling pathways.
Discussion
The goal of this work was to characterize the mutational genomic profile of triple-negative ETs using WES. Among the 17 triple-negative ETs, we considered that 7 had a polyclonal hematopoiesis in granulocytes as no mutation was detected by WES. This molecular approach has clear limitation because it cannot detect mutations in noncoding sequences and gene fusion and even more cannot detect mutations in coding sequences at low VAF. In this work, we defined clonal hematopoiesis as the presence of any somatic mutation either in a known driver of hematopoietic malignancies or in another gene (passenger mutation),32-35 but without using X chromosome inactivation assays except in 2 cases where results were concordant. It has been calculated that each normal hematopoietic stem cell accumulates 0.13 mutation per year in coding sequences.36 Thus, it was expected that in the case of clonal hematopoiesis in patients older than 50 years of age, several mutations would be found. However, the technique has a sensitivity of around 10% for VAF. Thus, it seems likely that these “polyclonal” triple-negative thrombocytosis cases can be MPNs with a low VAF in granulocytes similar to JAK2V617F ET in which VAF is much higher in platelet RNA than in granulocyte DNA.37 Therefore, it will be important in the future to directly perform WES or whole-genome sequencing on megakaryocytes or to sequence platelet RNA. Alternatively, some of these disorders can be other diseases than MPNs, more particularly hereditary thrombocytosis. In the companion article, germ-line gain-of-function mutations in JAK2 and MPL were found in triple-negative ETs.38 Hereditary thrombocytosis may arise without other familial cases if the patient is the founder. In 1 case, we detected a germ-line mutation in JAK2 (N1108S) that appears in in silico modeling as a possible gain-of-function mutation, suggesting a hereditary thrombocytosis. However, this mutation has also been described as a rare polymorphism. Thus, we cannot exclude that rare JAK2 or MPL variants, which may be present in the general population, can induce a MPN disease in certain individuals.
The enrichment in previously described rare signaling mutations9 was striking in triple-negative ETs. One was a homozygous nonsense mutation in SH2B3. Heterozygous SH2B3 mutations were reported in MPNs and it is discussed whether by themselves they could give rise to a MPN.14 In some cases, they are associated with JAK2V617F.39 Here, the mutation is homozygous and because mice totally deficient in Sh2b3 develop a myeloproliferative disorder,40 we can suggest that this homozygous mutation leading to an absence of LNK was really the driver of the MPN. Interestingly, a SF3B1 mutation was associated with this homozygous SH2B3 mutation, but the phenotype of the disease was an ET.
Four mutations in MPL were found in the first series of patients, including MPLS505N and MPLW515R located in exon 10 that are not commonly screened. The latter were somatic mutations in our study although they can also be identified as germ-line mutations in hereditary thrombocytosis.6,41
In 2 other patients, we detected an acquired MPLS204P mutation. This mutation has been previously described in a myelofibrosis, and a S204F mutation was described in another myelofibrosis case.10,12 In the companion article, S204P and S204F mutations were detected in 2 triple-negative patients.38 It is interesting to note that mouse MPL residue 204 is a proline instead of a serine in human MPL, thus the S204P mutation restores the mouse MPL sequence into the human MPL. Moreover, the germ-line mutation K39N (MPL Baltimore) present in a heterozygous form in 7% of an African-American population also restores a mouse sequence and is associated with a thrombocytosis.42 It is tempting to assume that differences between the human and mouse MPL sequences may contribute to the high platelet count in mice associated with an elevated plasma TPO level in comparison with the situation in humans. In the case of the K39N, there is evidence that the membrane expression level of the MPL mutant is lower than MPLWT and this can explain a thrombocytosis phenotype due to decreased TPO clearance activity of MPL, which is in great part related to the expression level of MPL on the surface of mature megakaryocytes and platelets.42 This has been well demonstrated in 3 murine models.43-45 Here, we clearly demonstrate that the thrombocytosis induced by MPLS204P is not due to a defect in cell trafficking of the receptor. In contrast, MPLS204P is a weak gain-of-function mutant in signaling, inducing constitutive STAT activation using sensitive approaches and an increased and more prolonged ligand-induced STAT phosphorylation than MPLWT. Using limiting dilution techniques, it was possible to demonstrate that MPLS204P could induce cytokine-independent growth with low efficiency in around 1 of 400 UT7 cells. Furthermore, UT7 MPLS204P cells were more resistant than UT7 MPLWT cells to 3-day cytokine deprivation. The 2 cases with MPLS204P were associated with several other mutations: 1 with ASXL1, the second with a mutation in an RNA helicase implicated in proliferation. Thus, we suspect that these mutants with very low oncogenic potential would need to be associated to other drivers to give rise to a MPN.
Finally, we detected a MPLY591N mutant in 1 patient. MPLY591D has been previously described in a polycythemia vera patient in association with a heterozygous JAK2V617F mutation10 as reported in the companion article. Here, we detected the MPLY591N in the absence of a JAK2 mutation. This mutation was assumed to be a gain-of-function for 2 reasons. Previously, it was shown that this tyrosine also called 78 (intracellular tyrosine) is phosphorylated on the MPLW515A and that the double-mutant MPLW515A/Y591F induced a much more severe MPN in the mouse than the single MPLW515A mutant.46 Furthermore, this tyrosine is also phosphorylated after TPO binding, and negatively regulates MPL signaling by promoting its internalization degradation as well as ERK signaling.46-48 Asparagine is a polar residue in contrast to the negative charge represented by tyrosine phosphorylation and, thus, the Y591N mutation resembles a Y591 loss-of-function, like Y591F, but possibly weaker because unlike phenylalanine, asparagine is polar. When the Y591N mutant was expressed in Ba/F3 cells it induced a slight TPO hypersensitivity, which was associated with constitutive activation of STAT3 and ERK. In the MPLY591N-positive patient, we did not perform WES to assess whether other driver mutations were present, as for MPLS204P. However, this mutant was detected in a case of ET occurring in a familial form of MPN where we have recently identified the predisposition locus consisting of a 700-kb duplication involving 6 genes. The overexpression of 2 of them, ATG2B and GSKIP, induces TPO hypersensitivity and cooperates with JAK2, MPL, and CALR mutations to give an ET.25 Thus, the predisposition locus could be the initiating event and the low gain-of-function MPLY591N could be sufficient to induce an overt ET.
Altogether, this study has shown that in a fraction of the so-called triple-negative ETs a significant proportion of patients have mutations in signaling molecules, more particularly in MPL. This group of patients appears heterogeneous including some with a polyclonal hematopoiesis as previously shown in pediatric ETs.49 Further studies using WES and RNA sequencing will be required to better understand the pathogenesis of these ETs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank E. Leroy for modeling JAK2N1108S and the bioinformatic platform of Gustave Roussy for analyzing the NGS experiments. The authors are grateful to P. Rameau for cell sorting experiments.
Support to S.N.C. is acknowledged from Ludwig Institute, Salus Sanguinis, Fondation Contre le Cancer, Fonds National de la Recherche Scientifique, Inter-University Attraction Pole and Action de Recherche Concertée Projects, Belgium. This work was supported by grants from the Agence Nationale pour la Recherche (Thrombocytosis [W.V.]), the Ligue Nationale contre le Cancer (Équipe Labellisée), the Fondation Laurette Fugain (W.V., I.P.), Agence Nationale pour la Recherche-Jeune Chercheur (I.P.), a specific grant from Gustave Roussy (Taxe d’Apprentissage) and from the INSERM. I.C. was supported by a Research Training in Industry and Agriculture fellowship. X.C. was supported by the University Paris-Diderot, F.F. by the INSERM (Poste d’Accueil), and F.P. by the Association pour la Recherche Médicale.
Authorship
Contribution: W.V. and I.P. designed and performed research, analyzed data, prepared figures, and wrote the paper; X.C. performed and interpreted pangenomic analysis, prepared figures, and wrote the paper; F.F. and F.P. performed functional analysis, prepared figures, and wrote the paper; K.M. performed functional analysis and prepared figures; J.P.D. and I.C. performed luciferase assays; J.C.I., V.U., and N.C. were involved in the clinical part; R.F. performed the TPO ELISA assays; C.M. performed the CALR sizing and provided genotyped samples; J.P.L.C. and N. Droin. performed the WES; M.K.D. did bioinformatics study of exome data; O.B. collected samples, performed the targeted NGS approach, and prepared figures; N. Debili., H.R., and S.N.C. gave experimental/intellectual input and corrected the manuscript; C.B.-C. provided samples, interpreted the NGS, and gave experimental/intellectual input; and all authors contributed to editing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: William Vainchenker, UMR U1170, Gustave Roussy, 114 rue Edouard Vaillant, 94805 Villejuif, France; e-mail: verpre@igr.fr.
References
Author notes
X.C., F.F., and F.P. contributed equally.
I.P. and W.V. contributed equally.