Key Points
GPR56 is a novel LSC marker for the majority of AML samples.
GPR56 expression levels correlate with genetic risk groups and clinical outcome in AML.
Abstract
Acute myeloid leukemia (AML) is a genetically heterogeneous hematologic malignancy, which is initiated and driven by a rare fraction of leukemia stem cells (LSCs). Despite the difficulties of identifying a common LSC phenotype, there is increasing evidence that high expression of stem cell gene signatures is associated with poor clinical outcome. Identification of functionally distinct subpopulations in this disease is therefore crucial to dissecting the molecular machinery underlying LSC self-renewal. Here, we combined next-generation sequencing technology with in vivo assessment of LSC frequencies and identified the adhesion G protein–coupled receptor 56 (GPR56) as a novel and stable marker for human LSCs for the majority of AML samples. High GPR56 expression was significantly associated with high-risk genetic subgroups and poor outcome. Analysis of GPR56 in combination with CD34 expression revealed engraftment potential of GPR56+ cells in both the CD34− and CD34+ fractions, thus defining a novel LSC compartment independent of the CD34+CD38− LSC phenotype.
Introduction
Characterization of the cell surface phenotype of normal human hematopoietic stem cells (HSCs) has been very successful, and purity greater than 1 in 10 cells can now be achieved.1 In contrast, acute myeloid leukemia (AML) stem cell phenotypes2,3 remain poorly defined and show enormous variability between specimens and genetic subgroups,4,5 thus compromising the isolation of leukemia stem cells (LSCs), which is the basis for developing LSC-eradicating therapies. Low LSC frequencies in currently available mouse models4,6 and rapid onset of differentiation upon ex vivo manipulation7 further limit ex vivo studies using primary patient material. Inability to identify surface markers to highly purify these cells therefore led to questioning of the existence of a consistent LSC surface marker for this disease. Most of the recent studies reporting novel LSC markers have extended the original definition of an LSC marker to cell surface antigens expressed preferentially on leukemic cells, but not on normal HSCs and progenitors to identify potential therapeutic targets or markers of minimal residual disease.8-10 Most of these markers have not been tested for their capacity to discriminate within 1 sample subpopulation with high repopulating capacity vs those lacking this stem cell–like feature using in vivo assays,4,5,8,10 so that the CD34+CD38− phenotype remains the most intensely studied LSC phenotype to date.2,11 However, development of more permissive immunodeficient mouse models revealed engraftment potential of leukemic populations that were previously considered devoid of LSC activity, such as the CD34low/− and CD38+ compartments,4,5 indicating that the LSC phenotype might not be uniform in different genetic AML subgroups. More recently, CD93 was identified as a novel LSC marker for MLL rearranged AML samples further supporting this hypothesis.12 Despite the phenotypic heterogeneity among AML samples, there is strong evidence that engraftment capacity in immunocompromised mice is highly associated with clinical outcome in adult and pediatric patients,13,14 emphasizing the need for better characterization of this functionally distinct subpopulation. Here we describe an approach in which deep sequencing technology was used in combination with functional in vivo experiments and mutational analyses to explore the relationship between genetic background, gene expression, and the frequency of LSCs in AML. With this integrative approach, we identified the surface protein GPR56 as a novel LSC marker independent of the CD34+CD38− LSC phenotype and linked its expression to genetic groups and clinical outcome.
Materials and methods
Patient samples
Specimens from adult AML patients were analyzed and cryopreserved at the Quebec Leukemia Cell Bank at Maisonneuve-Rosement Hospital, Montreal, after obtaining written informed consent from all patients. The Research Ethics Boards of Maisonneuve-Rosemont Hospital, Centre Hospitalier Universitaire de Québec, and University of Montreal approved the project. Patient and specimen characteristics of samples tested for LSC frequency are provided in supplemental Table 1, available on the Blood Web site. Human blood samples used for RNA-Sequencing (RNA-Seq) were collected by Hema-Quebec after obtaining written informed consent from all healthy volunteers and approval by the Research Ethics Board of Sainte-Justine Hospital and the University of Montreal. Cord blood CD34+ cells used to genetically engineer leukemia were obtained at the time of cesarean delivery of healthy, full-term infants, with consent according to procedures approved by the Research Ethics Board of the University of British Columbia.
Cell culture
For in vitro studies, AML cells were plated in 384-well plates (Greiner, 781182) at a density of 5000 cells in 50 µL volume per well. For in vivo experiments AML cells were seeded in T25 culture flasks (Sarstedt) at a density of 0.5 × 106 cells/mL before sorting and transplantation. Engineered NUP98-HOXD13 fusion gene (ND13)+MN1 CD34+ cord blood cells were generated and cultured as described.15 A total of 10 000 sorted cells was plated to compare the progeny generated by each fraction.
Cell lines
HEK293T and KG1a cell lines were purchased from ATCC and cultured in Dulbecco’s modified Eagle medium containing 10% heat inactivated fetal bovine serum.
Flow cytometry and cell sorting
Flow cytometry was performed on a BD LSR II cytometer equipped with an HTS device or on a BD Canto II cytometer (BD Bioscience), and results were analyzed with BD fluorescence-activated cell sorter (FACS) Diva 4.1 and FlowJo X softwares. Cell sorting was performed under sterile conditions on a BD Aria II cell sorter.
The following FACS antibodies were used: anti-human CD45 Pacific Blue (BioLegend 304029), CD45 fluorescein isothiocyanate (FITC; BioLegend 304006), CD33 phycoerythrin (PE; BD Bioscience 555450), CD33 BV421 (BD 562854), CD34 antigen-presenting cell (APC; BD Bioscience 555824), CD34 APC (Stem Cell Technologies 10613), CD3 FITC (BD Bioscience 555332), CD4 APC-Cy7 (BD 560158), CD8 APC (BD 555369), CD3 PE-Cy5 (BD 555334), CD19 PE-Cy7 (BD Bioscience 557835), GPR56 PE (BioLegend 358204; see supplemental Figure 1 and supplemental Methods for antibody validation), CD96 PE (BioLegend 338406), CD47 BV421 (BD 563760), TIM3 PE (R&D Systems, FAB2365P), CLL1 FITC (BioLegend 353608), CD44 PE (BD 550989), CD123 BV421 (BD 562517), and anti-mouse CD45.1 APC-efluor 730 (eBioscience 47-0453-82).
Xenotransplantation assays
NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice purchased from Jackson Laboratory were bred in a specific pathogen-free animal facility at the Institute for Research in Immunology and Cancer and CHU de Quebec. All experiments involving animals followed the recommendations of the Canadian Council on Animal Care and were approved by the Deontology Committee on Animal Experimentation at University of Montreal and University Laval. NSG mice were used for all experiments involving primary human AML samples. NSG mice transgenic for human interleukin-3, granulocyte macrophage colony-stimulating factor, and Steel factor (NSG-3GS)16 were bred in the animal resource center at the British Columbia Cancer Research Centre and experiments were carried out in accordance with Canadian Council on Animal Care guidelines with approval from the University of British Columbia. NSG-3GS mice were only used for transplantation of genetically engineered CD34+ cord blood cells.
Compounds
Stem Regenin1 (SR1) was purchased from Alichem (41864). UM729 was synthesized at the Medicinal Chemistry Core Facility at the Institute for Research in Immunology and Cancer. All powders were dissolved in dimethylsulfoxide (DMSO) and diluted in culture medium immediately before use to obtain a final concentration of 500 nM for SR1 and UM729. Final DMSO concentration was 0.1% in the in vitro experiments and 0.01% in the in vivo experiments.
RNA isolation
RNA was isolated from primary AML cells using Trizol reagent according to the manufacturer´s instructions (Invitrogen/Life Technologies), with an additional purification on RNeasy mini columns (Qiagen) to obtain high-quality RNA.
Exome and RNA-Seq
Libraries were constructed with the TruSeq DNA and RNA Sample Preparation Kits and exons were captured using TruSeq Exome Enrichment Kit (Illumina). Sequencing was performed using an Illumina HiSequation 2000. Sequence data were mapped to the reference genome hg19 using the Illumina Casava 1.8.2 package and Elandv2 mapping software according to the RefSeq database (University of California, Santa Cruz, April 16, 2014).
Publicly available RNA-Seq and microarray datasets
RNA-Seq data from 179 primary human AML samples were downloaded from The Cancer Genome Atlas (http://cancergenome.nih.gov). Sample and patient characteristics as published in the Cancer Genome Atlas Research Network work17 were used for sample annotation. Microarray and clinical data for 461 AML patients by Verhaak et al18 were available through www.leukemia-gene-atlas.org.19
Statistical analysis
Statistical analyses were done with GraphPad Prism v 6.01 and R. Mann-Whitney U tests were performed for comparison of 2 unpaired variables and Wilcoxon matched pairs signed-rank test for paired variables, when normal distribution could not be confirmed. Bars and error bars indicate means and standard deviations if not otherwise specified in figure legends. LSC frequencies with 95% confidence intervals (CI) were estimated with Extreme Limiting Dilution Analysis software (http://bioinf.wehi.edu.au/software/elda/) and significant differences in LSC frequency were calculated by χ2 test.20 In cases where all mice were positive or negative, a hypothetical positive or negative mouse was added to allow grouping into high, medium, and low LSC frequencies based on the mean estimate value. Fisher’s exact test was used to analyze sample term enrichment in subsets of AML samples. Survival analyses were done with the survival package for R. Log-rank test was used to determine significant differences in survival between 2 groups. Hazard ratios (HR) and 95% CI were calculated with the Mantel-Haenzsel method. P values < .05 were considered significant.
Results
Identification of LSC-associated genes
To identify genes associated with LSC activity in human AML, we determined LSC frequencies of 56 mainly normal karyotype AML specimens in NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mice through limiting dilution analyses (Figure 1A, Table 1, and supplemental Table 1 for specimen characteristics). We identified 10 samples with high engraftment levels in all mice injected with the lowest cell number (LSC frequencies >1 in 32 000) and 25 samples with no or very low engraftment at highest dose (LSC frequencies <1 in 3 × 106), hereafter called LSChigh and LSClow samples, respectively (supplemental Table 2). Our results confirmed a significant association between high LSC frequency and poor survival (P = .02, Figure 1B). There was no difference in age, sex, white blood cell count, percentage of blasts, and proportion of normal karyotype and FLT3-ITD–positive samples between the groups, but a higher number of samples with TP53 aberration in the LSChigh group (supplemental Figure 2A, supplemental Table 3). Through high-resolution transcriptome sequencing of these 56 specimens, we identified a set of genes significantly differentially expressed in LSChigh versus LSClow samples (supplemental Table 4). Genes more highly expressed in LSChigh samples were significantly enriched for published LSC and HSC gene sets (supplemental Figure 2B, supplemental Table 5) and comprised genes with known function in normal HSCs such as GATA2, DNMT3A, and DNMT3B.21,22 Conversely, genes downregulated in LSChigh samples were associated with granulocytic and monocytic differentiation such as CEBPD, CD14, IRF8, FCGR2A, FCER1G, and FCGRT. Ranking of genes from highest to lowest LSChigh/LSClow ratios revealed the adhesion G-protein–coupled receptor 56 (GPR56) as the most discriminatory candidate (6.5-fold difference, P = .0007, Figure 1C) with a mean reads per kilobase per million mapped reads (RPKM) expression value of 46 in LSChigh vs 7 in LSClow samples. GPR56 was also expressed in normal T cells and CD34+ subpopulations, with higher expression in the more immature CD34+CD45RA− compartment compared with committed CD34+CD33+ myeloid progenitors, but was absent in mature cells of the myeloid lineage and in B cells (Figure 1D). We observed no difference in the distribution of GPR56 transcript variants and resulting isoforms between leukemic and healthy cell populations (supplemental Figure 2C). Furthermore, we found that genes with known function in HSCs and LSCs such as MSI223 and DNMT3B,21 and the drug efflux transporters ABCG1, ABCC1, and ABCA2 correlated with GPR56 expression (Figure 1E; supplemental Table 6). To test whether proportions of positive cells determined by flow cytometry also correlated with LSC frequencies we stained 45 AML samples with antibodies against GPR56, CD34, and other described LSC markers. We observed a strong correlation between GPR56 gene expression and the percentage of positive cells determined by flow cytometry (Figure 1F) similar to what we observed for CD34 (supplemental Figure 2D; see supplemental Figure 1 and the supplemental Methods section for detailed antibody validation), suggesting that GPR56 might represent a novel LSC marker. Whereas CD34 alone did not correlate with LSC frequencies, proportions of GPR56+, CD34+GPR56+, and GPR56+ cells within the CD34+ compartment were significantly different in LSChigh vs LSClow samples in line with our gene expression analysis (Figure 1G). Gene expression levels of CD4710 were significantly higher in LSChigh compared with LSClow samples, but flow cytometry analysis revealed that >95% of total and of CD34+ blasts were CD47+ independent of LSC frequencies (supplemental Figure 3). CLL124 expression inversely correlated with LSC frequency at the gene and protein expression levels. All other tested LSC markers (TIM3, CD96, CD44, CD123)8,9,25-27 did not correlate with LSC frequency in our sample collection (supplemental Figure 3). Together, GPR56 appeared to be the most promising marker to identify subpopulations of primary human AML cells with different stem cell–like properties.
Identification of LSC-associated genes by RNA-Seq and in vivo data. (A) Experimental design (see our “Materials and methods” section for details). i.f., intrafemoral. (B) High LSC frequency is associated with poor survival (median overall survival 143 vs 369 days, P = .02 (log-rank), HR 3.2 (95% CI, 1.2-8.7), n = 56. (C, left) Average gene expression in LSChigh vs LSClow samples (log(RPKM+0.001) transformed). GPR56 is highlighted in red. (Right) Volcano plot displaying the average fold difference in gene expression in LSChigh (n = 10) vs LSClow samples (n = 26) for all genes with an average log10(RPKM+0.001) ≥1 in LSChigh samples in relation to the P value for each gene. P values were transformed as –log10 (P value). Candidate LSC genes are shown in red (criteria: average (log10(RPKM) in LSChigh samples ≥1, average ratio LSChigh/LSClow ≥2, P < .01 [Mann-Whitney U test]). Font and dot size indicate the average expression level of the genes in LSChigh samples. (D) GPR56 gene expression in hematopoietic cell populations. (E) Waterfall plot displaying correlation coefficients for all genes correlated to GPR56 based on RNA-Seq data from 437 AML samples. Highlighted in red are selected genes positively correlating with GPR56. Blue-annotated genes inversely correlate with GPR56. (F) Correlation between GPR56 gene expression by RPKM and percentage of positive cells measured by flow cytometry in 45 AML samples (log-scale). (G) Fractions of GPR56 and CD34+ subpopulations determined by flow cytometry in samples with high, medium, and low LSC frequency. Box-and-whisker plots displaying median, lower (Q1) and upper (Q3) quartile, minimum and maximum without outliers, and outliers identified as below Q1 − 1.5 (IQR) or above Q3 + 1.5 (IQR) (Tukey method). IQR, interquartile range = Q3-Q1.
Identification of LSC-associated genes by RNA-Seq and in vivo data. (A) Experimental design (see our “Materials and methods” section for details). i.f., intrafemoral. (B) High LSC frequency is associated with poor survival (median overall survival 143 vs 369 days, P = .02 (log-rank), HR 3.2 (95% CI, 1.2-8.7), n = 56. (C, left) Average gene expression in LSChigh vs LSClow samples (log(RPKM+0.001) transformed). GPR56 is highlighted in red. (Right) Volcano plot displaying the average fold difference in gene expression in LSChigh (n = 10) vs LSClow samples (n = 26) for all genes with an average log10(RPKM+0.001) ≥1 in LSChigh samples in relation to the P value for each gene. P values were transformed as –log10 (P value). Candidate LSC genes are shown in red (criteria: average (log10(RPKM) in LSChigh samples ≥1, average ratio LSChigh/LSClow ≥2, P < .01 [Mann-Whitney U test]). Font and dot size indicate the average expression level of the genes in LSChigh samples. (D) GPR56 gene expression in hematopoietic cell populations. (E) Waterfall plot displaying correlation coefficients for all genes correlated to GPR56 based on RNA-Seq data from 437 AML samples. Highlighted in red are selected genes positively correlating with GPR56. Blue-annotated genes inversely correlate with GPR56. (F) Correlation between GPR56 gene expression by RPKM and percentage of positive cells measured by flow cytometry in 45 AML samples (log-scale). (G) Fractions of GPR56 and CD34+ subpopulations determined by flow cytometry in samples with high, medium, and low LSC frequency. Box-and-whisker plots displaying median, lower (Q1) and upper (Q3) quartile, minimum and maximum without outliers, and outliers identified as below Q1 − 1.5 (IQR) or above Q3 + 1.5 (IQR) (Tukey method). IQR, interquartile range = Q3-Q1.
Sample characteristics of 56 primary AML samples
| ID . | Karyotype . | Molecular genetic aberrations . | FAB . | GPR56 RPKM . | GPR56+ % (FACS) . |
|---|---|---|---|---|---|
| 02H053 | 46,XY[20] | NPM1, FLT3-ITD, IDH2 | M1 | 0.94 | NA |
| 03H041 | 46,XX[22] | FLT3-ITD, NUP98-NSD1 fusion | M5 | 0.86 | 1.69 |
| 03H116 | 46,XX[21] | NPM1, FLT3-ITD, IDH2 | M1 | 7.69 | 3.73 |
| 03H119 | 46,XY[20] | RUNX1 | M1 | 3.04 | 3.02 |
| 04H024 | 46,XX[21] | NPM1, IDH2, DNMT3A (other) | M1 | 6.87 | 20.98 |
| 04H112 | 46,XX[21] | NPM1, FLT3-ITD, GATA2, DNMT3A (R882H) | M1 | 161.28 | 91.03 |
| 04H133 | 46,XX[20] | NPM1, FLT3-ITD, DNMT3A (other) | M1 | 143.72 | 84.95 |
| 05H050 | 46,XY[20] | FLT3-ITD, WT1, DNMT3A (R882H), MLL-PTD | M4 | 2.36 | 3.11 |
| 05H094 | 46,XY[23] | NPM1, TET2 | M5B | 0.90 | 1.55 |
| 05H149 | 46,XY[20] | IDH2, RUNX1, DNMT3A (R882H) | NC | 12.23 | 20.02 |
| 05H163 | 46,XY[22] | M1 | 6.35 | 20.17 | |
| 05H181 | 46,XX,inc[11] | NPM1, FLT3-ITD | M5B | 1.36 | NA |
| 06H028 | 46,XX[20] | NPM1, TET2, GATA2 | M1 | 1.34 | 1.62 |
| 06H088 | 46,XY,t(6;11)(q27;q23)[20] | M1 | 5.82 | 4.43 | |
| 06H135 | Complex karyotype | TP53 | M0 | 2.33 | NA |
| 06H144 | 46,XX[20] | NPM1, IDH2, DNMT3A (other) | M1 | 1.39 | NA |
| 07H099 | 46,XX,inv(16)(p13.1q22)[20] | NC | 1.02 | NA | |
| 07H135 | 46,XY[20] | NPM1, FLT3-ITD, IDH2, DNMT3A (other) | M1 | 37.56 | 91.48 |
| 08H012 | 47,XX,+11[21] | FLT3-ITD, IDH2, DNMT3A (other), MLL-PTD | M1 | 8.34 | 42.15 |
| 08H021 | 46,XY,t(9;11)(p22;q23)[20] | M5A | 0.54 | 0.46 | |
| 08H048 | 46,XY[21] | WT1 | M1 | 0.75 | NA |
| 08H112 | 46,XY[20] | IDH2, MLL-PTD | NC | 10.87 | 10.34 |
| 09H031 | 46,XX[20] | NPM1, FLT3-ITD | M1 | 16.34 | 78.73 |
| 09H043 | 46,XY[21] | NPM1, FLT3-ITD, DNMT3A (L566*) | M1 | 67.35 | 90.97 |
| 09H046 | 45,XY,add(16)(p13.1),-17[20] | TP53, TET2, RUNX1, DNMT3A (other) | NC | 11.81 | 14.97 |
| 09H083 | 46,XX[20] | NPM1, FLT3-ITD, IDH2, DNMT3A (R882H) | M1 | 40.04 | 84.19 |
| 09H113 | 46,XY[22] | IDH2, DNMT3A (other), MLL-PTD | M1 | 23.58 | 40.53 |
| 09H115 | 46,XY[24] | FLT3-ITD, IDH2, MLL-PTD | M1 | 1.90 | 3.51 |
| 10H031 | 46,XX[27] | MLL-MLLT4 (by FISH and RNA-Seq) | M5B | 0.45 | 0.29 |
| 10H038 | 46,XX[20] | WT1 | M0 | 5.52 | 31.86 |
| 10H056 | 46,XX[18] | IDH1, DNMT3A (other) | M1 | 6.21 | 9.53 |
| 10H072 | 46,XY[20] | NPM1, TET2, DNMT3A (R882C) | M5B | 0.91 | 1.44 |
| 10H089 | 46,XX[26] | WT1 | NC | 1.32 | NA |
| 10H092 | 46,XX[21] | NPM1, FLT3-ITD, DNMT3A (R882C) | M1 | 67.18 | 97.01 |
| 10H101 | 46,XX[22] | NPM1, FLT3-ITD, DNMT3A (R882H) | M2 | 47.97 | 83.44 |
| 10H109 | 45,XX,der(7)?t(7;18)(p12;q12),-18[17]/46,XX[3] | NPM1, TET2 | M1 | 1.72 | NA |
| 10H113 | Complex karyotype | TP53, IDH2 | M1 | 7.32 | 28.35 |
| 10H115 | 46,XY[23] | NPM1, FLT3-ITD, IDH2, WT1 | M1 | 4.65 | 6.88 |
| 10H127 | 46,XY,t(9;11)(p22;q23)[20] | M5A | 0.15 | 0.15 | |
| 10H161 | 47,XX,+8[9]/46,XX[4] | NPM1, TET2, DNMT3A (R882C) | M5B | 0.13 | 0.76 |
| 10H166 | 46,XY[20] | NPM1, FLT3-ITD, IDH2, DNMT3A (R882H) | NC | 41.00 | 69.93 |
| 11H006 | 46,XX[23] | NPM1, IDH1, DNMT3A (R882H) | M5A | 1.04 | 14.21 |
| 11H008 | 48,XY,+13,+15[3]/46,XY[14] | FLT3-ITD, MLL-PTD | NC | 6.37 | 5.59 |
| 11H009 | 46,XY[20] | FLT3-ITD, IDH1, DNMT3A (R882H) | M2 | 24.86 | 61.9 |
| 11H019 | 45,XX,add(10)(p13),-18[11]/46,XX[10] | NPM1, TET2 | M1 | 2.35 | 1.85 |
| 11H021 | 46,XX[20] | FLT3-ITD, MLL-PTD | M2 | 7.44 | NA |
| 11H046 | 92,XXYY[4]/46,XY[19] | IDH1, RUNX1 | M0 | 13.56 | 22.13 |
| 11H058 | 46,XY[20] | NPM1, FLT3-ITD, IDH1 | M1 | 44.24 | NA |
| 11H072 | 46,XX[20] | NPM1, FLT3-ITD, DNMT3A (R882H) | M2 | 73.17 | 95.2 |
| 11H083 | 46,XY[20] | NPM1, FLT3-ITD, DNMT3A (R882C) | M5A | 49.36 | 54.7 |
| 11H095 | 46,XY[20] | M5A | 0.23 | 0.13 | |
| 11H126 | 46,XY[21] | NPM1, TET2 | M5B | 1.32 | NA |
| 11H129 | 47,XY,+10[19]/46,XY[1] | GATA2 | M1 | 0.73 | 1.91 |
| 11H142 | 46,XX[21] | NPM1, FLT3-ITD, TET2 | M1 | 27.70 | 59.62 |
| 11H160 | 46,XX[22] | FLT3-ITD | M4 | 3.56 | 3.07 |
| 11H170 | Complex karyotype | RUNX1 | M0 | 44.80 | 50.33 |
| ID . | Karyotype . | Molecular genetic aberrations . | FAB . | GPR56 RPKM . | GPR56+ % (FACS) . |
|---|---|---|---|---|---|
| 02H053 | 46,XY[20] | NPM1, FLT3-ITD, IDH2 | M1 | 0.94 | NA |
| 03H041 | 46,XX[22] | FLT3-ITD, NUP98-NSD1 fusion | M5 | 0.86 | 1.69 |
| 03H116 | 46,XX[21] | NPM1, FLT3-ITD, IDH2 | M1 | 7.69 | 3.73 |
| 03H119 | 46,XY[20] | RUNX1 | M1 | 3.04 | 3.02 |
| 04H024 | 46,XX[21] | NPM1, IDH2, DNMT3A (other) | M1 | 6.87 | 20.98 |
| 04H112 | 46,XX[21] | NPM1, FLT3-ITD, GATA2, DNMT3A (R882H) | M1 | 161.28 | 91.03 |
| 04H133 | 46,XX[20] | NPM1, FLT3-ITD, DNMT3A (other) | M1 | 143.72 | 84.95 |
| 05H050 | 46,XY[20] | FLT3-ITD, WT1, DNMT3A (R882H), MLL-PTD | M4 | 2.36 | 3.11 |
| 05H094 | 46,XY[23] | NPM1, TET2 | M5B | 0.90 | 1.55 |
| 05H149 | 46,XY[20] | IDH2, RUNX1, DNMT3A (R882H) | NC | 12.23 | 20.02 |
| 05H163 | 46,XY[22] | M1 | 6.35 | 20.17 | |
| 05H181 | 46,XX,inc[11] | NPM1, FLT3-ITD | M5B | 1.36 | NA |
| 06H028 | 46,XX[20] | NPM1, TET2, GATA2 | M1 | 1.34 | 1.62 |
| 06H088 | 46,XY,t(6;11)(q27;q23)[20] | M1 | 5.82 | 4.43 | |
| 06H135 | Complex karyotype | TP53 | M0 | 2.33 | NA |
| 06H144 | 46,XX[20] | NPM1, IDH2, DNMT3A (other) | M1 | 1.39 | NA |
| 07H099 | 46,XX,inv(16)(p13.1q22)[20] | NC | 1.02 | NA | |
| 07H135 | 46,XY[20] | NPM1, FLT3-ITD, IDH2, DNMT3A (other) | M1 | 37.56 | 91.48 |
| 08H012 | 47,XX,+11[21] | FLT3-ITD, IDH2, DNMT3A (other), MLL-PTD | M1 | 8.34 | 42.15 |
| 08H021 | 46,XY,t(9;11)(p22;q23)[20] | M5A | 0.54 | 0.46 | |
| 08H048 | 46,XY[21] | WT1 | M1 | 0.75 | NA |
| 08H112 | 46,XY[20] | IDH2, MLL-PTD | NC | 10.87 | 10.34 |
| 09H031 | 46,XX[20] | NPM1, FLT3-ITD | M1 | 16.34 | 78.73 |
| 09H043 | 46,XY[21] | NPM1, FLT3-ITD, DNMT3A (L566*) | M1 | 67.35 | 90.97 |
| 09H046 | 45,XY,add(16)(p13.1),-17[20] | TP53, TET2, RUNX1, DNMT3A (other) | NC | 11.81 | 14.97 |
| 09H083 | 46,XX[20] | NPM1, FLT3-ITD, IDH2, DNMT3A (R882H) | M1 | 40.04 | 84.19 |
| 09H113 | 46,XY[22] | IDH2, DNMT3A (other), MLL-PTD | M1 | 23.58 | 40.53 |
| 09H115 | 46,XY[24] | FLT3-ITD, IDH2, MLL-PTD | M1 | 1.90 | 3.51 |
| 10H031 | 46,XX[27] | MLL-MLLT4 (by FISH and RNA-Seq) | M5B | 0.45 | 0.29 |
| 10H038 | 46,XX[20] | WT1 | M0 | 5.52 | 31.86 |
| 10H056 | 46,XX[18] | IDH1, DNMT3A (other) | M1 | 6.21 | 9.53 |
| 10H072 | 46,XY[20] | NPM1, TET2, DNMT3A (R882C) | M5B | 0.91 | 1.44 |
| 10H089 | 46,XX[26] | WT1 | NC | 1.32 | NA |
| 10H092 | 46,XX[21] | NPM1, FLT3-ITD, DNMT3A (R882C) | M1 | 67.18 | 97.01 |
| 10H101 | 46,XX[22] | NPM1, FLT3-ITD, DNMT3A (R882H) | M2 | 47.97 | 83.44 |
| 10H109 | 45,XX,der(7)?t(7;18)(p12;q12),-18[17]/46,XX[3] | NPM1, TET2 | M1 | 1.72 | NA |
| 10H113 | Complex karyotype | TP53, IDH2 | M1 | 7.32 | 28.35 |
| 10H115 | 46,XY[23] | NPM1, FLT3-ITD, IDH2, WT1 | M1 | 4.65 | 6.88 |
| 10H127 | 46,XY,t(9;11)(p22;q23)[20] | M5A | 0.15 | 0.15 | |
| 10H161 | 47,XX,+8[9]/46,XX[4] | NPM1, TET2, DNMT3A (R882C) | M5B | 0.13 | 0.76 |
| 10H166 | 46,XY[20] | NPM1, FLT3-ITD, IDH2, DNMT3A (R882H) | NC | 41.00 | 69.93 |
| 11H006 | 46,XX[23] | NPM1, IDH1, DNMT3A (R882H) | M5A | 1.04 | 14.21 |
| 11H008 | 48,XY,+13,+15[3]/46,XY[14] | FLT3-ITD, MLL-PTD | NC | 6.37 | 5.59 |
| 11H009 | 46,XY[20] | FLT3-ITD, IDH1, DNMT3A (R882H) | M2 | 24.86 | 61.9 |
| 11H019 | 45,XX,add(10)(p13),-18[11]/46,XX[10] | NPM1, TET2 | M1 | 2.35 | 1.85 |
| 11H021 | 46,XX[20] | FLT3-ITD, MLL-PTD | M2 | 7.44 | NA |
| 11H046 | 92,XXYY[4]/46,XY[19] | IDH1, RUNX1 | M0 | 13.56 | 22.13 |
| 11H058 | 46,XY[20] | NPM1, FLT3-ITD, IDH1 | M1 | 44.24 | NA |
| 11H072 | 46,XX[20] | NPM1, FLT3-ITD, DNMT3A (R882H) | M2 | 73.17 | 95.2 |
| 11H083 | 46,XY[20] | NPM1, FLT3-ITD, DNMT3A (R882C) | M5A | 49.36 | 54.7 |
| 11H095 | 46,XY[20] | M5A | 0.23 | 0.13 | |
| 11H126 | 46,XY[21] | NPM1, TET2 | M5B | 1.32 | NA |
| 11H129 | 47,XY,+10[19]/46,XY[1] | GATA2 | M1 | 0.73 | 1.91 |
| 11H142 | 46,XX[21] | NPM1, FLT3-ITD, TET2 | M1 | 27.70 | 59.62 |
| 11H160 | 46,XX[22] | FLT3-ITD | M4 | 3.56 | 3.07 |
| 11H170 | Complex karyotype | RUNX1 | M0 | 44.80 | 50.33 |
FAB, French-American-British classification; NA, not available; NC, not classifiable by FAB criteria.
GPR56 expression is associated with poor outcome and specific genetic lesions
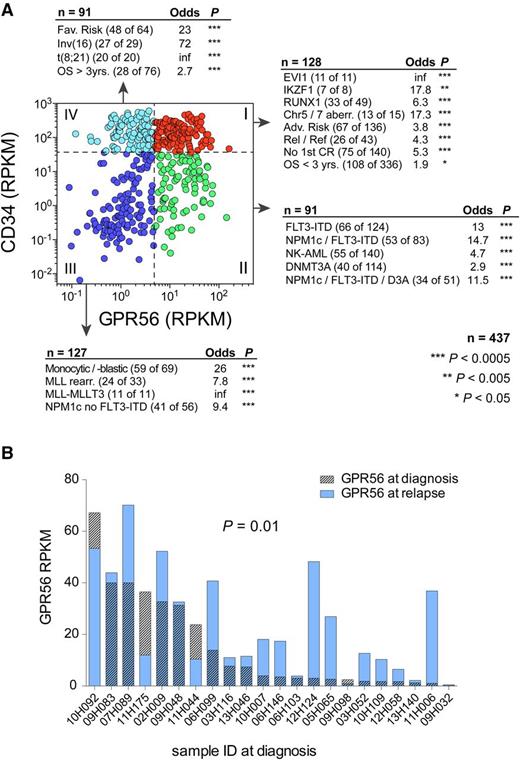
As part of the Leucegene Project (www.leucegene.ca), we performed RNA-Seq on a total of 437 primary AML samples. Because high GPR56 expression had been linked to EVI-mutated AML before,28 we questioned whether high GPR56 expression was an exclusive feature of this specific AML subgroup. We divided all 437 AML samples into 4 groups based on high (above the median) vs low (below the median) GPR56 and CD34 expression (Figure 2A) and found that each group was highly enriched for different sets of mutations: CD34highGPR56high specimens were significantly enriched for samples with high molecular and cytogenetic risk comprising all 11 EVI1/MECOM-rearranged samples, the majority of samples with chromosome 5 and 7 anomalies, and mutations in RUNX1 and IKZF1, which we had found to be frequently mutated in EVI1-rearranged AML.29 Moreover, this group was enriched for patients with poor survival (<3 years), relapsed or refractory AML, and no achievement of first complete remission. CD34lowGPR56high samples were enriched for FLT3-ITD with or without additional NPM1 mutation and DNMT3A mutated samples. The majority of MLL-rearranged and samples with monocytic/monoblastic morphology and NPM1-mutated samples without FLT3-ITD expressed low levels of both genes (CD34lowGPR56low), whereas the fourth group (CD34highGPR56low) comprised all 20 samples with t(8;21) and 27 of 29 samples with inv(16), both associated with favorable prognosis30 and known to rarely engraft in xenotransplantation assays.14 These observations were validated on the publicly available AML dataset from The Cancer Genome Atlas (supplemental Figure 4).31 RNA-Seq analysis of paired diagnosis-relapse samples revealed that GPR56 expression was similar or higher in 16 of 22 samples at relapse (Figure 2B). In line with these observations, high GPR56 expression was significantly associated with poor survival in patients of all ages and those younger than 60 years (supplemental Figure 5A). These results were confirmed in The Cancer Genome Atlas cohort and in 2 independent microarray datasets comprising 54932 and 46118 AML patients (supplemental Figure 5B-D).
GPR56 expression is associated with poor outcome and specific genetic lesions. (A) Scatterplot displaying GPR56 and CD34 expression (log10 scale) in 437 AML samples from the Leucegene sample collection. Adjacent text indicates enrichment of sample parameters in the 4 highlighted quadrants. Quadrants are defined by RPKM values below and above the medians of CD34 and GPR56 (dashed lines). Enrichment was calculated with Fisher’s exact test. Adv., adverse; Chr, chromosome; CR, complete remission; NK = normal karyotype; OS = overall survival; Rel/Ref, relapse/refractory. (B) RPKM values of GPR56 in 22 paired diagnosis-relapse samples (P = .01, Wilcoxon matched pairs signed-rank test).
GPR56 expression is associated with poor outcome and specific genetic lesions. (A) Scatterplot displaying GPR56 and CD34 expression (log10 scale) in 437 AML samples from the Leucegene sample collection. Adjacent text indicates enrichment of sample parameters in the 4 highlighted quadrants. Quadrants are defined by RPKM values below and above the medians of CD34 and GPR56 (dashed lines). Enrichment was calculated with Fisher’s exact test. Adv., adverse; Chr, chromosome; CR, complete remission; NK = normal karyotype; OS = overall survival; Rel/Ref, relapse/refractory. (B) RPKM values of GPR56 in 22 paired diagnosis-relapse samples (P = .01, Wilcoxon matched pairs signed-rank test).
GPR56 expression identifies the engrafting compartment in CD34+ AML
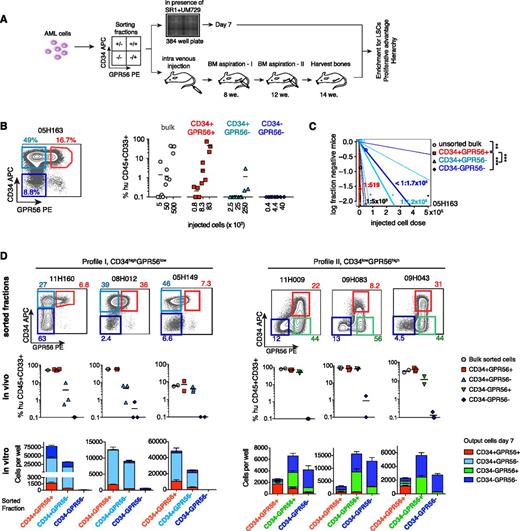
To determine whether GPR56 discriminates engrafting LSCs from non-LSCs we sorted GPR56+ and GPR56− cells within the CD34+ and CD34− compartments from selected specimens with known engraftment potential (outlined in Figure 3A). Sorted cells were injected in NSG mice at cell numbers respecting the percentage of each compartment in the bulk at the time of sorting (detailed description in our “Materials and methods” section and Figure legends). Equal cell numbers of each fraction were also analyzed in vitro to determine differences in proliferative potential. We performed a complete limiting dilution analysis on each sorted fraction for sample 05H163 and observed that CD34+GPR56+ cells, which represented only 16% of the bulk, yielded the same engraftment levels as bulk cells at all 3 tested cell doses (Figure 3B; eg, 5 × 104 bulk cells and 8 × 103 CD34+GPR56+ cells generated on average 35% and 46% human CD33+CD45+ cells, respectively). The engrafted cells displayed a similar immunophenotype compared with the original patient sample independent of whether bulk cells or sorted double-positive cells were injected (supplemental Figure 6A). Estimation of LSC frequencies in bulk and sorted cells revealed >50-fold higher purity of engrafting cells in the CD34+GPR56+ compared with the CD34+GPR56− compartment (Figure 3C; supplemental Table 7). When considering the proportions of subsets in the bulk, the CD34+GPR56+ fraction contributed at least 78-fold more to the total LSC pool than the other fractions (supplemental Figure 6B). Given that differences in engraftment capacity were seen at all 3 cell doses, we performed subsequent experiments at 1 cell dose only, at which we expected robust engraftment based on previous experiments. To assure that enough cells had engrafted, we performed bone marrow aspirations at 8 and 12 weeks before euthanizing the mice at 14 weeks (supplemental Figure 6C, supplemental Table 8). Combinatorial flow cytometric analysis of CD34 and GPR56 protein expression revealed distinct staining patterns (supplemental Figure 6D). Hypothesizing that differences in phenotype profiles might underlie different regulatory networks, we selected 3 samples in which GPR56+ cells were part of the larger CD34+ fraction (profile I; supplemental Figure 6D) and 3 in which CD34+ cells were part of the predominant GPR56+ fraction (profile II). Together, samples with detectable GPR56+ fraction comprised 32 of the 45 samples analyzed, whereas the remaining were considered negative (profile III). In samples in which GPR56+ cells represented a minor subset of total CD34+ cells, LSCs were enriched in the double-positive fraction (Figure 3D; supplemental Figure 6D). In that line, we observed in vitro that sorted CD34+GPR56+ cells generated significantly more total and double-positive cells than sorted CD34+GPR56− cells (Figure 3D, left panel, third row; P = .002, Mann-Whitney U test). The double-negative fraction did not contribute to relevant engraftment in vivo and had no proliferative capacity in vitro. A minor CD34−GPR56+ population representing <1% of total cells in these 3 samples failed to engraft and was not reproduced by the engrafting fractions. Flow cytometry analysis revealed that these cells were matching the CD56+CD3− phenotype of mature natural killer cells (supplemental Figures 1D and 6E) and were therefore not further studied. In CD34lowGPR56high AML samples (profile 2; Figure 3D, right panel; supplemental Figure 6C), both GPR56+ subsets (CD34+ and CD34−) contributed to leukemia propagation in mice (Figure 3D). GPR56+CD34− cells generated greater total but predominantly GPR56+CD34− and double-negative cells in vitro and yielded lower engraftment levels despite higher numbers of injected cells compared with GPR56+CD34+ cells, indicating that they were less enriched in LSCs than double-positive cells. Double-negative cells, which barely contributed to engraftment, were able to generate progeny in vitro when sorted from CD34lowGPR56high samples, but gave rise mostly to double-negative cells.
GPR56 expression identifies the engrafting compartment in CD34+ AML. (A) Experimental setup of in vitro and in vivo experiments. (B, left) Sorting strategy for sample 05H163. (Right) Engraftment levels (percentage of human CD33+CD45+ cells in mouse bone marrow) in 3 to 4 recipient mice 14 weeks posttransplantation of decreasing cell doses (cell doses are cell equivalents, eg, 5 × 103 bulk cells were equivalent to 800 CD34+GPR56+ (16%) cells). Bars indicate mean engraftment levels. (C) Absolute LSC frequencies of bulk cells and sorted fractions in AML 05H163 estimated by extreme limiting dilution analysis. **P < .005, ***P < .0005, χ2 test. (D) In vivo and in vitro analysis of sorted GPR56 subsets. (Top row) FACS profiles for CD34 and GPR56 and initial percentages of sorted fractions in sorted bulk cells. (Center row) Engraftment levels (% human CD45+CD33+ cells shown for each recipient, bars indicate mean engraftment levels) yielded by each sorted fraction after 14 weeks in NSG mice (see supplemental Figure 6C for 8 and 12 weeks). Fractions were injected at cell doses respecting the percentages in total cells. Mice with <0.1% human engraftment were positioned at 0.1% and considered negative. (Bottom row) The number of CD34+/− and GPR56+/− cells generated by the sorted fractions in 7 days in optimized in vitro conditions. Bars are stacked (ie, the sum of stacked bars indicates total cell number). Equal numbers (5000 cells/well) of the 3 predominant fractions were seeded per well; bars and error bars represent means and standard deviations of 3 to 6 replicate wells.
GPR56 expression identifies the engrafting compartment in CD34+ AML. (A) Experimental setup of in vitro and in vivo experiments. (B, left) Sorting strategy for sample 05H163. (Right) Engraftment levels (percentage of human CD33+CD45+ cells in mouse bone marrow) in 3 to 4 recipient mice 14 weeks posttransplantation of decreasing cell doses (cell doses are cell equivalents, eg, 5 × 103 bulk cells were equivalent to 800 CD34+GPR56+ (16%) cells). Bars indicate mean engraftment levels. (C) Absolute LSC frequencies of bulk cells and sorted fractions in AML 05H163 estimated by extreme limiting dilution analysis. **P < .005, ***P < .0005, χ2 test. (D) In vivo and in vitro analysis of sorted GPR56 subsets. (Top row) FACS profiles for CD34 and GPR56 and initial percentages of sorted fractions in sorted bulk cells. (Center row) Engraftment levels (% human CD45+CD33+ cells shown for each recipient, bars indicate mean engraftment levels) yielded by each sorted fraction after 14 weeks in NSG mice (see supplemental Figure 6C for 8 and 12 weeks). Fractions were injected at cell doses respecting the percentages in total cells. Mice with <0.1% human engraftment were positioned at 0.1% and considered negative. (Bottom row) The number of CD34+/− and GPR56+/− cells generated by the sorted fractions in 7 days in optimized in vitro conditions. Bars are stacked (ie, the sum of stacked bars indicates total cell number). Equal numbers (5000 cells/well) of the 3 predominant fractions were seeded per well; bars and error bars represent means and standard deviations of 3 to 6 replicate wells.
To determine whether the GPR56+ fraction overlapped with the previously described2 CD34+CD38− LSC phenotype, we costained 7 AML samples, for which LSC activity was assessed in sorted fractions, with antibodies against CD34, GPR56, and CD38. In CD34highGPR56low samples, in which LSC activity was predominantly found in the double-positive fraction, we observed highly variable proportions of GPR56+ cells within the CD34+CD38− compartment ranging from 7% to 84% (supplemental Figure 7A). In sample 05H163, in which LSCs were almost exclusively found in the CD34+GPR56+ fraction (Figures 3B-C), only 66% of the CD34+CD38− cells expressed GPR56. In CD34lowGPR56high samples, in which LSCs were found in both the CD34+GPR56+ and CD34−GPR56+ fractions (Figure 3D), we found that neither CD38 nor GPR56 further subdivided the CD34 fraction because nearly all CD34+ cells were at the same time CD38− and GPR56+ (supplemental Figure 7B). However, the CD34−GPR56+ LSC compartment, which we demonstrated to have repopulating potential in vivo, was not contained in the CD34+CD38− compartment and would be excluded by a sorting strategy based on CD34 and CD38. Together, these data showed that GPR56 either subdivided or identified LSCs beyond the CD34+CD38− LSC compartment.
GPR56 is a stable LSC marker in culture conditions
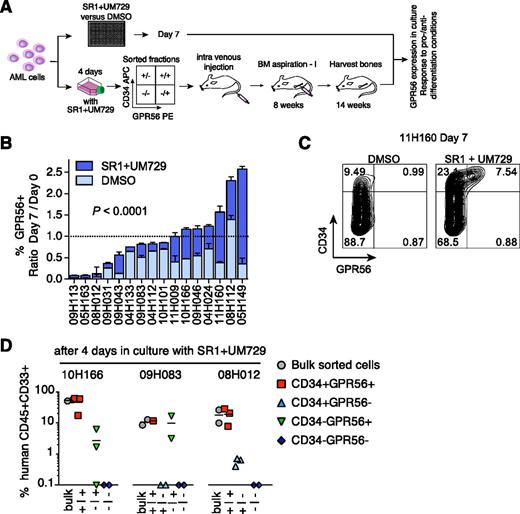
We next determined whether GPR56 stably identified LSCs under in vitro conditions (outlined in Figure 4A). In 15 of 16 specimens, GPR56 expression decreased within 7 days in cultures lacking the aryl-hydrocarbon receptor–antagonist SR1 and the pyrimidoindole UM729, but was partially or completely maintained in presence of the 2 compounds, which we had shown to support maintenance of repopulating activity ex vivo7 (Figure 4B, representative example shown in Figure 4C). A similar effect of the 2 compounds was observed for CD34 expression as previously described,7 but not for other tested LSC markers (supplemental Figure 8). Cell-sorting experiments performed with cells from 4-day cultures revealed that only GPR56+ cells contributed to >1% engraftment (Figure 4D). This shows the stability of GPR56 as an LSC marker under culture conditions, which is an important prerequisite for testing potential LSC-eradicating molecules in vitro.
GPR56 is a stable LSC marker in culture conditions. (A) Experimental setup of in vitro experiments to test stability of GPR56 in culture conditions. (B) Percentage of GPR56+ cells after 7 days in vitro in optimized culture condition (SR1+UM729) or vehicle DMSO compared with uncultured cells (given as ratios day 7/day 0, P < .0001, Wilcoxon matched pairs signed-rank test). (C) Representative FACS profile of sample 11H160 cultured for 7 days in optimized culture condition (SR1+UM729) or vehicle DMSO. Compare Figure 3D, left panel, for day 0 profile. (D) Human engraftment levels in recipient mice (horizontal bars indicate means) generated by sorted fractions after 14 weeks in NSG mice (lower panel) shown for 3 samples after 4 days in culture with SR1 and UM729.
GPR56 is a stable LSC marker in culture conditions. (A) Experimental setup of in vitro experiments to test stability of GPR56 in culture conditions. (B) Percentage of GPR56+ cells after 7 days in vitro in optimized culture condition (SR1+UM729) or vehicle DMSO compared with uncultured cells (given as ratios day 7/day 0, P < .0001, Wilcoxon matched pairs signed-rank test). (C) Representative FACS profile of sample 11H160 cultured for 7 days in optimized culture condition (SR1+UM729) or vehicle DMSO. Compare Figure 3D, left panel, for day 0 profile. (D) Human engraftment levels in recipient mice (horizontal bars indicate means) generated by sorted fractions after 14 weeks in NSG mice (lower panel) shown for 3 samples after 4 days in culture with SR1 and UM729.
These results were supported by a human leukemia model genetically engineered through overexpression of NUP98-HOXD13 (ND13) fusion gene and the oncogene MN1 in human CD34+ cord blood cells.15 Ten weeks after transduction, we detected GPR56 expression by quantitative reverse-transcription polymerase chain reaction and flow cytometry only in double transduced ND13+MN1 cells, which caused leukemia when injected in immunocompromised mice, whereas single-transduced ND13 cells lacked leukemogenic potential in vivo and maintained a CD34−GPR56− phenotype (supplemental Figure 9A-C). Similar to primary AML samples, only GPR56+CD34+ cells sorted from ND13+MN1 cells gave rise to all 4 fractions in vitro and in vivo, and maintained a blast-like morphology in culture (supplemental Figure 9D-G).
Discussion
In this study, we combined RNA-Seq analysis with functional in vivo assays to identify novel LSC determinants in primary human AML and validated GPR56 as a marker of leukemic subpopulations with high NSG engrafting capacity. Whereas LSCs were originally defined as a subset of cells within the leukemic bulk, which engraft in immunocompromised mice and reestablish the hierarchically organized human disease similar to normal HSCs,2,3 this definition has been modified by other groups to describe surface markers expressed preferentially on AML but not on normal CD34+ cells. We analyzed our RNA-Seq data and performed FACS analysis on 45 primary AML samples to compare GPR56 expression with the most cited LSC markers in the literature. As expected, we observed heterogeneity in cell surface marker expression but were able to recognize 2 repetitive patterns with regard to CD34 and GPR56 staining besides the minor fraction of AML samples that were CD34 and GPR56 double negative. In the first group, we found that GPR56 subdivided the CD34 population and identified the majority of LSCs. In this group of AML specimens GPR56 also further subdivided the CD34+CD38− and CD34+CD38+ populations. The second group was characterized by a large fraction of GPR56+ cells, which could be subdivided into CD34+ and CD34− subsets. We found LSC activity in both GPR56+ compartments, the CD34+ subset, which was at the same time CD38−, and in the GPR56+CD34− subset, but not in the GPR56− fraction. Thus, our data clearly reveal that GPR56+ populations are not identical to the CD34+CD38− compartment and define a novel LSC compartment besides the CD34+CD38− phenotype. The surface markers CD44 and CD47 were expressed at very high levels by the majority of leukemic blasts (>90% of bulk cells) as reported.8-10 Although such markers might be useful as therapeutic targets9,33 or markers of minimal residual disease, they obviously do not enrich for rare subsets of functionally distinct cells within the leukemic bulk. Other markers such as CLL1, CD96, or TIM3 were not associated with high LSC frequency in our dataset and did not correlate with loss of LSC activity during in vitro culture. Larger sample cohorts in these studies and analysis of genetic and morphologic subtypes enriched by these markers would be required to better elucidate these discrepant findings. Recently, CD93 was identified as a novel LSC marker in MLL-rearranged leukemias,12 which have been suggested to represent a distinct AML entity based on differential gene expression studies.34 We found that most MLL-rearranged and monocytic AML specimens were CD34−GPR56− double-negative and consequently did not contain a GPR56+ LSC compartment, thus supporting the hypothesis that LSC phenotypes might not be uniform across genetically distinct AML subgroups. Interestingly, absence or low expression of GPR56 was not only associated with MLL-rearranged AML, but also with inv(16) and t(8;21), 2 cytogenetic aberrations associated with favorable prognosis,30 which are known to rarely engraft in immunocompromised mice15 and were therefore not assessed in our study. Whereas high GPR56 expression had been linked to EVI1-mutated AML before,28 we found significant enrichment also for other genetic groups associated with poor prognosis, including FLT3-ITD and mutations in RUNX1 and TP53,35 suggesting that GPR56 might be part of a common stem cell network induced by different mutational events. Accordingly, GPR56 expression on its own was associated with poor survival in our and several other published datasets. Given the significant association with various high-risk genetic lesions, assessment of GPR56 expression as an independent prognostic factor in AML becomes obsolete.
Identification of LSC markers not expressed on normal hematopoietic stem and progenitor cells would obviously be ideal because LSCs could be targeted without affecting normal hematopoiesis. However, there is strong evidence for a correlation between normal HSC gene expression, LSC activity, and bad outcome.4 We found that GPR56 is highly expressed on the majority of normal CD34+ cells and consequently does not further segregate normal human stem and progenitor compartments. Moreover, LSChigh samples best clustered with total CD34+ and CD34+CD33+ cells than with the more immature CD34+CD45RA− compartment and expressed a set of lymphoid genes, which is in line with reports on a lymphoid-primed multipotential progenitor–like phenotype of LSCs.36 Although GPR56 has been attributed a role in homing of murine HSCs28 and endothelial to hematopoietic stem cell transition,37 its functional role in leukemia propagation remains to be determined.
In conclusion, we established GPR56 as a novel surface marker identifying leukemic subpopulations with high repopulating capacity, a key feature attributed to LSCs, in primary AML samples both in the CD34+ and CD34− compartments, demonstrating its utility apart from the CD34+CD38− LSC-associated phenotype. We showed that high GPR56 expression is not restricted to EVI1-mutated samples as previously described, but a characteristic shared by various AML subtypes with high-risk genetic lesions and poor outcome.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors acknowledge M. Fréchette and V. Blouin-Chagnon for assistance with xenotransplantation experiments, N. Mayotte and S. Fortier for technical assistance with mice analyses, D. Gagné and G. Dulude for technical support with cell sorting, and M. Arteau for technical support with A and exome sequencing. Clinical specimens were collected and analyzed by the Banque de Cellules Leucémiques du Québec, which is supported by the Cancer Research Network of the Fonds de Recherche du Québec en Santé.
This work was supported by the Government of Canada through Genome Canada and the Ministère de l’économie, de l’innovation et de l’exportation du Québec through Génome Québec; Canada Research Chair in Molecular Genetics of Stem Cells (G.S.) and a Research Chair in Leukemia supported by Industrielle-Alliance (Université de Montréal) (J.H.); of postdoctoral fellowships from the German Cancer Aid (Deutsche Krebshilfe) and the Cole Foundation and a Max-Eder-Grant from the German Cancer Aid (C.P.); and a postdoctoral fellowship from Vetenskapsrådet (G.L.N.).
Authorship
Contribution: C.P. designed, executed, and analyzed experiments, wrote the paper, and generated the figures; A.B. and J.S. designed and performed in vivo experiments; V.-P.L. and I.B. analyzed mutations; J.Y. performed flow cytometry, analyzed mice, and edited the manuscript; P.G. analyzed RNA-Seq data and generated figures; G.L.N., S.I., and K.H. designed, performed, and analyzed experiments with engineered cord blood cells; J.K. performed in vitro experiments, analyzed mice, and edited the manuscript; I.B. validated mutations; K.E., T.H., and S.K.B. performed microarray and survival analyses; E.D. sorted and sequenced cord blood samples; G.B. and S.L. helped with RNA-Seq and statistical analyses; and F.B., G.S., and J.H. contributed to project conception, experimental design, and editing of the manuscript. J.H. analyzed cytogenetic data and provided all AML samples.
Conflicts-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Frédéric Barabé, Centre de Recherche en Infectiologie du CHU de Québec–Université Laval, 2705 Boul Laurier local RC709, Québec, QC, Canada, G1V 4G2; e-mail: frederic.barabe@crchul.ulaval.ca.
References
Author notes
J.H., G.S., and F.B. contributed equally to this study.

![Figure 1. Identification of LSC-associated genes by RNA-Seq and in vivo data. (A) Experimental design (see our “Materials and methods” section for details). i.f., intrafemoral. (B) High LSC frequency is associated with poor survival (median overall survival 143 vs 369 days, P = .02 (log-rank), HR 3.2 (95% CI, 1.2-8.7), n = 56. (C, left) Average gene expression in LSChigh vs LSClow samples (log(RPKM+0.001) transformed). GPR56 is highlighted in red. (Right) Volcano plot displaying the average fold difference in gene expression in LSChigh (n = 10) vs LSClow samples (n = 26) for all genes with an average log10(RPKM+0.001) ≥1 in LSChigh samples in relation to the P value for each gene. P values were transformed as –log10 (P value). Candidate LSC genes are shown in red (criteria: average (log10(RPKM) in LSChigh samples ≥1, average ratio LSChigh/LSClow ≥2, P < .01 [Mann-Whitney U test]). Font and dot size indicate the average expression level of the genes in LSChigh samples. (D) GPR56 gene expression in hematopoietic cell populations. (E) Waterfall plot displaying correlation coefficients for all genes correlated to GPR56 based on RNA-Seq data from 437 AML samples. Highlighted in red are selected genes positively correlating with GPR56. Blue-annotated genes inversely correlate with GPR56. (F) Correlation between GPR56 gene expression by RPKM and percentage of positive cells measured by flow cytometry in 45 AML samples (log-scale). (G) Fractions of GPR56 and CD34+ subpopulations determined by flow cytometry in samples with high, medium, and low LSC frequency. Box-and-whisker plots displaying median, lower (Q1) and upper (Q3) quartile, minimum and maximum without outliers, and outliers identified as below Q1 − 1.5 (IQR) or above Q3 + 1.5 (IQR) (Tukey method). IQR, interquartile range = Q3-Q1.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/127/16/10.1182_blood-2015-11-683649/4/m_2018f1.jpeg?Expires=1769810227&Signature=3P8sPByqAPNel2z6rVwzFkCGqTTFmdHIXPbmwy76lMH8dAW8bkTjBUK1juvcaiU7ii-h09i7vG7AR0MJ9CipM0d34Y2MCEPkzEcAcJ0T3ZKvKT2-BkQkpG2rj8GECDxC8Jf322dW9i7uiYwCnern53t4Q4CwQfX6ImCqUJlSI0KOZv8KtSJGoUBEaCpHyVPuAVHG0VwB21Tgf~maycjXyEN8PY8nncx9EhaH-OgO~LlvLV2p9W7fImJX6kpIFWSFwNvvzgewixL-Dp3yMMzu8J0xbaq6HtWPULQfqcd5RDO0NhqCDigbOE9DqaqiGJkyM5IU~woyP-elPd9FoTBb9w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal