Key Points
P falciparum STEVORs interact with the erythrocyte cytoskeletal ankyrin complex.
Infected erythrocyte deformability is regulated by PKA-mediated phosphorylation of STEVOR cytoplasmic domain.
Abstract
Deformability of Plasmodium falciparum gametocyte-infected erythrocytes (GIEs) allows them to persist for several days in blood circulation and to ensure transmission to mosquitoes. Here, we investigate the mechanism by which the parasite proteins STEVOR (SubTElomeric Variable Open Reading frame) exert changes on GIE deformability. Using the microsphiltration method, immunoprecipitation, and mass spectrometry, we produce evidence that GIE stiffness is dependent on the cytoplasmic domain of STEVOR that interacts with ankyrin complex at the erythrocyte skeleton. Moreover, we show that GIE deformability is regulated by protein kinase A (PKA)–mediated phosphorylation of the STEVOR C-terminal domain at a specific serine residue (S324). Finally, we show that the increase of GIE stiffness induced by sildenafil (Viagra) is dependent on STEVOR phosphorylation status and on another independent mechanism. These data provide new insights into mechanisms by which phosphodiesterase inhibitors may block malaria parasite transmission.
Introduction
For decades, the strategies to fight malaria have focused on preerythrocytic and pathogenic asexual blood stages. However, achievement of malaria elimination requires development of novel strategies targeting the parasite sexual stages (gametocytes) that are responsible for transmission to mosquitoes. Plasmodium falciparum immature gametocyte-infected erythrocytes (GIEs) sequester in the human bone marrow,1-3 and appear only as mature stages in the peripheral blood where they are accessible for mosquitoes that ensure transmission. Release into the peripheral blood is accompanied by an increase in GIE deformability that allows mature stages to persist several days in the bloodstream and avoid the clearance by the spleen.4-6 These observations led to a new paradigm where gametocyte sequestration in the bone marrow would depend on mechanical retention due to the important stiffness of immature GIE, whereas an increase in deformability may allow mature stages to cross the vascular endothelium in order to reach the peripheral circulation.6-8 Distinct mechanical properties of immature and mature GIE therefore provide insights into their interactions with the human bone marrow and resolve key knowledge gaps. However, the mechanisms underlying the switch in GIE deformability late in the maturation process remain elusive. In asexual stages, the progressive decrease in erythrocyte deformability upon infection is due to the presence of the large intracellular parasite and to a decrease in membrane viscoelasticity mediated by parasite-encoded proteins.9 These proteins associate with the erythrocyte membrane skeleton where they are proposed to induce cross-linking of cytoskeletal proteins.10-13 In sexual stages, the switch in deformability is linked to expression and localization of STEVOR (SubTElomeric Variable Open Reading frame) proteins at the erythrocyte membrane.6 The stevor multicopy gene family is 1 of the 3 major families of variant genes in P falciparum, and comprises 35 genes that are clonally expressed.14-16 The encoded STEVOR proteins exhibit a semiconserved N-terminal domain and a central variable domain exposed on the erythrocyte surface, whereas the conserved C-terminal domain is cytoplasmic.17,18 Besides their role in invasion, adhesion, and rosetting in asexual stages,19-22 STEVOR proteins have been shown to impact the deformability of the infected erythrocyte in mature asexual parasites and immature sexual stages.6,23 However, the mechanism underlying STEVOR-mediated changes in erythrocyte deformability remains unclear.
A recent study has demonstrated that GIE deformability is regulated by the cyclic adenosine monophosphate (cAMP)–signaling pathway.24 Protein kinase A (PKA) activity contributes to the stiffness of immature GIE and a drop in cAMP concentration triggers the switch in deformability in mature stages. These observations have the potential to be translated into therapies for blocking malaria transmission. Indeed, raising cAMP levels with sildenafil (Viagra) or derived analogs may impair mature GIE circulation through the spleen.24 The substrates phosphorylated by PKA at the parasite and/or erythrocyte membrane in immature stages remain, however, unidentified. Their identification would clarify an important process in the parasite life cycle and open the way for the design of optimal interfering compounds.
Here, we show that the cytoplasmic domain of STEVOR interacts with erythrocyte ankyrin complex and is phosphorylated by PKA, and that GIE deformability is impacted by this interaction and related phosphorylation.
Methods
Parasite culture, gametocyte stage-specific purification, and transfection
P falciparum NF54 clones B10, A12,6 and transgenic lines were cultured in O+ human blood and RPMI 1640 medium supplemented with 10% heat-inactivated human serum under hypoxic conditions. Synchronous production of highly specific gametocytes stages was achieved according to a described protocol.25 Transgenic P falciparum strains were obtained by transfection of plasmids into B10 parasites as described in supplemental Methods (available on the Blood Web site). Transgenic parasites were cultivated with blasticidin (BSD) to ensure episomal overexpression of plasmids carrying a blasticidin (bsd) expression cassette.
Microsphiltration
Microsphiltration experiments were performed on tips or on microplates as described previously.26-28 Briefly, calibrated metal microspheres with 2 different size distributions (5- to 15-µm diameter and 15- to 25-µm diameter) composed a matrix used to assay infected erythrocyte deformability under flow. Suspensions of synchronized cultures containing 1% to 5% parasites were perfused through the microsphere matrix at a flow rate of 60 mL per hour using an electric pump (Syramed SP6000; arcomed ag) or were vacuum aspirated using a manifold system (Beckman Coulter) coupled to an electric high-output vacuum pump (Millipore) via a 10-L trap. The upstream and downstream samples were collected and parasitemia was assayed to determine parasite retention vs flow-through. Detailed protocols of microsphiltration assays are provided in supplemental Methods.
Drug treatments
Synchronized cultures containing 1% to 5% GIEs were incubated 30 minutes at 37°C with 100 μM 8-Bromide-cAMP (8Br-cAMP), 50 nM calyculin A (Ser/Thr protein phosphatase inhibitor), or 100 μM sildenafil citrate. All reagents were purchased from Sigma-Aldrich.
Immunofluorescence assays
Synchronous cultures were air-dried on glass blood smears and methanol-fixed at −20°C for 3 minutes. After 1 hour of preincubation in 1× phosphate-buffered saline/2% bovine serum albumin, slides were incubated overnight with a mouse monoclonal antibody anti-Ty1 antibody (Diagenode) at 1/50 000 and with Alexa Fluor 488–conjugated goat anti-mouse antibody (Molecular Probes) for 1 hour. Samples were observed at ×100 magnification using a Leica DM 5000 B.
Western blot analyses
Synchronous stage III GIE or 40-hour postinvasion (hpi) schizonts were purified by magnetic isolation and denatured in protein loading buffer 5 minutes at 95°C. Samples were probed overnight with different antibodies. For detailed description of different antibodies used, see supplemental Methods. A detection step was performed using the Pierce chemoluminescence system (Pierce) following the manufacturer’s instructions.
Immunoprecipitation and capillary liquid chromatography tandem mass spectrometry
Immunoprecipitation assays were performed on 6 × 106 magnetic-activated cell sorting–purified parasites. Total parasite extracts were incubated with agarose beads coated with 2 µg of antibodies and antibody-bound protein G-coated beads were analyzed by western blotting. For mass spectrometry (MS), 0.5 µg of antibody were used and coimmunoprecipitated proteins were analyzed by liquid chromatography (LC)-MS/MS. MS/MS spectra were identified by the Mascot 2.5 (Matrix Science) server against the Swiss-Prot database and the PlasmoDB database. Detailed protocols for extracts and LC-MS/MS are provided in supplemental Methods.
In vitro phosphorylation assay
Three synthetic peptides (Kemptide: Biotin-Ahx-LRRASLG, Stevor S324: Biotin-Ahx-RRKNSWK, Stevor A324: Biotin-Ahx-RRKNAWK), generated by Proteogenix, were used for in vitro phosphorylation assays. Each peptide was incubated with bovine PKA (Sigma-Aldrich) in 7.5 µL of ice-cold kinase buffer. The compounds used for peptide inhibition were 50 µM H89 and 10 µM KT5720. The reaction was initiated by the addition of 15 µM adenosine triphosphate (ATP), 3 µCi of [γ-32P]ATP (3000 Ci/mmol; Perkin Elmer). After 30-minute incubation at 30°C, the reaction was stopped with 5 µL of guanidine hydrochloride at 7.5 M (Sigma-Aldrich). Ten microliters of mixture were spotted on 1 cm2 SAM2 Biotin Capture membrane (Promega) and washed several times. Membranes were incubated overnight in a Storage Phosphor Screen (Molecular Dynamics) and signal was detected by Typhoon device. A detailed protocol of the phosphorylation assay is provided in supplemental Methods.
Statistical analysis
Statistical significance for differences in retention rates was established using the Wilcoxon Mann-Whitney rank sum test.
Results
The cytoplasmic domain of STEVOR is essential for infected erythrocyte stiffness
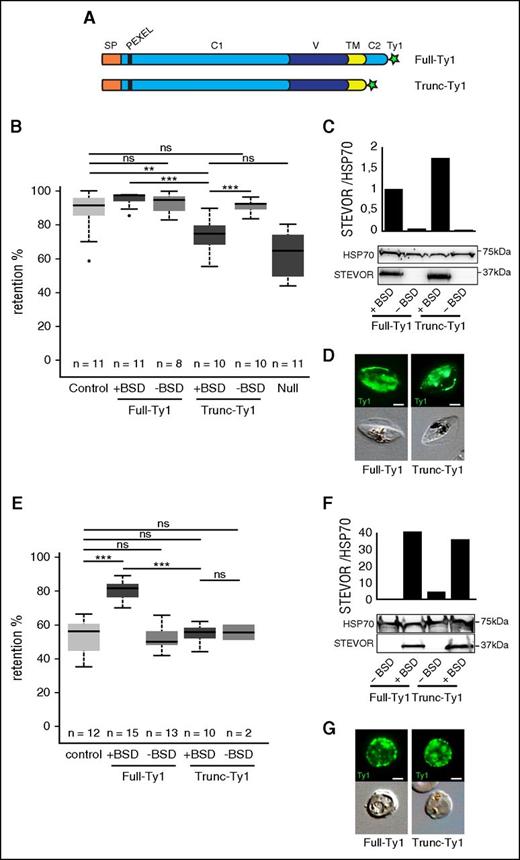
Based on the topology of the protein,18 we hypothesized that STEVOR impacts the stiffness of infected erythrocytes via its conserved cytoplasmic domain. To address this hypothesis, we generated 2 transgenic parasite lines that episomally overexpress, driven by the constitutive promoter of calmodulin (PF14_0323/PF3D7_1434200), either a Ty1-tagged copy of the PFF1550w (PF3D7_0631900) stevor gene (Full-Ty1) or a Ty1-tagged copy of PFF1550w truncated for the cytoplasmic domain (Trunc-Ty1) (Figure 1A). As a control, the B10 clone that expresses an endogenous stevor gene6 was transfected with an empty plasmid (control line). We measured the filterability of immature stage III GIE from these lines by using the microsphiltration method which mimics the physical constraints experienced by infected erythrocytes in the splenic microcirculation.26,28 In immature GIE, expression of endogenous STEVOR in wild-type parasites leads to retention rates in microbeads around 90%.6 This implies that saturation of the system does not allow detection of a significant increase in retention rates. In the transgenic lines, however, overexpression of STEVOR results in downregulation of endogenous stevor expression (supplemental Figure 1). This suggests that differences in retention rates between Trunc-Ty1 and Full-Ty1 lines should be measurable. As expected, the retention rates of the Trunc-Ty1 line were significantly lower than that of Full-Ty1 and control lines (74.1% vs 95.1% and 90.4%, P = .0001 and .0086) (Figure 1B), whereas the truncated protein expression was slightly higher than the full STEVOR expression (Figure 1C) and both proteins were efficiently exported at the erythrocyte membrane (Figure 1D). This phenotype was reverted to the levels of retention associated with endogenous STEVOR expression when we promoted shedding of the overexpressing episome by cultivating the Full-Ty1 line for several generations without BSD selection (Figure 1B). Importantly, retention rates of Trunc-Ty1 line were not significantly different from the ones of the Null transgenic line (74.1% vs 63.1%, P = .09) that downregulates the entire stevor gene family due to an epigenetic knockdown29 (Figure 1B; supplemental Figure 2). These results indicate that the stiffness phenotype mediated by STEVOR is essentially due to the cytoplasmic domain of the protein. We further confirmed these observations in 22-hpi asexual stages before endogenous expression of STEVOR.16,30,31 At these stages, overexpression of the full copy of STEVOR in the Full-Ty1 line substantially increased the retention rates compared with the control line (78% vs 51.9%, P = 0), whereas the retention rates of the Trunc-Ty1 line were at the same level as the control line (54.9% vs 51.9%, P = .9; Figure 1E-G). Altogether, these results indicate that STEVOR-mediated rigidity of infected erythrocytes is dependent on the C-terminal domain in both asexual and gametocytes stages.
The cytoplasmic domain of STEVOR contributes to infected erythrocyte stiffness. (A) Schematic representation of STEVOR recombinant proteins overexpressed in the Full-Ty1 and in the Trunc-Ty1 lines. Signal peptide (orange, SP), PEXEL/HT motif (black bar), N-terminal semiconserved region (light blue, C1), hypervariable region (dark blue, V), transmembrane domain (yellow, TM), C-terminal conserved cytoplasmic region (light blue, C2), and Ty1-tag (green star) are indicated. (B,E) Retention rates in microsphilters of stage III GIE (B) and asexual stages (E) from the control (light gray), the Full-Ty1, the Trunc-Ty1, and the Null lines cultivated with (+BSD, dark gray) or without (−BSD, medium gray) BSD. Error bars denote the standard error of the mean. Outliers are shown as open circles. Stars represent highly significant differences in retention rates (***P < .001; **P < .01). n, number of experiments; ns, nonsignificant differences in retention rates. (C,F) Western blot analysis of STEVOR expression in stage III GIE (C) and asexual stages (F) from the Full-Ty1 and the Trunc-Ty1 lines cultivated with (+BSD) or without (−BSD) BSD. Immunoblots were probed with a rabbit polyclonal antibody directed against STEVOR (PFC0025c) and with a rat polyclonal antibody directed against HSP70 to normalize expression. Quantitation of signal intensities was realized using Quantity One software (Bio-Rad). (D,G) Immunofluorescence analysis of stage III GIE (D) and asexual stages (G) from the Full-Ty1 and the Trunc-Ty1 lines. Infected erythrocytes were stained with anti-Ty1 antibodies followed by anti-mouse Alexa 488–conjugated IgG. Pictures were taken under identical exposure conditions. The bars represent 2 µm.
The cytoplasmic domain of STEVOR contributes to infected erythrocyte stiffness. (A) Schematic representation of STEVOR recombinant proteins overexpressed in the Full-Ty1 and in the Trunc-Ty1 lines. Signal peptide (orange, SP), PEXEL/HT motif (black bar), N-terminal semiconserved region (light blue, C1), hypervariable region (dark blue, V), transmembrane domain (yellow, TM), C-terminal conserved cytoplasmic region (light blue, C2), and Ty1-tag (green star) are indicated. (B,E) Retention rates in microsphilters of stage III GIE (B) and asexual stages (E) from the control (light gray), the Full-Ty1, the Trunc-Ty1, and the Null lines cultivated with (+BSD, dark gray) or without (−BSD, medium gray) BSD. Error bars denote the standard error of the mean. Outliers are shown as open circles. Stars represent highly significant differences in retention rates (***P < .001; **P < .01). n, number of experiments; ns, nonsignificant differences in retention rates. (C,F) Western blot analysis of STEVOR expression in stage III GIE (C) and asexual stages (F) from the Full-Ty1 and the Trunc-Ty1 lines cultivated with (+BSD) or without (−BSD) BSD. Immunoblots were probed with a rabbit polyclonal antibody directed against STEVOR (PFC0025c) and with a rat polyclonal antibody directed against HSP70 to normalize expression. Quantitation of signal intensities was realized using Quantity One software (Bio-Rad). (D,G) Immunofluorescence analysis of stage III GIE (D) and asexual stages (G) from the Full-Ty1 and the Trunc-Ty1 lines. Infected erythrocytes were stained with anti-Ty1 antibodies followed by anti-mouse Alexa 488–conjugated IgG. Pictures were taken under identical exposure conditions. The bars represent 2 µm.
The cytoplasmic domain of STEVORs interacts with the ankyrin complex at the erythrocyte cytoskeleton
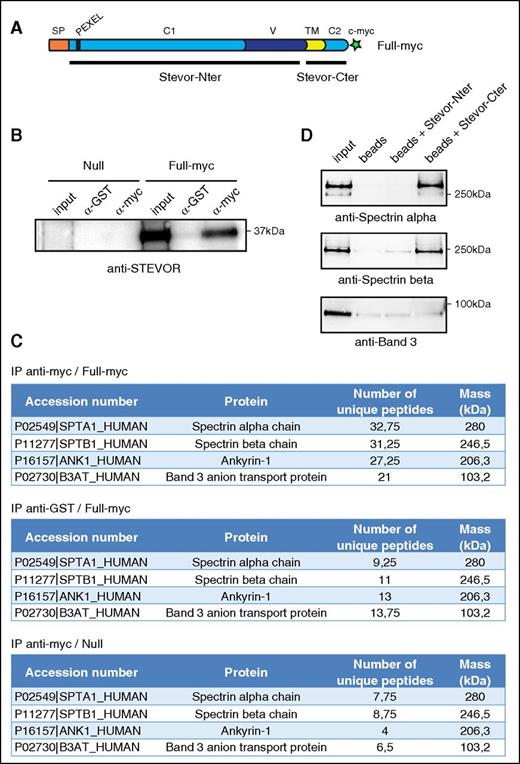
According to the results detailed in the previous section and to STEVOR topology at the erythrocyte membrane, we postulated that alteration of immature GIE filterability might be mediated by interactions between the STEVOR cytoplasmic domain and components of the host cell membrane. To investigate this hypothesis, we combined immunoprecipitation assays and a MS analysis to identify the putative interacting partner(s) of STEVORs. The immunoprecipitation was performed on stage III GIE from the Full-myc line that overexpressed a c-myc–tagged STEVOR protein (PFF1550w) and has the same filterability phenotype as the Full-Ty1 line (Figure 2A; supplemental Figure 3). The Null transgenic line was used as a control pulldown to ensure specificity of the interactions. The efficiency and specificity of the immunoprecipitation on stage III GIE lysates using anti-glutathione S-transferase (GST) and anti-c-myc antibodies were evaluated by western blot analysis using an anti-STEVOR antibody (Figure 2B). MS analysis of proteins that coprecipitated with anti-c-myc in the Full-myc revealed that 4 proteins composing the ankyrin complex, spectrin α, spectrin β, ankyrin, and band 3, were consistently detected with significant high scores across 4 independent experiments (Figure 2C). These proteins were not significantly coprecipitated with anti-GST antibodies in the Full-myc line nor with both antibodies in the Null line, confirming the specificity of our results. To validate these results, we performed a pulldown analysis using recombinant his-tagged STEVOR C-terminal (Stevor-Cter) or N-terminal (Stevor-Nter) domains and recombinant spectrin dimer or band 3 (Figure 2D). In contrast to the Stevor N-ter domain, the Stevor-Cter domain was able to pull down spectrin α and spectrin β, whereas neither of the STEVOR domains had the ability to bind band 3. These data confirm that the cytoplasmic domain of STEVOR directly interacts with at least 1 cytoskeletal proteins composing the ankyrin complex.
STEVOR proteins interact with components of the ankyrin complex. (A) Schematic representation of STEVOR recombinant protein overexpressed in the Full-myc line. Black bars represent recombinant his-tagged Stevor-Cter and N-terminal (Stevor-Nter) domains. (B) Immunoprecipitation of stage III V GIE lysates from the Full-myc and the Null lines with anti-myc and anti-GST antibodies. Immunoblot was probed with a rabbit polyclonal antibody directed against STEVOR (PFL2610w). (C) MS analysis of immunoprecipitates from the Full-myc and the Null line with anti-myc and anti-GST antibodies. *Average number of peptides for 4 independent experiments. (D) Pulldown assays of recombinant spectrin dimer (Sigma-Aldrich) (top and middle panel) or recombinant Band 3 (bottom panel) with recombinant his-tagged STEVOR C-terminal (Stevor-Cter) or N-terminal (Stevor-Nter) domains on nickel beads. Input represents 10% of recombinant proteins used for pulldowns. Immunoblot were probed with a rabbit anti-spectrin α antibody (top panel), a rabbit anti-spectrin β antibody (middle panel) or a rabbit anti-band 3 antibody (bottom panel).
STEVOR proteins interact with components of the ankyrin complex. (A) Schematic representation of STEVOR recombinant protein overexpressed in the Full-myc line. Black bars represent recombinant his-tagged Stevor-Cter and N-terminal (Stevor-Nter) domains. (B) Immunoprecipitation of stage III V GIE lysates from the Full-myc and the Null lines with anti-myc and anti-GST antibodies. Immunoblot was probed with a rabbit polyclonal antibody directed against STEVOR (PFL2610w). (C) MS analysis of immunoprecipitates from the Full-myc and the Null line with anti-myc and anti-GST antibodies. *Average number of peptides for 4 independent experiments. (D) Pulldown assays of recombinant spectrin dimer (Sigma-Aldrich) (top and middle panel) or recombinant Band 3 (bottom panel) with recombinant his-tagged STEVOR C-terminal (Stevor-Cter) or N-terminal (Stevor-Nter) domains on nickel beads. Input represents 10% of recombinant proteins used for pulldowns. Immunoblot were probed with a rabbit anti-spectrin α antibody (top panel), a rabbit anti-spectrin β antibody (middle panel) or a rabbit anti-band 3 antibody (bottom panel).
cAMP-mediated changes in GIE deformability are linked to STEVOR expression
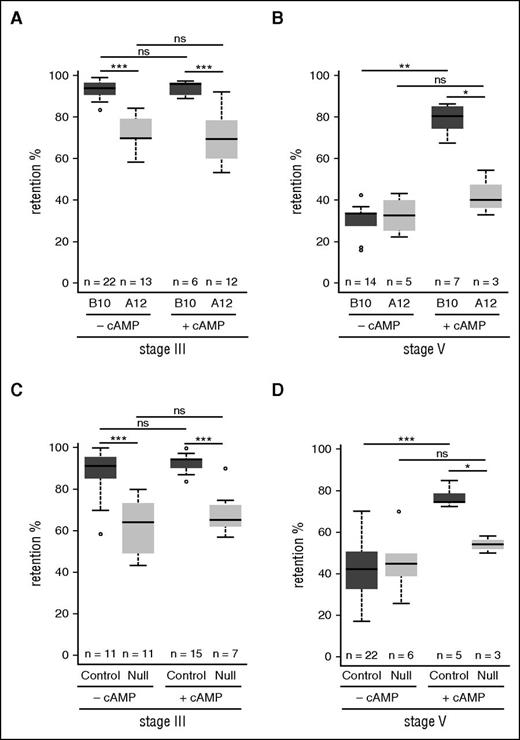
Our recent results showing that GIE deformability is regulated by the cAMP-signaling pathway24 suggest that STEVOR interaction with the ankyrin complex may be regulated by PKA-mediated phosphorylation. To test this hypothesis, we first addressed whether the cAMP-mediated changes in GIE deformability contribute to the stiffness phenotype induced by STEVOR expression. We measured the filterability of stage III and stage V GIE from parasite lines expressing different levels of STEVOR in the presence or absence of an analog of cAMP, 8Br-cAMP. We first use 2 sibling wild-type clones of the NF54 parasite strain that express different levels of STEVOR: while B10 endogenously expresses STEVOR, messenger RNA production of all stevor genes is downregulated in the A12 line leading to a decrease in STEVOR expression.6,16 Microsphiltration experiments on immature GIEs showed a significant decrease in retention rates in A12 compared with B10 clones (93.7% vs 73.5%, P = 0), whereas their retention rates remained unchanged upon incubation with 8Br-cAMP (Figure 3A). However, in mature GIEs, the significant increase in retention rates upon incubation of the B10 clone with 8Br-cAMP (30% vs 73%, P = 0) was not observed in the A12 clone (32.5% vs 41.7%, P = .4). This indicates that 8Br-cAMP specifically affects the filterability of P falciparum mature GIEs that expressed STEVORs (Figure 3B). We then confirmed this result by using the transgenic control and Null parasite lines (Figure 3C-D). As expected, the treatment with 8Br-cAMP affected the deformability of stage V from the control transgenic line (41% vs 76.7%, P = 0) but not from the Null line (45.5% vs 54.1%, P = .17). These results indicate that changes in GIE filterability mediated by STEVOR expression and by cellular cAMP signaling are involved in the same molecular mechanism.
cAMP-mediated changes in GIE deformability are linked to STEVOR expression. (A-B) Retention in microsphilters of stages III (A) and stages V (B) GIE from the B10 (dark gray) and the A12 (medium gray) wild-type clones preincubated at 37°C during 30 minutes with (+cAMP) or without (–cAMP) 100 µM 8Br-cAMP. (C-D) Retention in microsphilters of stages III (C) and stages V (D) GIE from the control (dark gray) and the Null (medium gray) parasite line. GIEs were preincubated at 37°C 30 minutes with (+cAMP) or without (−cAMP) 100 µM 8Br-cAMP. Error bars denote the standard error of the mean. Outliers are shown as open circles. Stars represent significant differences in retention rates (***P < .001; **P < .01; *P < .05). ns, nonsignificant differences in retention rates.
cAMP-mediated changes in GIE deformability are linked to STEVOR expression. (A-B) Retention in microsphilters of stages III (A) and stages V (B) GIE from the B10 (dark gray) and the A12 (medium gray) wild-type clones preincubated at 37°C during 30 minutes with (+cAMP) or without (–cAMP) 100 µM 8Br-cAMP. (C-D) Retention in microsphilters of stages III (C) and stages V (D) GIE from the control (dark gray) and the Null (medium gray) parasite line. GIEs were preincubated at 37°C 30 minutes with (+cAMP) or without (−cAMP) 100 µM 8Br-cAMP. Error bars denote the standard error of the mean. Outliers are shown as open circles. Stars represent significant differences in retention rates (***P < .001; **P < .01; *P < .05). ns, nonsignificant differences in retention rates.
STEVORs are phosphorylated by PKA
The results obtained suggest that changes in cellular cAMP levels influence GIE deformability via PKA phosphorylation of STEVOR proteins. In support of this hypothesis, an in silico analysis using NetPhos 2.0 (cbs.dtu.dk/services/NetPhosK) led to the prediction of several phosphorylation sites in a consensus sequence resulting from an alignment of all members of the STEVOR family (Figure 4A). Among the several predicted phosphorylation sites that showed a high score, only serine S123, S165, and S324 residues are conserved across the entire STEVOR family. In contrast to the serine S123 and S165 residues that are localized within the N-terminal semiconserved region, the S324 residue located within the C-terminal domain is surrounded by a sequence (RKNSW) similar to the consensus sequence for PKA phosphorylation sites (R-R-X-S/T-Φ, where Φ represents a hydrophobic residue).32 We then assessed the ability of PKA to phosphorylate the sequence RKNSW in vitro. The analysis of an in vitro phosphorylation assay on STEVOR S324 peptide (RRKNSW), STEVOR A324 peptide (RRKNAW) and control Kemptide (LRRASLG) substrates demonstrated that a recombinant bovine PKA protein phosphorylated STEVOR S324 peptide with a better efficiency than the Kemptide. As expected, the phosphorylation was decreased upon addition of the PKA inhibitors KT5720 and H89 and was totally abolished after substitution of the serine by an alanine residue in the STEVOR A324 peptide (Figure 4B). In addition, the STEVOR S324 peptide was also phosphorylated by schizont extracts and phosphorylation was decreased upon addition of H89, indicating that the sequence RKNSW can be phosphorylated by human or parasite PKA in the infected cell (Figure 4C). These results indicate that STEVOR proteins can be phosphorylated by PKA at the serine residue S324 located within the C-terminal domain.
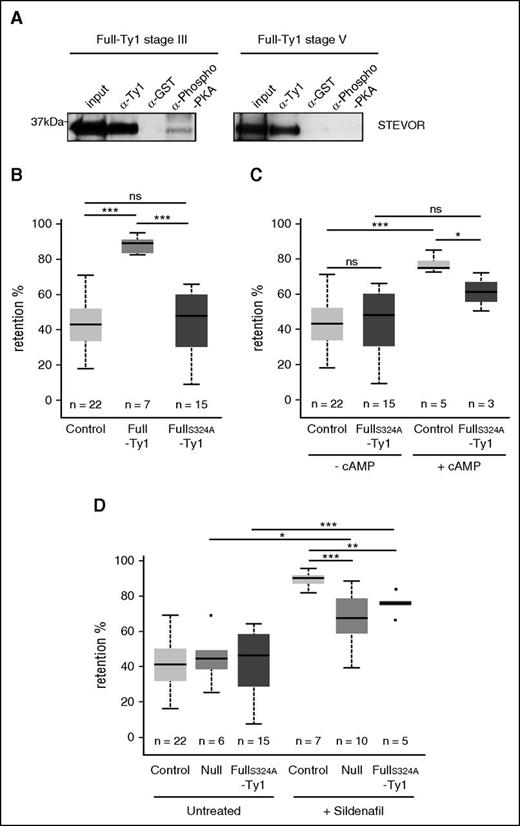
The cytoplasmic domain of STEVOR is phosphorylated by PKA. (A) Schematic representation of STEVOR proteins and alignment of flanking sequences of the 3 NetPhos-predicted serine phosphorylation sites for all members of the STEVOR family. Serine S123, S165, and S324 (in red) are numbered regarding the consensus sequence. (B) In vitro PKA phosphorylation assay for 3 peptides corresponding to a typical PKA phosphorylation substrate (Kemptide) and to the predicted-phosphorylation site on the C-terminal domain of STEVOR (Stevor S324). As a negative control, an alanine residue was substituted to the serine 324 (Stevor A324). The 3 peptides were incubated with a recombinant bovine PKA in the presence of 32[P]ATP, with or without PKA inhibitors H89 or KT5720. Phosphorylation signal (mean ± standard deviation [SD]) under different conditions is reported relative to kemptide phosphorylation from 3 independent experiments. a.u., arbitrary units. (C) In vitro PKA phosphorylation assay for the the Stevor S324 and the Stevor A324 peptides incubated with 40-hpi schizont extracts (control line) in the presence of 32[P]ATP, with or without H89. Phosphorylation signal (mean ± SD) under different conditions is reported relative to Stevor S324 peptide phosphorylation from 3 independent experiments. (D) Immunoprecipitation of 40-hpi schizont lysates from the Full-myc line with anti-myc, anti-GST, and anti-phospho-PKA antibodies. Immunoblot was probed with a rat antibody directed against STEVOR (PFC0025c). (E) Immunoprecipitation of stage III GIE lysates from the Full-Ty1 line with anti-GST and anti-phospho-PKA antibodies. Immunoblot was probed with a rabbit antibody directed against STEVOR (PFC0025c). (F) Immunoprecipitation of 40-hpi schizont lysates from the Full-myc line with anti-GST and anti-myc antibodies. Immunoblot was probed with a rabbit anti-phospho-PKA antibody.
The cytoplasmic domain of STEVOR is phosphorylated by PKA. (A) Schematic representation of STEVOR proteins and alignment of flanking sequences of the 3 NetPhos-predicted serine phosphorylation sites for all members of the STEVOR family. Serine S123, S165, and S324 (in red) are numbered regarding the consensus sequence. (B) In vitro PKA phosphorylation assay for 3 peptides corresponding to a typical PKA phosphorylation substrate (Kemptide) and to the predicted-phosphorylation site on the C-terminal domain of STEVOR (Stevor S324). As a negative control, an alanine residue was substituted to the serine 324 (Stevor A324). The 3 peptides were incubated with a recombinant bovine PKA in the presence of 32[P]ATP, with or without PKA inhibitors H89 or KT5720. Phosphorylation signal (mean ± standard deviation [SD]) under different conditions is reported relative to kemptide phosphorylation from 3 independent experiments. a.u., arbitrary units. (C) In vitro PKA phosphorylation assay for the the Stevor S324 and the Stevor A324 peptides incubated with 40-hpi schizont extracts (control line) in the presence of 32[P]ATP, with or without H89. Phosphorylation signal (mean ± SD) under different conditions is reported relative to Stevor S324 peptide phosphorylation from 3 independent experiments. (D) Immunoprecipitation of 40-hpi schizont lysates from the Full-myc line with anti-myc, anti-GST, and anti-phospho-PKA antibodies. Immunoblot was probed with a rat antibody directed against STEVOR (PFC0025c). (E) Immunoprecipitation of stage III GIE lysates from the Full-Ty1 line with anti-GST and anti-phospho-PKA antibodies. Immunoblot was probed with a rabbit antibody directed against STEVOR (PFC0025c). (F) Immunoprecipitation of 40-hpi schizont lysates from the Full-myc line with anti-GST and anti-myc antibodies. Immunoblot was probed with a rabbit anti-phospho-PKA antibody.
To determine the status of STEVOR phosphorylation by PKA in infected erythrocytes, we performed immunoprecipitation assays on schizonts and stage III GIE lysates from the Full-myc and the Full-Ty1 lines, respectively (Figure 4D-F). We used a monoclonal antibody specific for canonical phospho-PKA sites that specifically recognizes S324 phosphorylation on the Stevor-Cter domain (supplemental Figure 4). We first observed that an anti-STEVOR antibody specifically revealed a 37-kDa band among proteins immunoprecipitated with the anti-phospho-PKA antibody in both asexual and sexual stages lysates, suggesting that STEVORs are PKA substrates (Figure 4D-E). We confirmed this result by showing that a myc-tagged STEVOR protein immunoprecipitated with an anti-myc antibody can be detected with an anti-phospho PKA antibody (Figure 4F), validating that STEVOR proteins are phosphorylated by PKA in infected erythrocytes.
Phosphorylation of STEVOR cytoplasmic domain regulates immature GIE stiffness
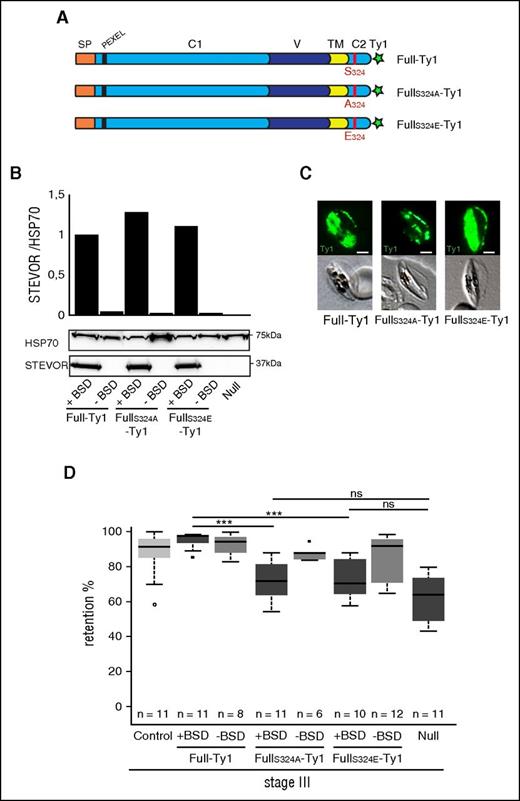
Pulldown analysis of recombinant spectrin dimer or band 3 using either the phosphorylated or unphosphorylated Stevor-Cter domain showed that phosphorylation of S324 did not impact on interaction between STEVOR cytoplasmic domain and cytoskeletal proteins in vitro (supplemental Figure 5). However, in the infected cell, these interactions may also be dependent on phosphorylation of cytoskeletal proteins and in vitro results may not reflect the impact of S324 phosphorylation on infected cell deformability. To address whether phosphorylation of the STEVOR Cter domain regulates GIE rigidity, we generated 2 transgenic lines that overexpressed Ty1-tagged STEVORS324A and Ty1-tagged STEVORS324E proteins where the serine S324 was mutated by an alanine and a glutamate residue, respectively (Figure 5A). This widely used approach allows for mimicking of an unphosphorylated and a constitutively phosphorylated serine residue of a protein.33 Immunoblotting and immunostaining of GIEs with anti-STEVOR and anti-Ty1 antibodies indicated that the transgenic parasites have a similar level of STEVOR expression (Figure 5B) and that the proteins are properly exported to the erythrocyte membrane of stage III GIE (Figure 5C). Microsphiltration analysis of FullS324A-Ty1 immature GIE showed a significant decrease in retention rates compared with Full-Ty1 (71.5% vs 95.1%, P = 0), at a level almost similar to that observed in absence of STEVOR expression in the Null line (P = .080) (Figure 5D). Removing the episomal vector by cultivation without BSD selection restored retention rates to the phenotype of the control line (87.5% vs 90.40%, P = .28), indicating that increases in deformability were specific to the overexpression of mutated STEVOR protein (Figure 5D). Although we expected that the phosphomimetic S324E mutation could trigger a similar phenotype as observed for Full-Ty1 GIE, retention rates of FullS324E-Ty1 GIE were identical to that of FullS324A-Ty1 GIE (72% vs 71.5%, P = .6). This suggests that phosphomimetic mutation of S324 failed to reproduce the changes caused to STEVOR by phosphorylation, as observed in other systems.34 However, this result strengthens the importance of mutation at STEVOR S324 in GIE rigidity. The role of the S324 residue in the stiffness phenotype was further confirmed in 22-hpi asexual parasites (supplemental Figure 6), thus validating that the deformability of infected erythrocytes is associated with mutation at STEVOR S324. Altogether, these results suggest that STEVOR-mediated rigidity of infected erythrocytes is dependent on the phosphorylation by PKA of the serine residue S324 at the C-terminal domain.
Immature GIE stiffness is dependent on STEVOR S324 phosphorylation. (A) Schematic representation of STEVOR recombinant protein overexpressed in the Full-Ty1, the FullS324A-Ty1, and the FullS324E-Ty1 lines. Red bar, The serine 324 in the Full-Ty1 line is mutated in alanine 324 in the FullS324A-Ty1 line and in glutamate 324 in the FullS324E-Ty1 line, respectively. (B) Western blot analysis of STEVOR expression in stage III GIE from the Full-Ty1, the FullS324A-Ty1, the FullS324E-Ty1 and the Null lines cultivated with (+BSD) or without (−BSD) blasticidin. Immunoblots were probed with a rabbit antibody directed against STEVOR (PFC0025c) and with a rat antibody directed against HSP70 to normalize expression. Quantitation of signal intensities was realized using Quantity One software (Bio-Rad). (C) Immunofluorescence analysis of stage III GIE from the Full-Ty1, the FullS324A-Ty1, and the FullS324E-Ty1 lines. Infected erythrocytes were stained with anti-Ty1 antibodies followed by anti-mouse Alexa 488–conjugated IgG. Pictures were taken under identical exposure conditions. The bars represent 2 µm. (D) Retention rates in microsphilters of stage III GIE from the control (light gray), the Full-Ty1, the FullS324A-Ty1, the FullS324E-Ty1, and the Null lines cultivated with (+BSD, dark gray) or without (−BSD, medium gray) BSD. Error bars denote the standard error of the mean. Outliers are shown as open circles. Stars represent highly significant differences in retention rates (***P < .001). ns, nonsignificant differences in retention rates.
Immature GIE stiffness is dependent on STEVOR S324 phosphorylation. (A) Schematic representation of STEVOR recombinant protein overexpressed in the Full-Ty1, the FullS324A-Ty1, and the FullS324E-Ty1 lines. Red bar, The serine 324 in the Full-Ty1 line is mutated in alanine 324 in the FullS324A-Ty1 line and in glutamate 324 in the FullS324E-Ty1 line, respectively. (B) Western blot analysis of STEVOR expression in stage III GIE from the Full-Ty1, the FullS324A-Ty1, the FullS324E-Ty1 and the Null lines cultivated with (+BSD) or without (−BSD) blasticidin. Immunoblots were probed with a rabbit antibody directed against STEVOR (PFC0025c) and with a rat antibody directed against HSP70 to normalize expression. Quantitation of signal intensities was realized using Quantity One software (Bio-Rad). (C) Immunofluorescence analysis of stage III GIE from the Full-Ty1, the FullS324A-Ty1, and the FullS324E-Ty1 lines. Infected erythrocytes were stained with anti-Ty1 antibodies followed by anti-mouse Alexa 488–conjugated IgG. Pictures were taken under identical exposure conditions. The bars represent 2 µm. (D) Retention rates in microsphilters of stage III GIE from the control (light gray), the Full-Ty1, the FullS324A-Ty1, the FullS324E-Ty1, and the Null lines cultivated with (+BSD, dark gray) or without (−BSD, medium gray) BSD. Error bars denote the standard error of the mean. Outliers are shown as open circles. Stars represent highly significant differences in retention rates (***P < .001). ns, nonsignificant differences in retention rates.
The switch in deformability in mature stages occurs upon STEVOR dephosphorylation
The mechanism underlying the switch in deformability from immature to mature stages depends on a drop in cellular cAMP concentration and reduced PKA phosphorylation of membrane proteins.24 To determine the status of STEVOR phosphorylation by PKA in mature GIE, we performed an immunoprecipitation on stage III and stage V GIE lysates from the Full-Ty1 line using a phospho-PKA antibody (Figure 6A). Although an anti-STEVOR antibody revealed a 37-kDa band in phospho-PKA immunoprecipitates from immature GIE lysates, STEVOR was not detected in phospho-PKA immunoprecipitates from mature GIE lysates. These results indicate that STEVORs are phosphorylated by PKA in stiff immature GIE but not in deformable mature stages.
The switch in deformability in mature stages occurs upon STEVOR S324 dephosphorylation. (A) Immunoprecipitation of stage III and stage V GIE lysates from the Full-Ty1 line with anti-Ty1, anti-GST, and anti-phospho-PKA antibodies. Immunoblots were probed with a rabbit antibody directed against STEVOR (PFC0025c). (B) Retention rates in microsphilters of stage V GIE from the control (light gray), the Full-Ty1 (medium gray), and the FullS324A-Ty1 (dark gray) lines. (C) Retention rates in microsphilters of stage V GIE from the control (light gray) and the FullS324A-Ty1 (dark gray) lines preincubated at 37°C 30 minutes with (+cAMP) or without (−cAMP) 100 µM 8Br-cAMP. (D) Retention rates in microsphilters of stage V GIE from the control (light gray), the Null (medium gray), and the FullS324A-Ty1 (dark gray) lines preincubated at 37°C 30 minutes with (+Sildenafil) or without (Untreated) 100 µM sildenafil citrate. Error bars denote the standard error of the mean. Outliers are shown as open circles. Stars represent significant differences in retention rates (***P < .001; **P < .01; *P < .05). ns, nonsignificant differences in retention rates.
The switch in deformability in mature stages occurs upon STEVOR S324 dephosphorylation. (A) Immunoprecipitation of stage III and stage V GIE lysates from the Full-Ty1 line with anti-Ty1, anti-GST, and anti-phospho-PKA antibodies. Immunoblots were probed with a rabbit antibody directed against STEVOR (PFC0025c). (B) Retention rates in microsphilters of stage V GIE from the control (light gray), the Full-Ty1 (medium gray), and the FullS324A-Ty1 (dark gray) lines. (C) Retention rates in microsphilters of stage V GIE from the control (light gray) and the FullS324A-Ty1 (dark gray) lines preincubated at 37°C 30 minutes with (+cAMP) or without (−cAMP) 100 µM 8Br-cAMP. (D) Retention rates in microsphilters of stage V GIE from the control (light gray), the Null (medium gray), and the FullS324A-Ty1 (dark gray) lines preincubated at 37°C 30 minutes with (+Sildenafil) or without (Untreated) 100 µM sildenafil citrate. Error bars denote the standard error of the mean. Outliers are shown as open circles. Stars represent significant differences in retention rates (***P < .001; **P < .01; *P < .05). ns, nonsignificant differences in retention rates.
To address whether dephosphorylation of S324 in stage V GIE contributes to the increase in deformability of mature GIE, we measured the retention rate of stage V from the Full-Ty1 and the FullS324A-Ty1 lines. We first confirmed our previous results showing that STEVOR overexpression impaired the switch in deformability observed in wild-type parasites,6 with retention rates significantly higher in the Full-Ty1 than in the control line (86.4% vs 41% P = 0) (Figure 6B). Importantly, mutation of S324 in the FullS324A-Ty1 line restored the deformable phenotype of mature GIE to levels similar to that of the control line (41.8% vs 41%, P = .66) (Figure 6B). In addition, incubation of the FullS324A-Ty1 line with 8Br-cAMP did not significantly impair the filterability of mature GIE as observed in the control line (59.5% vs 76.7%, P = .03) (Figure 6C). These results confirm that the switch in GIE deformability is dependent on STEVOR C-terminal domain dephosphorylation at the S324 residue in mature stages.
Sildenafil-mediated changes of GIE deformability are partially linked to STEVOR S324 phosphorylation
We have previously demonstrated that sildenafil increases cAMP cellular levels and impair filterability in mature GIE.24 To address whether these effects are associated to STEVOR phosphorylation, we measured filterability of mature GIE from the control, Null, and FullS324A-Ty1 lines upon incubation with 100 µM sildenafil citrate (Figure 6D). First, we confirmed that incubation of the control line with sildenafil drastically impairs the filterability of mature GIE (89.5% vs 41%, P = 0). Importantly, the retention rates of control parasites upon sildenafil treatment were significantly higher than that of the sildenafil-treated Null and FullS324A -Ty1 lines (89.5% vs 66.7% and 75.8%, P = .0001 and .005), indicating that STEVOR phosphorylation contributes to the sildenafil-mediated stiffness phenotype. However, the filterability of sildenafil-treated Null and FullS324A-Ty1 lines was significantly affected compared with untreated stage V GIEs (66.7% and 75.8% vs 45.4% and 41.8%, P = .034 and 0), suggesting that sildenafil also increases mature GIE stiffness via a STEVOR-independent pathway. These results suggest that sildenafil has an effect on GIE deformability not only by modifying the STEVORs phosphorylation status via cAMP signaling pathway but also via an independent unknown pathway.
Discussion
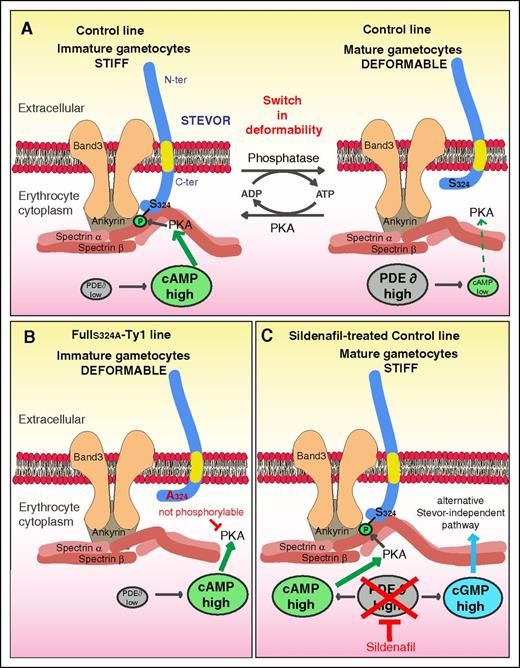
The ability of P falciparum mature GIEs to persist for several days into the peripheral circulation and pass through splenic slits depends on their extreme deformability. In this study, we investigate the mechanisms by which STEVORs exert changes on the mechanical properties of infected erythrocytes. We demonstrate that STEVORs interact with the cytoskeletal ankyrin complex and that GIE deformability depends on PKA-mediated phosphorylation of the STEVOR cytoplasmic tail at a specific serine residue (S324) in both asexual and gametocyte stages. Our results raise a new model where the stiffness of immature GIEs depends on the interaction between the phosphorylated STEVOR Cter domain and 1 or several proteins within the ankyrin complex (Figure 7). According to this model, the drop of cAMP level in mature GIE may reduce the phosphorylation of both STEVORs and their cytoskeletal partner(s) and consequently weaken or abolish their interactions, thereby increasing the deformability of infected erythrocytes. These data decipher how chemical compounds such as sildenafil impair GIE filterability, which opens new perspectives in the drug-induced blockade of malaria transmission.
Model for STEVOR phosphorylation-mediated regulation of GIE deformability. (A) Regulation of deformability in immature and mature GIE from the control line. (B) Deformable phenotype in immature GIE from the FullS324-Ty1 line. (C) Stiff phenotype in sildenfil-treated mature GIE from the control line.
Model for STEVOR phosphorylation-mediated regulation of GIE deformability. (A) Regulation of deformability in immature and mature GIE from the control line. (B) Deformable phenotype in immature GIE from the FullS324-Ty1 line. (C) Stiff phenotype in sildenfil-treated mature GIE from the control line.
In response to external forces, erythrocytes are able to maintain their shape, stability, and deformability.35 This remarkable feature relies on the dynamic rearrangement of the erythrocyte membrane and cytoskeleton. The cytoskeleton is a hexagonal lattice made of spectrin tetramers (αβ)2 tied by actin, protein 4.1R, and other proteins, and is tethered to the fluid lipid bilayer via transmembrane proteins such as band 3, glycoproteins, and glycophorins. These integral membrane proteins have a lateral mobility allowing the skeleton and the bilayer to slide against each other.35,36 Maintenance of the physiological deformability of the erythrocyte membrane is highly dependent on interactions of cytoskeletal proteins with different integral membrane proteins, such as the interaction of ankyrin and β-spectrin with band 3 within the ankyrin complex.37-40 STEVOR binding to spectrin or other components of the ankyrin complex might interfere with these interactions and impede translational diffusion of transmembrane proteins.36,41 Such interactions could conceivably render the membrane more rigid. In erythrocytes infected with P falciparum asexual stages, the transmembrane protein PfEMP1 is anchored to the skeleton via binding to KAHRP.42-44 KAHRP associates with both ankyrin and spectrin within the ankyrin complex and may cross-link spectrin, resulting in increased membrane rigidity.10,11,45,46 The rise in the knob density in mature asexual stages results in the increased number of vertical constraints between the spectrin network and the lipid bilayer, which further stiffens the membrane.47 In GIEs, KAHRP and PfEMP1 are not expressed48,49 and STEVORs may perform an analogous role by increasing vertical constraints between the cytoskeleton and the lipid bilayer.
Protein-protein interactions essential for the erythrocyte membrane mechanical properties are modulated by posttranscriptional modifications of either cytoskeleton or parasite proteins.50 For instance, the phosphorylation of cytoskeletal proteins by PKA or other kinases decreases the membrane stability.51-53 We propose that PKA-mediated phosphorylation of STEVOR S324 may change the conformation of the STEVOR C-terminal domain and increase its interaction with the skeleton partner, thus increasing stiffness of the membrane. These interactions are also likely dependent on phosphorylation or other posttranslational modifications of host membrane proteins. Regulation of STEVOR phosphorylation status may allow the parasite to adapt GIE deformability to mechanical constraints encountered in the bone marrow parenchyma or in the peripheral circulation. It remains hypothetical whether P falciparum PKA subunits are exported into the erythrocyte membrane to phosphorylate STEVORs because they both lack a recognizable secretion signature. However, it is plausible that the parasite hijacks human PKA activity to promote STEVOR phosphorylation and increase erythrocyte stiffness.
Our data confirm that sildenafil renders transmissible mature gametocytes rigid and less likely to circulate through the spleen. This increase in rigidity is dependent on STEVOR S324 phosphorylation, suggesting that sildenafil interferes with STEVOR phosphorylation status by raising cAMP levels. However, we establish that a STEVOR-independent unknown pathway also contributes to the sildenafil-mediated stiffness phenotype. This pathway may be related to cyclic guanosine monophosphate (cGMP) signaling because GIE deformability is also regulated by cGMP levels that can be raised upon sildenafil treatment.24 The absence of any cAMP-mediated increase in stiffness in parasites that do not express STEVOR rules out a potential crosstalk between cGMP and cAMP signaling. One could hypothesize that the sildenafil-mediated stiffness phenotype may be triggered by cGMP-mediated posttranslational modifications of cytoskeleton proteins constituting the ankyrin complex. It would be of interest to identify the actors involved in this pathway to improve our understanding of GIE deformability regulation mechanisms.
Altogether, our study deciphers a mechanism that might be crucial for sequestration of immature gametocytes in the bone marrow and for persistence of mature gametocytes in blood circulation. Moreover, it provides new insights into the mechanism by which PDE inhibitors may block malaria parasite transmission.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kirk Deitsch and Artur Scherf for providing the pSBH and the pLN-Ty1C plasmids, respectively. The authors thank Emmanuel Bischoff for his support with statistical analysis. The authors thank François Guilloneau and Cédric Broussard from the Mass Spectrometry facility 3P5, Institut Cochin for technical assistance and support with data analysis.
C.L., B.N., F.D., A.L., and Y.D. acknowledge the financial support from the Fonds Inkermann, the Centre National de la Recherche Scientifique (CNRS; ATIP-Avenir grant), and the Bill & Melinda Gates Foundation (grant OPP1043892). C.L. acknowledges INSERM, CNRS, and the Labex Gr-Ex.
Authorship
Contribution: B.N., F.D., Y.D., A.L., J.D., and J.S. performed experiments; B.N., F.D., A.M., and C.L. analyzed data and performed statistical analysis; P.B., A.B., and A.M. contributed new reagents or analytical tools; B.N. and C.L. designed the research; and B.N. and C.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Catherine Lavazec, INSERM U1016, Institut Cochin, 22 Rue Méchain, 75015 Paris, France; e-mail: catherine.lavazec@inserm.fr.
References
Author notes
B.N. and F.D. contributed equally.

![Figure 4. The cytoplasmic domain of STEVOR is phosphorylated by PKA. (A) Schematic representation of STEVOR proteins and alignment of flanking sequences of the 3 NetPhos-predicted serine phosphorylation sites for all members of the STEVOR family. Serine S123, S165, and S324 (in red) are numbered regarding the consensus sequence. (B) In vitro PKA phosphorylation assay for 3 peptides corresponding to a typical PKA phosphorylation substrate (Kemptide) and to the predicted-phosphorylation site on the C-terminal domain of STEVOR (Stevor S324). As a negative control, an alanine residue was substituted to the serine 324 (Stevor A324). The 3 peptides were incubated with a recombinant bovine PKA in the presence of 32[P]ATP, with or without PKA inhibitors H89 or KT5720. Phosphorylation signal (mean ± standard deviation [SD]) under different conditions is reported relative to kemptide phosphorylation from 3 independent experiments. a.u., arbitrary units. (C) In vitro PKA phosphorylation assay for the the Stevor S324 and the Stevor A324 peptides incubated with 40-hpi schizont extracts (control line) in the presence of 32[P]ATP, with or without H89. Phosphorylation signal (mean ± SD) under different conditions is reported relative to Stevor S324 peptide phosphorylation from 3 independent experiments. (D) Immunoprecipitation of 40-hpi schizont lysates from the Full-myc line with anti-myc, anti-GST, and anti-phospho-PKA antibodies. Immunoblot was probed with a rat antibody directed against STEVOR (PFC0025c). (E) Immunoprecipitation of stage III GIE lysates from the Full-Ty1 line with anti-GST and anti-phospho-PKA antibodies. Immunoblot was probed with a rabbit antibody directed against STEVOR (PFC0025c). (F) Immunoprecipitation of 40-hpi schizont lysates from the Full-myc line with anti-GST and anti-myc antibodies. Immunoblot was probed with a rabbit anti-phospho-PKA antibody.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/24/10.1182_blood-2016-01-690776/4/m_e42f4.jpeg?Expires=1769103276&Signature=RXfdIrZf5O-hU-mKuCsdDQZLbhbdAM4Bmno5zD8dDo6blsGh5kaLDFoEJBkur0l43SBI-~b6ZyXS2x1I3fZVhXa2xeaq1dWhOkE26zVascXtNSCVM80CNcjTAuBBQGHyL4Ybcl6fvPHlHnoUtogw2xRKt9BG97X6e5BmgpiPhbZ0m7MnE0eHN9jSLLw8nayrxVZfA8URG9wtlDkL4VWdmIJI7HxcawMoiT5IcQSav2EMpEpqeCTugK05RHhP6oD-cqRaw3KR3ciTAPEyOSaK--40LqRXBtFgecbHZ4VYfV6HfwS0MFtLIvvbLK4tRa7erfzfxM6RJW0HV3sfYzSL7w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal