Key Points
Thrombocytopenia on intensive care unit admission is independently associated with increased mortality in patients with sepsis.
Thrombocytopenia is associated with a more disturbed host response in critically ill patients with sepsis independent of disease severity.
Abstract
Preclinical studies have suggested that platelets influence the host response during sepsis. We sought to assess the association of admission thrombocytopenia with the presentation, outcome, and host response in patients with sepsis. Nine hundred thirty-one consecutive sepsis patients were stratified according to platelet counts (very low <50 × 109/L, intermediate-low 50 × 109 to 99 × 109/L, low 100 × 109 to 149 × 109/L, or normal 150 × 109 to 399 × 109/L) on admission to the intensive care unit. Sepsis patients with platelet counts <50 × 109/L and 50 × 109 to 99 × 109/L presented with higher Acute Physiology and Chronic Health Evaluation scores and more shock. Both levels of thrombocytopenia were independently associated with increased 30-day mortality (hazard ratios with 95% confidence intervals 2.00 [1.32-3.05] and 1.72 [1.22-2.44], respectively). To account for baseline differences besides platelet counts, propensity matching was performed, after which the association between thrombocytopenia and the host response was tested, as evaluated by measuring 17 plasma biomarkers indicative of activation and/or dysregulation of pathways implicated in sepsis pathogenesis and by whole genome blood leukocyte expression profiling. In the propensity matched cohort, platelet counts < 50 × 109/L were associated with increased cytokine levels and enhanced endothelial cell activation. All thrombocytopenic groups showed evidence of impaired vascular integrity, whereas coagulation activation was similar between groups. Blood microarray analysis revealed a distinct gene expression pattern in sepsis patients with <50 × 109/L platelets, showing reduced signaling in leukocyte adhesion and diapedesis and increased complement signaling. These data show that admission thrombocytopenia is associated with enhanced mortality and a more disturbed host response during sepsis independent of disease severity, thereby providing clinical validity to animal studies on the role of platelets in severe infection.
Introduction
Thrombocytopenia is a common finding in critically ill patients with an incidence ranging from 20% to 50%.1-3 A low platelet count has been associated with mortality in patients admitted to the intensive care unit (ICU).1-5 Current knowledge of the relation between platelet counts and ICU outcome is mainly based on heterogeneous populations of critically ill medical, surgical, and trauma patients. Multiple risk factors for the development of thrombocytopenia have been identified in ICU patients, among which sepsis prominently features.1,2,4 A study focusing on critically ill patients with sepsis also reported an association between thrombocytopenia and mortality6 ; however, this relation was not confirmed in a larger investigation.7
Although the role of platelets in blood clotting has been recognized for a long time, in more recent years multiple biological functions apart from hemostasis have been attributed to platelets.8-10 For example, platelets have been shown to limit bacterial growth and dissemination in experimental sepsis11-14 ; to influence leukocyte recruitment and functions,11,15-17 cytokine responses,13,18-20 and to influence activation of the vascular endothelium21 and the coagulation system.22 Moreover, platelets aid in maintaining vascular integrity, especially in the context of a strong proinflammatory environment.13,23,24 Our group recently demonstrated that thrombocytopenia results in an exaggerated host response to pneumonia-derived sepsis in mice, as reflected by enhanced activation of the cytokine network, the vascular endothelium, and the coagulation system.13,14
Although preclinical evidence indicating that platelets can influence key host responses to sepsis is abundant, clinical studies addressing this pathophysiological link are limited. We here sought to assess the association between ICU admission platelet counts and the presentation and outcome of sepsis patients. Moreover, we determined the association between ICU admission thrombocytopenia and the host response in critically ill patients with sepsis. For this, we measured 17 plasma biomarkers indicative of activation of the cytokine network, the vascular endothelium, and the coagulation system, and in an unbiased approach analyzed whole-blood leukocyte transcriptomes in sepsis patients stratified by platelet counts.
Patients and methods
Study design, setting, patient identification
This study was conducted as part of the Molecular Diagnosis and Risk Stratification of Sepsis (MARS) project, a prospective observational study in the mixed ICUs of 2 tertiary teaching hospitals (Academic Medical Center in Amsterdam and University Medical Center Utrecht in Utrecht).25,26 All consecutive patients above 18 years of age admitted between January 2011 and July 2013 with an expected length of stay longer than 24 hours were included via an opt-out method approved by the medical ethical committees of the participating hospitals. For every admitted patient, the plausibility of an infection was assessed using a 4-point scale (ascending from none, possible, probable, to definite) using Center for Disease Control and Prevention27 and International Sepsis Forum consensus definitions,28 making use of all clinical, radiological, and microbiological data, as described in detail.25 Sepsis was defined as the presence of infection diagnosed within 24 hours after ICU admission with a probable or definite likelihood, accompanied by at least one additional parameter as described in the 2001 International Sepsis Definitions Conference.29 Dedicated research physicians prospectively collected demographic, comorbidity (including the Charlson comorbidity index30 ; for definitions see supplemental Material, available on the Blood Web site), and daily clinical (including microbiology) and interventional data. Severity indices included Acute Physiology and Chronic Health Evaluation (APACHE) IV31 and modified Sequential Organ Failure Assessment (SOFA) scores. The contribution of coagulation (platelet counts) and central nervous system (not reliable due to sedation on ICU) was excluded. Specific organ failures were defined as a modified SOFA score of 3 or greater, except for cardiovascular failure for which a score of 1 or more was used.32 Shock was defined by the use of vasopressors (noradrenaline) for hypotension in a dose of >0.1 µg/kg/min during at least 50% of the ICU day. Acute kidney injury and acute lung injury were assessed using strict preset criteria.33,34 ICU-acquired complications were defined as events that started 2 days or more after ICU admission. The following admissions were excluded: transfers from other ICUs (except when transferred on the day of ICU admission), ICU readmissions within the same hospital admission or within 30 days after the first hospital admission, and patients with a hematologic malignancy, liver cirrhosis, splenectomy, thrombocytosis (≥400 × 109/L), or unknown platelet counts in the first 24 hours after ICU admission.
The lowest platelet count within the first 24 hours after ICU admission was used to stratify patients in groups with very low platelet counts (<50 × 109/L), intermediate-low platelet counts (50× 109 to 99 × 109/L), low platelet counts (100 × 109 to 149 × 109/L), or normal platelet counts (150× 109 to 399 × 109/L); boundaries were based on previous studies.3,6,35 In a sensitivity analysis, patients were stratified in groups based on platelet count quartiles.
Plasma protein assays
On admission, plasma was stored within 4 hours after blood draw at −80°C. Measurements were done in EDTA anticoagulated plasma. Tumor necrosis factor-α, interleukin-6 (IL-6), IL-8, IL-1β, IL-10, IL-13, interferon-γ, fractalkine, soluble intercellular adhesion molecule-1 (ICAM-1), and soluble E-selectin were measured by FlexSet cytometric bead array (BD Biosciences, San Jose, CA) using FACS Calibur (Becton Dickinson, Franklin Lakes, NJ). Angiopoietin-1, angiopoietin-2, protein C, antithrombin (all R&D Systems, Abingdon, UK), and D-dimer (Procartaplex; eBioscience, San Diego, CA) were measured by Luminex multiplex assay using BioPlex 200 (BioRad, Hercules, CA). Prothrombin time (PT) and activated partial thromboplastin time (aPTT) were determined by using a photometric method with Dade Innovin Reagent or by Dade Actin FS Activated PTT Reagent, respectively (both Siemens Healthcare Diagnostics). Normal values were generated from plasma from 27 age- and gender-matched healthy volunteers (included after written informed consent), except for PT and aPTT (reference values of routine laboratory).
Blood gene expression microarrays
Whole blood was collected in PAXgene tubes (Becton-Dickinson, Breda, The Netherlands) within 24 hours after ICU admission. Total RNA was isolated using the PAXgene blood mRNA kit (Qiagen, Venlo, The Netherlands) in combination with the QIAcube automated system (Qiagen), according to the manufacturer’s instructions. RNA (RNA integrity number > 6.0) was processed and hybridized to the Affymetrix Human Genome U219 96-array and scanned by using the GeneTitan instrument at the Cologne Center for Genomics, Cologne, Germany, as described by the manufacturer (Affymetrix). See supplemental data file for detailed methods used in microarray data preprocessing, differential gene expression analysis, bioinformatics, and biological pathway analysis. Benjamini-Hochberg (BH) adjusted P values defined significant differential gene expression and pathway enrichment.
Statistical analysis
All data were analyzed using R studio built under R version 3.2.2 (R Core Team 2013, Vienna, Austria).36 Data distribution was assessed by Shapiro-Wilk tests and histogram plots. Categorical variables are presented as absolute numbers (percentages); parametric quantitative variables are presented as means ± standard deviation (SD), and nonparametric quantitative variables are presented as median and interquartile ranges (IQR; 25th and 75th percentiles). A Mann-Whitney U test or a Kruskal-Wallis test was used to analyze continuous nonparametric data, whereas continuous parametric data were analyzed using a Student t test or analysis of variance where appropriate. Post-hoc testing of nonparametric data was performed using a Dunn's test of multiple comparisons using rank sums and by using Tukey post-hoc testing for parametric data. Categorical data were analyzed by χ2 tests. P < .05 was considered to represent a statistically significant difference. A multivariable Cox proportional hazard model was used to determine the association between differences in admission platelet counts and mortality by day 30. The covariables included in the model, based on baseline differences, were patient age, gender, APACHE IV acute physiology score (APS), shock on admission, and the baseline comorbidities: cardiovascular insufficiency, hypertension, malignancy, and peripheral vascular disease. R package survival was used for this analysis.
Considering that the release of host response biomarkers in sepsis often is proportional to disease severity,37,38 we used propensity score matching to create groups with distinct platelet counts yet with comparable disease severity on ICU admission to determine the association between thrombocytopenia and the host response. For this, we used a logistic regression implemented in the R library MatchIt (version 2.4-21), including variables associated with disease severity and baseline variables that were different between groups or could be of influence on our parameter of interest or outcome. The propensity score included variables also used in the multivariable Cox proportional hazard model (see above). In order to enable inclusion of as many patients with severe thrombocytopenia as possible in the propensity matched analyses, first subjects in the very low platelet count group were matched 1:6 to patients in the normal platelet count group using nearest matching with a caliper of 0.20 SD of the normally distributed propensity score. In a next run, the matched patients of the normal platelet count group were matched to the intermediate-low platelet count group in the same way, using a caliper of 0.20 SD of the normally distributed propensity score, this time using 1:1 matching. Last, the remaining matched patients in the normal group were identified and used to 1:1 match to the low platelet count group, also using a caliper of 0.20 SD. This approach was taken to be able to compare all patients in each group to the patients in the other groups. In a sensitivity analysis (groups based on quartiles), propensity score matching was performed with similar methods, but using baseline variables age, gender, APACHE IV APS, shock on admission, malignancy, and chronic obstructive pulmonary disease, as these were significantly different between groups.
Results
Patient characteristics
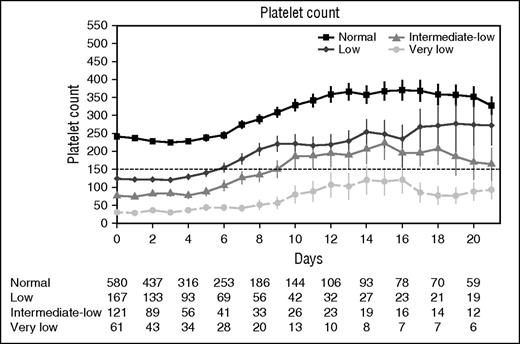
The 2.5-year study period encompassed 1483 patients admitted to the ICU with sepsis. In total, 552 patients were excluded because they involved readmissions (n = 174), transfers from other ICUs (n = 129), hematologic malignancies (n = 92), liver cirrhosis (n = 23), splenectomy (n = 8), or thrombocytosis (n = 96), or because platelet counts within the first 24 hours of ICU admission were not available (n = 30). Of the remaining 931 admissions for sepsis, 61 (6.6%) presented platelet counts of <50 × 109/L, 121 (13.0%) of 50 × 109 to 99 × 109/L, 167 (17.9%) of 100 × 109 to 149 × 109/L, and 580 (62.3%) in the normal range (150 × 109 to 399 × 109/L). Platelet counts remained relatively stable during the first 4-6 days after ICU admission across all groups (Figure 1). Thereafter, platelet counts increased, although patients with very low platelet counts remained thrombocytopenic for prolonged periods of time while in the ICU.
Platelet count over time stratified according to platelet counts on ICU admission. Data are mean and standard error of the mean. Numbers below x-axis indicate number of patients still present on the ICU for each group.
Platelet count over time stratified according to platelet counts on ICU admission. Data are mean and standard error of the mean. Numbers below x-axis indicate number of patients still present on the ICU for each group.
Patients with very low platelet counts were younger than patients with low (100 × 109 to 149 × 109/L) or normal platelet counts (150 × 109 to 399 × 109/L), had fewer comorbidities, and were more often admitted for medical reasons (Table 1). The primary source of infection was largely similar between groups with a few exceptions: patients with normal platelet counts were more often admitted with pulmonary sepsis and less often with urinary sepsis. Patients with very low or intermediate-low platelet counts were more severely ill on ICU admission, as reflected by higher APACHE IV scores, higher APACHE IV APS, higher modified SOFA scores, and more shock (Table 1).
Baseline characteristics and disease severity of sepsis patients stratified according to platelet counts on ICU admission
| . | Very low (<50 × 109/L) . | Intermediate-low (50 × 109 to 99 × 109/L) . | Low (100 × 109 to 149 × 109/L) . | Normal (150 × 109 to 399 × 109/L) . | P . |
|---|---|---|---|---|---|
| Patients, n (%) | 61 (6.6%) | 121 (13.0%) | 167 (17.9%) | 580 (62.3%) | |
| Demographics | |||||
| Age (years), mean (SD) | 56.4 (14.6)*† | 58.5 (16.4)* | 62.2 (13.9) | 62.6 (14.4) | .002 |
| Gender male, n (%) | 34 (55.7%) | 66 (54.5%) | 112 (67.1%) | 349 (60.2%) | .14 |
| White race, n (%) | 52 (85.2%) | 105 (86.8%) | 147 (88%) | 509 (87.8%) | .41 |
| Chronic comorbidity | |||||
| Modified Charlson comorbidity index,§ median (IQR) | 0 (0-1)*† | 1 (0-2)† | 1 (0-3) | 1 (0-2) | .0002 |
| Cardiovascular insufficiency, n (%) | 9 (14.8%)† | 27 (22.3%) | 55 (32.9%) | 146 (25.2%) | .03 |
| Chronic obstructive pulmonary disease, n (%) | 5 (8.2%) | 11 (9.1%) | 24 (14.4%) | 100 (17.2%) | .05 |
| Diabetes mellitus, n (%) | 7 (11.5%) | 21 (17.4%) | 38 (22.8%) | 121 (20.9%) | .22 |
| Hypertension, n (%) | 9 (14.8%)*† | 35 (28.9%) | 59 (35.3%) | 191 (32.9%) | .02 |
| Malignancy, n (%) | 6 (9.8%) | 16 (13.2%) | 31 (18.6%) | 113 (19.5%) | .13 |
| Peripheral vascular disease, n (%) | 6 (9.8%) | 17 (14.0%) | 34 (20.4%)* | 69 (11.9%) | .03 |
| Renal insufficiency, n (%) | 11 (18.0%) | 22 (18.2%) | 27 (16.2%) | 86 (14.8%) | .76 |
| Use of antiplatelet drugs¶, n (%) | 10 (16.4%) | 31 (25.6%) | 49 (29.3%) | 172 (29.7%) | .27 |
| Medical admission type, n (%) | 53 (86.9%)*†‡ | 83 (68.6%) | 113 (67.7%) | 425 (73.3%) | .02 |
| Primary source of infection, n (%) | |||||
| Respiratory tract | 22 (36.1%)* | 37 (30.6%)* | 63 (37.7%)* | 305 (52.6%) | .001 |
| Abdomen | 16 (26.2%) | 35 (28.9%) | 37 (22.2%) | 116 (20.0%) | .16 |
| Cardiovascular | 8 (13.1%) | 19 (15.7%) | 29 (17.4%) | 80 (13.8%) | .63 |
| Urinary tract | 10 (16.4%) | 23 (19.0%)* | 27 (16.2%) | 57 (9.8%) | .01 |
| Skin | 5 (8.2%) | 7 (5.8%) | 11 (6.6%) | 22 (3.8%) | .23 |
| Severity of disease | |||||
| APACHE IV score, median (IQR) | 104 (84-131)*†‡ | 85 (69-112)† | 77 (63-99) | 76 (60-94) | <.0001 |
| APACHE IV APS, median (IQR) | 89 (73-119)*†‡ | 75 (55-94)*† | 65 (50-85) | 64 (48-79) | <.0001 |
| Modified SOFA‖, median (IQR) | 10 (7-12)*† | 8 (6-10)*† | 7 (5-9)* | 7 (4-8) | <.0001 |
| Septic shock, n (%) | 33 (54.1%)*† | 60 (49.6%)*† | 62 (37.1%)* | 165 (28.4%) | .001 |
| Mechanical ventilation, n (%) | 44 (72.1%) | 85 (70.2%) | 115 (68.9%) | 406 (70%) | .98 |
| Organ failure, n (%) | 53 (86.9%) | 100 (82.6%) | 143 (85.6%) | 479 (82.6%) | .20 |
| Acute kidney injury, n (%) | 34 (55.7%)* | 61 (50.4%)* | 75 (44.9%)* | 177 (30.5%) | .001 |
| Acute lung injury, n (%) | 22 (36.1%) | 36 (29.8%) | 38 (22.8%) | 132 (22.8%) | .07 |
| . | Very low (<50 × 109/L) . | Intermediate-low (50 × 109 to 99 × 109/L) . | Low (100 × 109 to 149 × 109/L) . | Normal (150 × 109 to 399 × 109/L) . | P . |
|---|---|---|---|---|---|
| Patients, n (%) | 61 (6.6%) | 121 (13.0%) | 167 (17.9%) | 580 (62.3%) | |
| Demographics | |||||
| Age (years), mean (SD) | 56.4 (14.6)*† | 58.5 (16.4)* | 62.2 (13.9) | 62.6 (14.4) | .002 |
| Gender male, n (%) | 34 (55.7%) | 66 (54.5%) | 112 (67.1%) | 349 (60.2%) | .14 |
| White race, n (%) | 52 (85.2%) | 105 (86.8%) | 147 (88%) | 509 (87.8%) | .41 |
| Chronic comorbidity | |||||
| Modified Charlson comorbidity index,§ median (IQR) | 0 (0-1)*† | 1 (0-2)† | 1 (0-3) | 1 (0-2) | .0002 |
| Cardiovascular insufficiency, n (%) | 9 (14.8%)† | 27 (22.3%) | 55 (32.9%) | 146 (25.2%) | .03 |
| Chronic obstructive pulmonary disease, n (%) | 5 (8.2%) | 11 (9.1%) | 24 (14.4%) | 100 (17.2%) | .05 |
| Diabetes mellitus, n (%) | 7 (11.5%) | 21 (17.4%) | 38 (22.8%) | 121 (20.9%) | .22 |
| Hypertension, n (%) | 9 (14.8%)*† | 35 (28.9%) | 59 (35.3%) | 191 (32.9%) | .02 |
| Malignancy, n (%) | 6 (9.8%) | 16 (13.2%) | 31 (18.6%) | 113 (19.5%) | .13 |
| Peripheral vascular disease, n (%) | 6 (9.8%) | 17 (14.0%) | 34 (20.4%)* | 69 (11.9%) | .03 |
| Renal insufficiency, n (%) | 11 (18.0%) | 22 (18.2%) | 27 (16.2%) | 86 (14.8%) | .76 |
| Use of antiplatelet drugs¶, n (%) | 10 (16.4%) | 31 (25.6%) | 49 (29.3%) | 172 (29.7%) | .27 |
| Medical admission type, n (%) | 53 (86.9%)*†‡ | 83 (68.6%) | 113 (67.7%) | 425 (73.3%) | .02 |
| Primary source of infection, n (%) | |||||
| Respiratory tract | 22 (36.1%)* | 37 (30.6%)* | 63 (37.7%)* | 305 (52.6%) | .001 |
| Abdomen | 16 (26.2%) | 35 (28.9%) | 37 (22.2%) | 116 (20.0%) | .16 |
| Cardiovascular | 8 (13.1%) | 19 (15.7%) | 29 (17.4%) | 80 (13.8%) | .63 |
| Urinary tract | 10 (16.4%) | 23 (19.0%)* | 27 (16.2%) | 57 (9.8%) | .01 |
| Skin | 5 (8.2%) | 7 (5.8%) | 11 (6.6%) | 22 (3.8%) | .23 |
| Severity of disease | |||||
| APACHE IV score, median (IQR) | 104 (84-131)*†‡ | 85 (69-112)† | 77 (63-99) | 76 (60-94) | <.0001 |
| APACHE IV APS, median (IQR) | 89 (73-119)*†‡ | 75 (55-94)*† | 65 (50-85) | 64 (48-79) | <.0001 |
| Modified SOFA‖, median (IQR) | 10 (7-12)*† | 8 (6-10)*† | 7 (5-9)* | 7 (4-8) | <.0001 |
| Septic shock, n (%) | 33 (54.1%)*† | 60 (49.6%)*† | 62 (37.1%)* | 165 (28.4%) | .001 |
| Mechanical ventilation, n (%) | 44 (72.1%) | 85 (70.2%) | 115 (68.9%) | 406 (70%) | .98 |
| Organ failure, n (%) | 53 (86.9%) | 100 (82.6%) | 143 (85.6%) | 479 (82.6%) | .20 |
| Acute kidney injury, n (%) | 34 (55.7%)* | 61 (50.4%)* | 75 (44.9%)* | 177 (30.5%) | .001 |
| Acute lung injury, n (%) | 22 (36.1%) | 36 (29.8%) | 38 (22.8%) | 132 (22.8%) | .07 |
Significant vs normal platelet count (150 × 109 to 399 × 109/L) using a Dunn's Test of multiple comparisons using rank sums.
Significant vs low platelet count (100 × 109 to 149 × 109/L) using a Dunn's Test of multiple comparisons using rank sums.
Significant vs intermediate-low platelet count (50 × 109 to 99 × 109/L) using a Dunn's Test of multiple comparisons using rank sums.
Modified Charlson comorbidity was calculated without the contribution of age.
Modified SOFA was calculated without the contribution of SOFA coagulation and SOFA central nervous system.
Use of antiplatelet drugs: use prior to ICU admission as chronic medication (carbasalate calcium, acetylsalicylic acid, clopidogrel, dipyridamol).
Sepsis course and outcome
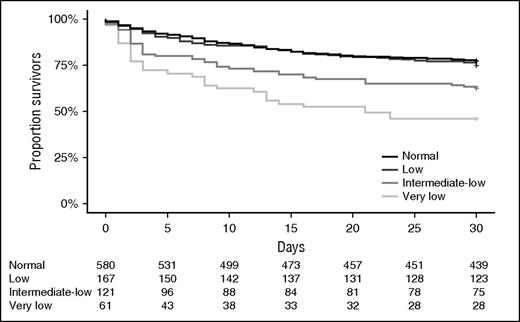
ICU and hospital lengths of stay were similar between groups (Table 2). Admission thrombocytopenia was not associated with an altered incidence of ICU-acquired acute kidney or lung injury. Very low and intermediate-low platelet counts on ICU admission were associated with higher ICU mortality (Table 2), as well as with higher mortality at day 30 (Figure 2) and up to 1 year after ICU admission (Table 2). In a multivariable Cox regression analysis, patients with very low and intermediate-low platelet counts showed an increased risk for mortality by day 30 compared with patients with normal platelet counts (hazard ratios with 95% confidence intervals 2.00 [1.32-3.05] and 1.72 [1.22-2.44], respectively).
Outcome of sepsis patients stratified according to platelet counts on ICU admission
| . | Very low (<50 × 109/L) . | Intermediate-low (50 × 109 to 99 × 109/L) . | Low (100 × 109 to 149 × 109/L) . | Normal (150 × 109 to 399 × 109/L) . | P . |
|---|---|---|---|---|---|
| Patients, n (%) | 61 (6.6%) | 121 (13.0%) | 167 (17.9%) | 580 (62.3%) | |
| Length of stay median (IQR) | |||||
| Length of ICU stay (d) | 5 (2-9) | 3 (2-8) | 5 (2-10) | 5 (2-10) | .83 |
| Length of hospital stay (d) | 20 (8-33) | 21 (9-45) | 24 (11-49) | 23 (12-45) | .21 |
| ICU-acquired complications, n (%) | |||||
| None | 51 (83.6%) | 105 (86.8%) | 133 (79.6%) | 476 (82.1%) | .43 |
| Acute kidney injury | 2 (3.3%) | 6 (5.0%) | 17 (10.2%) | 50 (8.6%) | .19 |
| Acute lung injury | 2 (3.3%) | 2 (1.7%) | 8 (4.8%) | 29 (5.0%) | .40 |
| Mortality, n (%) | |||||
| ICU | 29 (47.5%)*†‡ | 40 (33.1%)*† | 27 (16.2%) | 82 (14.1%) | <.001 |
| Hospital | 34 (55.7%)*† | 56 (46.3%)*† | 46 (27.5%) | 156 (26.9%) | <.001 |
| 30 d | 33 (54.1%)*†‡ | 45 (37.2%)*† | 42 (25.1%) | 132 (22.8%) | <.001 |
| 60 d | 34 (55.7%)*†‡ | 54 (44.6%)*† | 48 (28.7%) | 160 (27.6%) | <.001 |
| 90 d | 38 (62.3%)*† | 60 (49.6%)*† | 53 (31.7%) | 180 (31.0%) | <.001 |
| 1 y | 43 (70.5%)*† | 68 (56.2%)*† | 71 (42.5%) | 244 (42.1%) | <.001 |
| . | Very low (<50 × 109/L) . | Intermediate-low (50 × 109 to 99 × 109/L) . | Low (100 × 109 to 149 × 109/L) . | Normal (150 × 109 to 399 × 109/L) . | P . |
|---|---|---|---|---|---|
| Patients, n (%) | 61 (6.6%) | 121 (13.0%) | 167 (17.9%) | 580 (62.3%) | |
| Length of stay median (IQR) | |||||
| Length of ICU stay (d) | 5 (2-9) | 3 (2-8) | 5 (2-10) | 5 (2-10) | .83 |
| Length of hospital stay (d) | 20 (8-33) | 21 (9-45) | 24 (11-49) | 23 (12-45) | .21 |
| ICU-acquired complications, n (%) | |||||
| None | 51 (83.6%) | 105 (86.8%) | 133 (79.6%) | 476 (82.1%) | .43 |
| Acute kidney injury | 2 (3.3%) | 6 (5.0%) | 17 (10.2%) | 50 (8.6%) | .19 |
| Acute lung injury | 2 (3.3%) | 2 (1.7%) | 8 (4.8%) | 29 (5.0%) | .40 |
| Mortality, n (%) | |||||
| ICU | 29 (47.5%)*†‡ | 40 (33.1%)*† | 27 (16.2%) | 82 (14.1%) | <.001 |
| Hospital | 34 (55.7%)*† | 56 (46.3%)*† | 46 (27.5%) | 156 (26.9%) | <.001 |
| 30 d | 33 (54.1%)*†‡ | 45 (37.2%)*† | 42 (25.1%) | 132 (22.8%) | <.001 |
| 60 d | 34 (55.7%)*†‡ | 54 (44.6%)*† | 48 (28.7%) | 160 (27.6%) | <.001 |
| 90 d | 38 (62.3%)*† | 60 (49.6%)*† | 53 (31.7%) | 180 (31.0%) | <.001 |
| 1 y | 43 (70.5%)*† | 68 (56.2%)*† | 71 (42.5%) | 244 (42.1%) | <.001 |
Significant vs normal platelet count (150 × 109 to 399 × 109/L) using a Dunn's test of multiple comparisons using rank sums.
Significant vs low platelet count (100 × 109 to 149 × 109/L) using a Dunn's test of multiple comparisons using rank sums.
Significant vs intermediate-low platelet count (50 × 109 to 99 × 109/L) using a Dunn's test of multiple comparisons using rank sums.
Thirty-day Kaplan-Meier survival plots of sepsis patients stratified according to platelet counts on ICU admission.
Thirty-day Kaplan-Meier survival plots of sepsis patients stratified according to platelet counts on ICU admission.
Activation of the cytokine network
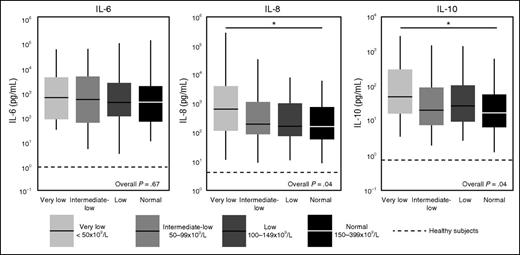
As disease severity can strongly influence the release of host response biomarkers in sepsis,37,38 we used propensity score matching to create groups with distinct platelet counts yet with comparable disease severity on ICU admission. Demographics, chronic comorbidities, and disease severity were not different between propensity matched groups (supplemental Table 1). As expected,37,38 sepsis patients displayed a profound activation of the cytokine network, as reflected by elevated plasma levels of IL-6, IL-8, and IL-10 (Figure 3). In the unmatched cohort, patients with thrombocytopenia presented with increased levels of IL-6, IL-8, and IL-10 (supplemental Table 2). In the matched cohort, patients with very low platelet counts still had higher IL-8 and IL-10 levels (Figure 3). The plasma concentrations of TNF-α, interferon-γ, IL-1β, and IL-13 were undetectable or very low in the vast majority of patients and not different between groups (data not shown).
Cytokine levels in sepsis patients on ICU admission stratified according to platelet counts in the propensity matched cohort. Data are expressed as box-and-whisker diagrams depicting the median and lower quartile, upper quartile, and their respective 1.5 IQR as whiskers (as specified by Tukey). Dotted lines indicate median values obtained in 27 healthy aged matched subjects. *P ≤ .05 using a Dunn's test of multiple comparisons using rank sums.
Cytokine levels in sepsis patients on ICU admission stratified according to platelet counts in the propensity matched cohort. Data are expressed as box-and-whisker diagrams depicting the median and lower quartile, upper quartile, and their respective 1.5 IQR as whiskers (as specified by Tukey). Dotted lines indicate median values obtained in 27 healthy aged matched subjects. *P ≤ .05 using a Dunn's test of multiple comparisons using rank sums.
Activation of the vascular endothelium
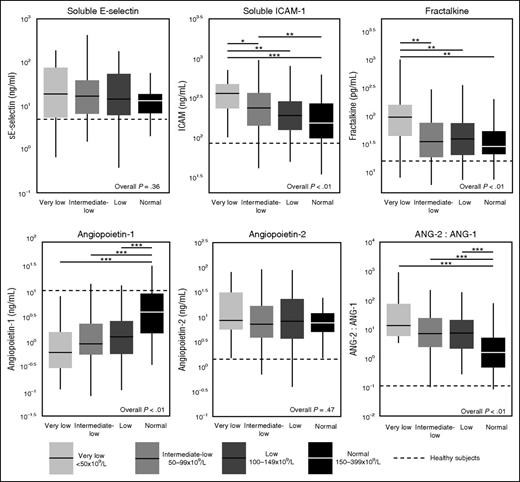
Sepsis patients displayed activation of the vascular endothelium (elevated plasma concentrations of soluble E-selectin, soluble ICAM-1, fractalkine) and loss of vascular integrity (increased levels of angiopoietin-2 and reduced levels of angiopoietin-1) (Figure 4). In the unmatched cohort, patients with thrombocytopenia presented with higher levels of soluble E-selectin, soluble ICAM-1, fractalkine, and angiopoietin-2, as well as a more profound loss of angiopoietin-1 resulting in an increased angiopoetin-2:1 ratio (supplemental Table 2). The associations between platelet counts and these markers of endothelial activation and integrity largely remained in the matched cohort; only soluble E-selectin did not differ between groups anymore (Figure 4).
Endothelial cell activation markers in sepsis patients on ICU admission stratified according to platelet counts in the propensity matched cohort. Data are expressed as box-and-whisker diagrams depicting the median and lower quartile, upper quartile, and their respective 1.5 IQR as whiskers (as specified by Tukey). Dotted lines indicate median values obtained in 27 healthy aged-matched subjects. *P ≤ .05 using a Dunn's test of multiple comparisons using rank sums; **P ≤ .01 using a Dunn's test of multiple comparisons using rank sums; ***P ≤ .001 using a Dunn's test of multiple comparisons using rank sums.
Endothelial cell activation markers in sepsis patients on ICU admission stratified according to platelet counts in the propensity matched cohort. Data are expressed as box-and-whisker diagrams depicting the median and lower quartile, upper quartile, and their respective 1.5 IQR as whiskers (as specified by Tukey). Dotted lines indicate median values obtained in 27 healthy aged-matched subjects. *P ≤ .05 using a Dunn's test of multiple comparisons using rank sums; **P ≤ .01 using a Dunn's test of multiple comparisons using rank sums; ***P ≤ .001 using a Dunn's test of multiple comparisons using rank sums.
Activation of the coagulation system
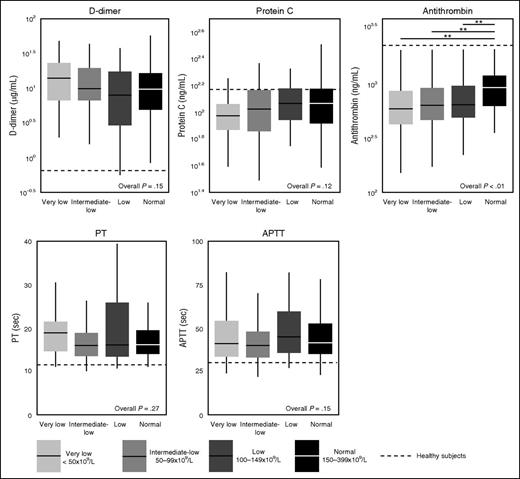
Patients with sepsis had prolonged aPTT and PT, elevated plasma levels of D-dimer, and reduced levels of the anticoagulants antithrombin and protein C indicative of a net procoagulant state (Figure 5).
Coagulation markers in sepsis patients on ICU admission stratified according to platelet counts, in the propensity matched cohort. Data are expressed as box-and-whisker diagrams depicting the median and lower quartile, upper quartile, and their respective 1.5 IQR as whiskers (as specified by Tukey). Dotted lines indicate median values obtained in 27 healthy age matched subjects. *P ≤ .05 using a Dunn's test of multiple comparisons using rank sums.
Coagulation markers in sepsis patients on ICU admission stratified according to platelet counts, in the propensity matched cohort. Data are expressed as box-and-whisker diagrams depicting the median and lower quartile, upper quartile, and their respective 1.5 IQR as whiskers (as specified by Tukey). Dotted lines indicate median values obtained in 27 healthy age matched subjects. *P ≤ .05 using a Dunn's test of multiple comparisons using rank sums.
In the unmatched cohort, thrombocytopenia was associated with prolonged aPTT and PT, higher D-dimer, and lower antithrombin concentrations (supplemental Table 2). In the matched cohort, the association between platelet counts and coagulation only remained for antithrombin levels (Figure 5).
Blood leukocyte transcriptome analysis
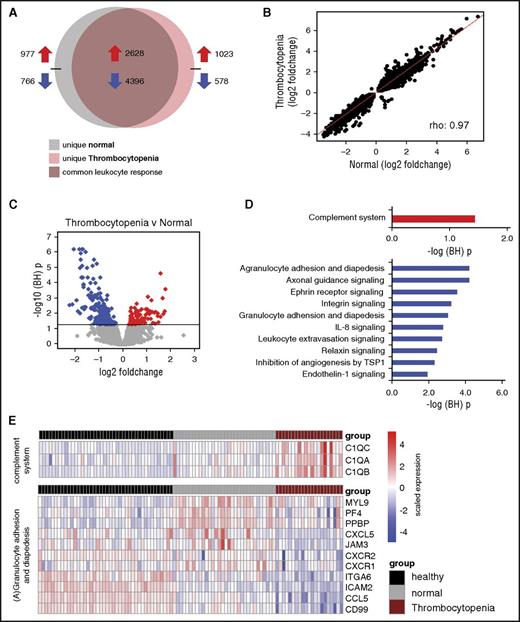
Using an unbiased approach, we compared the blood leukocyte transcriptome of patients with very low platelet counts (<50 × 109/L, n = 21) to those with normal platelet counts (150 × 109 to 399 × 109/L, n = 32); this analysis comprised the propensity matched cohort, using a subgroup of consecutive patients enrolled during the first 1.5 years of this study. Genome-wide blood gene expression profiles of these sepsis patients were initially compared with 42 healthy controls. Both thrombocytopenic patients and patients with normal platelet counts displayed strong transcriptional alterations (Figure 6A). Of the altered transcriptomes, 80% were common to both sepsis groups (Figure 6B). Pathway analysis revealed common overexpressed genes associated with typical proinflammatory, anti-inflammatory, and Toll-like receptor signaling pathways (supplemental Figure 1). Common underexpressed genes associated with primarily T-cell signaling pathways as well as EIF2 (translation) and MTOR (metabolic) signaling pathways (supplemental Figure 1). Unique gene expression signatures were also uncovered for each sepsis group (Figure 6A). Indeed, differential gene expression analysis revealed 234 genes (multiple-comparison adjusted, P < .05) (Figure 6C). Pathway analysis showed that overexpressed genes in thrombocytopenic patients were significantly associated with the complement system pathway, including subcomponents to complement component 1 (C1QA, C1QB, and C1QC; Figure 6D-E). Underexpressed genes associated with predominantly leukocyte mobility pathways, including agranulocyte adhesion and diapedesis (Figure 6D-E).
The leukocyte genomic responses and associated biological pathways in sepsis patients with thrombocytopenia, in the propensity matched cohort. (A) Venn-Euler representation of differentially expressed genes in sepsis patients with very low platelet counts (<50 × 109/L, thrombocytopenia) and normal platelet counts (150 × 109 to 399 × 109/L, normal) vs healthy subjects (adjusted P < .05). Red arrows denote overexpressed genes; blue arrows denote underexpressed genes. (B) Dot plot depicting the common response (log2 foldchanges) of patients with very low and normal platelet counts as compared with healthy subjects. ρ, Spearman’s correlation coefficient. (C) Volcano plot illustrating the differences in leukocyte genomic responses (integrating log2 foldchanges and multiple-test adjusted probabilities) between sepsis patients with thrombocytopenia (<50 × 109/L) and normal counts (150 × 109 to 399 × 109/L). Considering adjusted P < .05, 234 genes were identified as differentially expressed. Red dots denote significantly overexpressed genes, whereas blue dots denote significantly underexpressed genes in thrombocytopenic patient samples. Horizontal black line indicates multiple-test adjusted (BH) P < .05 threshold. (D) Overexpressed genes in thrombocytopenic sepsis patients associated with the complement signaling pathway (red bar). Underexpressed genes associated with predominantly leukocyte mobility, adhesion, and extravasation pathways (blue bars). –log (BH) p, negative log-transformed BH adjusted P values. (E) Heatmap plots of significantly (adjusted P < .05) differential gene expression indices pertaining to the complement system and (A) granulocyte adhesion and diapedesis pathways. Red rows denote overexpression; blue rows denote underexpression.
The leukocyte genomic responses and associated biological pathways in sepsis patients with thrombocytopenia, in the propensity matched cohort. (A) Venn-Euler representation of differentially expressed genes in sepsis patients with very low platelet counts (<50 × 109/L, thrombocytopenia) and normal platelet counts (150 × 109 to 399 × 109/L, normal) vs healthy subjects (adjusted P < .05). Red arrows denote overexpressed genes; blue arrows denote underexpressed genes. (B) Dot plot depicting the common response (log2 foldchanges) of patients with very low and normal platelet counts as compared with healthy subjects. ρ, Spearman’s correlation coefficient. (C) Volcano plot illustrating the differences in leukocyte genomic responses (integrating log2 foldchanges and multiple-test adjusted probabilities) between sepsis patients with thrombocytopenia (<50 × 109/L) and normal counts (150 × 109 to 399 × 109/L). Considering adjusted P < .05, 234 genes were identified as differentially expressed. Red dots denote significantly overexpressed genes, whereas blue dots denote significantly underexpressed genes in thrombocytopenic patient samples. Horizontal black line indicates multiple-test adjusted (BH) P < .05 threshold. (D) Overexpressed genes in thrombocytopenic sepsis patients associated with the complement signaling pathway (red bar). Underexpressed genes associated with predominantly leukocyte mobility, adhesion, and extravasation pathways (blue bars). –log (BH) p, negative log-transformed BH adjusted P values. (E) Heatmap plots of significantly (adjusted P < .05) differential gene expression indices pertaining to the complement system and (A) granulocyte adhesion and diapedesis pathways. Red rows denote overexpression; blue rows denote underexpression.
Sensitivity analysis
We performed an alternative analysis dividing patients into quartiles of platelet counts (quartile 1, 0 × 109 to 116 × 109/L, n = 235; quartile 2, 117 × 109 to 181 × 109/L, n = 234; quartile 3, 182 × 109 to 251 × 109/L, n = 228; quartile 4, 251 × 109 to 399 × 109/L, n = 232) (supplemental Table 3). Notably, this approach is clinically less relevant because only quartile 1 exclusively consists of patients with thrombocytopenia, and even in this quartile, patients with various degrees of thrombocytopenia are grouped together. Nonetheless, most results from our preferred analysis were reproduced. Patients with the lowest platelet counts (quartile 1) presented with more severe disease, as indicated by higher APACHE IV scores and more shock, and had the highest mortality rates up to 1 year after ICU admission (supplemental Table 3). We performed propensity matching to enable analysis of the host response in patients in different platelet count quartiles with similar disease severities (supplemental Table 4). Akin to the analysis shown in Figures 3-5, patients with the lowest platelet counts (quartile 1) had higher IL-10, soluble E-selectin, soluble ICAM-1, fraktalkine, and angiopoetin-2:1 ratios and lower antithrombin levels when compared with patients with normal platelet counts (quartiles 3 and 4) (supplemental Table 3). In addition, patients from quartile 1 had a unique gene expression signature compared with those in quartile 4 (supplemental Figure 2), which copied the differences in the analysis based on stratification according to predefined platelet count strata (shown in Figure 6).
Discussion
Platelets are versatile effector cells involved in hemostasis, inflammation, leukocyte functions, and endothelial cell activation. Although multiple preclinical investigations have indicated that these pleiotropic platelet functions can influence the course and outcome of experimental sepsis,10,13,14,19 the role of platelets in the host response to human sepsis is less well studied. To our knowledge, this is the first investigation in humans to study the association between platelet counts and activation of host response pathways relevant for sepsis pathogenesis. We measured 17 plasma biomarkers indicative of activation of the cytokine network, the vascular endothelium, and the coagulation system, and, in an unbiased approach, we evaluated the genomic response of blood leukocytes in a well-documented patient population with a sample size that allowed for propensity matching and thereby analyses of patients with different platelet counts and otherwise similar baseline characteristics. Our main finding was that severe thrombocytopenia is associated with enhanced activation of the cytokine network and the vascular endothelium, a more profound loss of vascular integrity, and a distinct whole-blood leukocyte transcriptome pattern revealing decreased leukocyte adhesion, diapedesis, and extravasation signaling. These results suggest that low platelet counts independently associate with a more disturbed host response and provide indirect proof for the clinical validity of animal studies on the role of platelets in the pathogenesis of sepsis.
Our study, encompassing 931 consecutive sepsis patients, is the largest to date to determine the association between thrombocytopenia and sepsis presentation and outcome. Patients with low platelet counts (<100 × 109/L) were more severely ill, as indicated by higher APACHE IV and SOFA scores, and had more shock and organ failure. Moreover, patients with platelet counts < 100 × 109/L had an increased mortality up to 1 year after ICU admission, and in a multivariable Cox regression analysis, these low platelet counts were independently associated with an enhanced mortality at day 30. These findings are in accordance with a previous study in 69 patients with septic shock that reported an association between admission thrombocytopenia and higher SOFA scores, vasopressor requirement, and mortality.6 Another investigation, encompassing 304 sepsis patients, found no association between thrombocytopenia and mortality, but did report an independent association between nonresolution of thrombocytopenia and mortality.7 This latter study7 differed from the current analysis by its retrospective nature and inclusion of almost exclusively septic shock patients (93% vs 40% in the present study).
We measured 17 plasma biomarkers to obtain insight on the activation of distinct host response pathways known to contribute to the pathological sequelae of sepsis. Most differences in host response biomarkers between patients with thrombocytopenia and those with normal platelet counts remained after propensity matching, suggesting that low platelet counts on ICU admission may influence distinct host response pathways during human sepsis independent of disease severity. Although the current observational findings do not prove a causal relationship between thrombocytopenia and a more disturbed host response in sepsis, investigations in mice suggest that platelets may indeed modify pivotal inflammatory reactions during severe infection. In accordance with increased IL-8 and IL-10 levels in patients with <50 × 109/L platelets, mice depleted of platelets had increased cytokine concentrations during endotoxemia19 and sepsis,13 and blood from platelet-depleted mice showed increased cytokine production in response to Klebsiella pneumoniae.13 In murine sepsis platelets, attenuated cytokine release at least in part via platelet glycoprotein 1b, the von Willebrand factor receptor.39 We found that patients with thrombocytopenic sepsis showed evidence of more profound activation of endothelial cells (elevated plasma soluble ICAM-140 and fractalkine levels41 ) and a reduced vascular integrity (higher angiopoietin-2:1 ratios)40 relative to patients with normal platelet counts. The angiopoietin-2:1 ratio is an indicator of acute vascular dysfunction, supported by clinical studies and investigations in knockout mice.42,43 This interaction between platelets and endothelial cells is in line with several in vitro and animal studies. Platelets can physically block gaps in the vascular lining, promote endothelial cell growth and ultrastructure, and secrete soluble factors that enhance barrier function, such as serotonin and angiopoietin-1.44 Platelets can adhere to the endothelium and secrete mediators such as IL-1β and CD154, which can promote activation of the endothelium,9,45 and platelets aid in maintaining vascular integrity, especially in a strong proinflammatory environment.13,23,24 Finally, the fact that the plasma concentrations of D-dimer, a split product of crosslinked fibrin, were similar in patients with different platelet numbers suggests that the coagulation cascade was not hindered by decreased platelet counts, which is corroborated by similar findings in thrombocytopenic mice with sepsis. 13,14
By using whole-blood genome-wide transcriptional profiling of leukocytes, we were able to assess the association between thrombocytopenia and thousands of molecular signaling events. The vast majority of the leukocyte genomic response to sepsis was common in patients with and without thrombocytopenia. This common response included typical proinflammatory, anti-inflammatory, metabolic, and T-cell signaling pathways, which is in accordance with previous studies.26,46 Pathways with reduced expression in patients with <50 × 109/L platelets relative to patients with normal platelet counts predominantly involved leukocyte adhesion, extravasation, and diapedesis. In this respect, it should be noted that the axonal guidance signaling pathway largely entails genes involved in cytoskeletal reorganization (for example, TUBA8, TUBB, and PXN), while Ephrin receptor signaling is important for leukocyte adhesion.47-49 During inflammation, leukocytes are recruited to the site of infection, where a multistep process takes place, which includes rolling, binding, and integrin activation, leading to extravasation from the vessel. Various murine and ex vivo studies have identified a role for platelets in all steps of leukocyte recruitment and extravasation.8,10 Platelets can aid in recruitment of neutrophils to the site of infection50,51 and can drive neutrophil migration, crawling, and cytoskeletal reorganization.52-54 In addition, activated platelets can increase leukocyte integrin activation, which allows firm adhesion of leukocytes to the endothelium before extravasation.8,55-57 Our array analysis supports these preclinical data and suggests that platelets contribute to these leukocyte functions in patients with sepsis. Complement signaling was the only pathway that was upregulated in leukocytes of patients with thrombocytopenia. Complement activation is a widely reported host response in patients with sepsis.58,59 We did not measure complement activation products in our cohort, which requires immediate blood processing in anticoagulants containing protease inhibitors. Therefore, it remains to be established whether thrombocytopenia is associated with enhanced complement activation in patients with sepsis. Notably, because arrays were performed in whole blood, it is possible that platelet RNA contributes to differences in transcriptional profiles in patients with and without thrombocytopenia. However, as leukocytes contain approximately a 12 500-fold higher concentration of RNA compared with platelets,60 it is likely that transcripts are mainly leukocyte derived.
Our study has strengths and limitations. We studied a large prospectively enrolled cohort in which patients were meticulously characterized according to strict criteria. We implemented propensity matching to correct for differences in disease severity between patients with different platelet counts. We performed a sensitivity analysis that yielded similar results. Nonetheless, a bias may have remained after propensity matching due to unmeasured confounders. Data were collected in 2 ICUs in The Netherlands, which may limit the generalizability.
To conclude, critically ill patients with sepsis with thrombocytopenia (<50 × 109/L and 50 × 109 to 99 × 109/L) on ICU admission demonstrated increased disease severity and mortality. Admission thrombocytopenia (<50 × 109/L) was associated with elevated plasma levels of IL-8 and IL-10, increased endothelial cell activation, enhanced disturbance of the vascular integrity, and decreased leukocyte adhesion and migration signaling, independent of disease severity. These observational data taken together with functional data in animal sepsis models suggest that thrombocytopenia may aggravate part of the disturbed host response during sepsis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
T.A.M.C. was funded by a grant from the Landsteiner Foundation for Blood Transfusion Research (LSBR grant 1351). This work was supported by the Center for Translational Molecular Medicine (http://www.ctmm.nl), project MARS (grant 04I-201).
Authorship
Contribution: T.A.M.C., L.A.v.V., and T.v.d.P. designed the study. L.A.v.V., M.A.W., and P.M.C.K.K. acquired all the data. A.J.H. performed protein biomarker measures. B.P.S. performed the microarray analysis. L.A.v.V. is the guarantor of this work, had full access to all data, and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors were involved in the interpretation of the data. T.A.M.C., L.A.v.V., and T.v.d.P. drafted the manuscript, and all authors reviewed and revised it critically for important intellectual content. All authors gave final approval of this version to be submitted.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the MARS Consortium appears in the supplemental Materials.
Correspondence: Theodora A. M. Claushuis, Center for Experimental and Molecular Medicine, Academic Medical Center, Meibergdreef 9, Room F0-117, 1105 AZ Amsterdam, The Netherlands; e-mail: t.a.claushuis@amc.uva.nl.
References
Author notes
T.A.M.C. and L.A.v.V. contributed equally to this study.