Key Points
Donor-derived allogeneic CAR T cells are functional and eradicate ALL.
Allogeneic CD4+ CAR T cells can mediate acute GVHD but only when CD19+ leukemia is present.
Abstract
Acute lymphoblastic leukemia (ALL) persisting or relapsing following bone marrow transplantation (BMT) has a dismal prognosis. Success with chimeric antigen receptor (CAR) T cells offers an opportunity to treat these patients with leukemia-redirected donor-derived T cells, which may be more functional than T cells derived from patients with leukemia but have the potential to mediate graft-versus-host disease (GVHD). We, together with others, have previously demonstrated tumor-specific T-cell dysfunction in the allogeneic environment. Here, we studied CAR T-cell function following BMT using an immunocompetent murine model of minor mismatched allogeneic transplantation followed by donor-derived CD19-CAR T cells. Allogeneic donor-derived CD19-CAR T cells eliminated residual ALL with equal potency to those administered after syngeneic BMT. Surprisingly, allogeneic CAR T cells mediated lethal acute GVHD with early mortality, which is atypical for this minor mismatch model. We demonstrated that both allogeneic and syngeneic CAR T cells show initial expansion as effector T cells, with a higher peak but rapid deletion of allogeneic CAR T cells. Interestingly, CAR-mediated acute GVHD was only seen in the presence of leukemia, suggesting CAR-target interactions induced GVHD. Indeed, serum interleukin (IL)-6 was elevated only in the presence of both leukemia and CAR T cells, and IL-6 neutralization ameliorated the severity of GVHD in a delayed donor lymphocyte infusion model. Finally, allogeneic CD4+ CAR T cells were responsible for GVHD, which correlated with their ability to produce IL-6 upon CAR stimulation. Altogether, we demonstrate that donor-derived allogeneic CAR T cells are active but have the capacity to drive GVHD.
Introduction
Allogeneic blood or bone marrow transplantation (allo-BMT) can cure acute lymphoblastic leukemia (ALL), but is typically reserved for patients in remission after high-risk recurrence or, less commonly, very high risk disease in first remission.1-4 The curative effect of allo-BMT results from the high dose chemo/radiotherapy administered for conditioning, as well as an immune effect from the allogeneic graft, termed graft-versus-leukemia (GVL).5,6 Allogeneic T cells play a key role in GVL but have the potential to mediate allogeneic graft-versus-host disease (GVHD), a major cause of morbidity and mortality.6,7 Furthermore, whereas donor lymphocyte infusion (DLI) can induce remission in myeloid malignancies relapsing following allo-BMT,8,9 DLI has limited success in ALL even when GVHD occurs.10 Thus, although GVL has been clearly demonstrated for ALL, improved potency and specificity are needed.
Promising results have been obtained from protocols using genetically modified T cells expressing chimeric antigen receptors (CAR) to redirect specificity toward B-cell–associated proteins expressed on the surface of ALL. CARs typically contain an extracellular antibody-derived single-chain Fv linked to a T-cell receptor (TCR) signaling component (ζ chain of CD3), and 1 to 2 co-stimulatory molecules (ie, CD28, 41BB, and OX40).11-14 This provides major histocompatibility complex (MHC)-independent T-cell activation and co-stimulation. Initial reports of CAR T cells targeting CD19 for ALL showed remarkable results with remission rates of over 70% in relapsed refractory patients.15-17 Although cells used to generate the CAR T cells were harvested from the patient in these trials, 25% to 60% of participants had received a prior allo-BMT such that the collected T cells were derived from the allograft. Nonetheless, recipient-derived allogeneic-CAR T cells could induce remission, with no GVHD observed. Interestingly, from the published National Cancer Institute (NCI) pediatric experience, only 4/8 previously allografted patients achieved remission following CD19 CAR, compared with 10/13 patients with no prior BMT.15 Because the number of patients reported on these early trials is small, it is unclear whether this will portend a lower remission rate and, if so, whether this is related to the allogeneic T cells being dysfunctional or to more aggressive disease in post–allo-BMT patients. Hence, with the additional challenges associated with collecting T cells from patients treated with chemotherapy, protocols are now exploring the use of donor-derived CAR T cells.18,19
CAR T cells retain the endogenous TCR with potential specificity for recipient antigens in the allogeneic setting and thus, potential to induce GVHD. The capacity for allogeneic CAR T cells to cause GVHD and the impact of endogenous TCR specificity on CAR T-cell function cannot be easily evaluated in xenograft systems typically employed for pre-clinical CAR studies. Using murine minor mismatch allo-BMT models, we previously demonstrated that allogeneic T cells have diminished capacity to respond to vaccines and mediate antitumor responses even with mild subclinical GVHD,20-22 and that adoptively transferred T cells with dual specificity for tumor and alloantigen through a single receptor demonstrate reduced but variable effectiveness dependent on the distribution of the allogeneic antigen.23 Thus, we evaluated the efficacy of allogeneic CD19-targeted CAR T cells for the prevention of pre–B-cell ALL relapse in an immunocompetent murine model of HLA-matched, minor mismatched allo-BMT.
Materials and methods
Mice
C57BL/6 (H-2b CD45.2+) and B6-Ly5.2 (H-2b CD45.1+) were purchased from the NCI Animal Production Program (Frederick, MD). C3H.SW (H-2b CD45.2+) and interleukin (IL)-6−/− mice (B6.129S2-IL6tm1Kopf/J) strains were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were used between 6 and 10 weeks of age. All animals were kept in a pathogen-free facility under protocols approved by the Animal Care and Use Committee at the NCI.
Tumor cell lines
A murine pre–B-ALL carrying the human E2a:PBX transgene crossed to a CD3ε−/− was generously provided by Dr Janetta Bijl.24 These cells were developed into a stable cell line expressing pre-B markers. Leukemia cells were cultured in 10% complete mouse media .20 For in vivo assays, 106 cells were administered via tail-vein to nonirradiated mice unless otherwise specified. The EL4 cell line was purchased from American Type Culture Collection and was used as CD19− control for in vitro assays.
T-cell isolation, activation, and CAR transduction
The murine CD19 CAR construct on a mouse stem cell virus-based splice-gag vector retroviral backbone was previously described25 and used to make a CD19 CAR stable producer line. Splenocytes harvested from CO2-euthanized mice were passed via a 70-μm filter, erythrocyte depleted using ACK-lysis buffer (Lonza, Walkersville, MD), and transferred over a mouse CD3+ T cell enrichment column (R&D Systems, Minneapolis, MN) according to the manufacturers’ instructions. T cells were incubated in complete mouse media and activated with Mouse T-Activator CD3/CD28 Dynabeads (Life Technologies, Grand Island, NY) in the presence of IL-2 (30 U/mL) and IL-7 (10 ng/mL). On days 2 and 3 of ex vivo T cell culture, retroviral supernatant was spun at 2000 g for 2 hours on plates coated with retronectin (Clontech, Mountain View, CA). Supernatant was removed and T cells were incubated for an additional 24 hours. Following the second transduction, T cells were expanded for 24 hours before magnetic CD3/CD28 bead removal. Twelve to 24 hours after bead removal, T cells were IV injected. When indicated, splencoytes were selected using CD4 or CD8 magnetic microbeads on a Miltenyi LD column (Miltenyi Biotec, San Diego, CA) prior to culture, activation, and transduction.
BMT and adoptive T-cell therapy
BMT was as previously described,23 with the exception of 1000 cGy as the lethal irradiation dose. Adoptive T cell transfer was performed 2 days following BMT (unless otherwise specified). Mice were followed daily for survival by a veterinarian team blinded to the study design, and were euthanized when moribund. Mice were weighed at least twice weekly following BMT and assessed for skin ruffling, back hunching, and/or skin loss, as signs of GVHD.26
Functional assessment of in vivo T cells
Splenocytes were harvested from euthanized recipient mice on day 7 following BMT. CD45.1 positive-selection was performed using biotin-labeled anti-CD45.1 with anti-biotin magnetic beads through AutoMACS (Miltenyi Biotec). Target cells were stained with CellTrace Violet (Life Technologies, Grand Island, NY) for 30 minutes, and washed and co-incubated with sorted splenocytes for 4 hours. Following incubation, T cells were washed and incubated with monensin and with antigen-presenting cell (APC)-conjugated anti-CD107a or isotype control (BD Biosciences, San Jose, CA). Co-culture supernatants were harvested and analyzed for cytokine production using mouse interferon (IFN)-γ Quantikine enzyme-linked immmunosorbent assay (ELISA) according to the manufacturer’s instructions (R&D Systems). Plates were read on SpectraMax M5 (Molecular Devices, Sunnyvale, CA) at 450 nm with correction at 538 nm, and analyzed using SoftMax Pro Software version 5.2 (Molecular Devices).
In vivo cytokine assessment
Anesthetized mice were bled and 50 to 100 μL of serum harvested. For experiments requiring larger volumes, mice were euthanized followed by terminal bleeding. Serum was frozen in −80°C, then thawed once for cytokine assessment. Mouse cytokine array Q1 (RayBiotech, Norcross, GA) was used according to the manufacturer’s instructions. Plates were read using GenePix 4000B (Molecular Devices). For confirmation, serum was evaluated with mouse IFN-γ Quantikine ELISA and mouse IL-6 Quantikine ELISA (R&D systems), as previously described.
Antibodies and flow cytometry
For in vivo experiments, anti-murine IL-6R (clone 15A7; Bio X Cell, West Lebanon, NH) was given at a dose of 500 mcg every other day starting on day 1 (prior to adoptive T-cell transfer). Control mice were injected with rat immunoglobulin (Ig)G2b isotype control (clone LTF-2; Bio X Cell). For flow cytometry, the following conjugated anti-murine antibodies were used: fluorescein isothiocyanate–anti-CD45.1, phycoerythrin (PE)-Cy7–anti-CD45.2, APC-Cy7–anti-B220 (BioLegend, San Diego, CA), PerCP-Cy5.5–anti-CD8a, PE-Cy7–anti-CD44, APC–anti-CD62L, eflour450–anti-CD19 (eBioscience, San Diego, CA), Pacific Blue–anti-CD4, APC–anti-B220, and APC–anti-CD107a (BD Biosciences). CAR detection was performed using protein-L with PE-Streptavidin (BD Biosciences). Samples were analyzed on a BD LSRFortessa (BD Biosciences) using FACS Diva software and analyzed using FloJo version 9.6.4 software (Tree Star, Ashland, OR).
Histopathology
Liver and gut were harvested from euthanized mice, fixed with 10% buffered neutral formalin, and transferred to 70% enthanol. Specimens were sent for paraffin block generation, sectioned at 5 µm, and stained with hematoxylin and eosin (American Histo Labs).
Statistical analysis
Survival of recipients was analyzed through the Kaplan–Meier method, using Wilcoxon rank test. For continuous variables (weight, cell enumeration, and in vitro assays), unpaired Student t test was performed. For weight measurement, the last recorded weight of a mouse was used for calculation at each time point after death, as long as members of its group were still alive. Statistical analysis was performed using GraphPad Prism version 6 for Macintosh (San Diego, CA). P values < .05 were considered significant.
Results
Murine CD19 CAR T cells eliminate CD19+ leukemia in vivo but activity is dependent on lymphodepletion
We incorporated a murine CD19 CAR25 into a pre–B-ALL model, generated from C57/Bl6 mice expressing an E2a:PBX transgene23,24 expressing CD45.2, CD19, B220, CD43, CD127, and CD93, consistent with a pre–B ALL subtype. In vitro CD3/CD28 bead-induced T-cell activation and retroviral transduction resulted in preferential expansion of CD8+ cells and a CD19 CAR transduction efficiency of over 75% (see supplemental Figure 1A, available on the Blood Web site). We next established the in vivo efficacy of CD19 CAR T cells against murine pre–B-cell ALL. CD19-CAR T cells were active at doses as low as 3 × 105. Similar to TCR-based immunotherapy,27,28 the effectiveness of CAR T cells required a lymphodepleting preparative regimen, either 500 cGy of irradiation or 4 mg of cyclophosphamide given 1 day prior to cell infusion (supplemental Figure 1B-C).
Allogeneic CD19-CAR T cells induce remission of residual posttransplant leukemia but can generate lethal acute GVHD
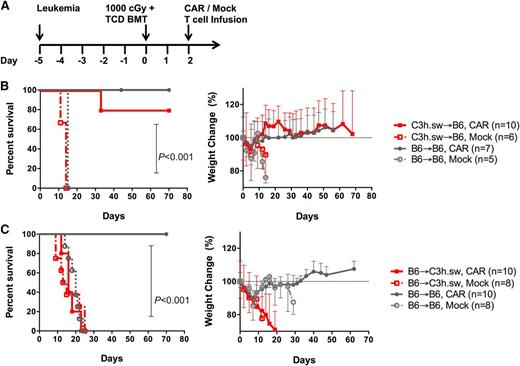
After confirming CAR T-cell efficacy in vivo in the syngeneic setting, we next tested CAR efficacy in an HLA-matched minor-mismatched BMT model. Recipient mice were injected with 106 murine pre–B-ALL cells on day −5, and received 1000 cGy radiation on day 0 followed by infusion of T-cell–depleted (TCD) bone marrow (BM). CD19 CAR transduced or mock-transduced T cells were given IV on day +2 (Figure 1A). Because the ALL is on a B6 background, we first used a model in which B6 recipients matching the leukemia received allogeneic C3h.sw marrow and T-cell donors (both donors and recipients, H-2b). Allogeneic CAR T cells were able to clear leukemia with prolonged survival compared with recipients of mock T cells (median survival: >100 days for CAR treated and 14 days for mock treated; Figure 1B) without evidence of GVHD.
Treatment with allogeneic CAR T cells following B6 → C3h.sw BMT resulted in early mortality. (A) Treatment protocol: mice received 106 E2a:PBX leukemia cells on day −5, followed by myeloablative radiation (1000 cGy) and a TCD BMT (3.5-4 × 106 cells) on day 0, followed by adoptive transfer of 106 CAR or mock T cells on day +2. (B) Leukemia-bearing B6 recipients received B6 (black) or C3h.sw (red) BM and T cells (CAR transduced in solid lines and mock T cells in dashed lines). Survival and weight change from baseline were monitored. (C) Allogeneic transplant was now with C3h.sw as recipients of B6 CAR (red, solid) or mock (red, dashed) T cells. Survival and weight change from baseline are shown. Survival graphs indicate pooled data from 2 replicate experiments.
Treatment with allogeneic CAR T cells following B6 → C3h.sw BMT resulted in early mortality. (A) Treatment protocol: mice received 106 E2a:PBX leukemia cells on day −5, followed by myeloablative radiation (1000 cGy) and a TCD BMT (3.5-4 × 106 cells) on day 0, followed by adoptive transfer of 106 CAR or mock T cells on day +2. (B) Leukemia-bearing B6 recipients received B6 (black) or C3h.sw (red) BM and T cells (CAR transduced in solid lines and mock T cells in dashed lines). Survival and weight change from baseline were monitored. (C) Allogeneic transplant was now with C3h.sw as recipients of B6 CAR (red, solid) or mock (red, dashed) T cells. Survival and weight change from baseline are shown. Survival graphs indicate pooled data from 2 replicate experiments.
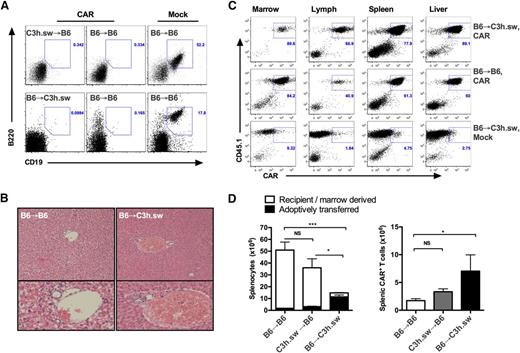
Next, to eliminate any contribution from a GVL effect originating from an allogeneic TCR, we assessed CAR activity in C3h.sw recipients and B6 allogeneic donors. Because the leukemia is matched to the donor in this model, the entire antileukemic effect is mediated by the CD19 CAR on donor T cells. Surprisingly, recipients of allogeneic B6 CAR T cells demonstrated rapid mortality as early as day 9 (median survival: 14 days for B6 → C3h.sw CAR, 23 days B6 → B6 mock, unreached for B6 → B6 CAR; Figure 1C). This was preceded by rapid weight loss suggestive of GVHD occurring at T-cell doses as low as 3 × 105. No leukemia was identified on day 16 in all CAR-treated recipients but was easily identified in mock-treated mice (Figure 2A), indicating preserved CAR T-cell activity and demonstrating that mortality was not due to leukemia. To further investigate for cause of lethality, liver, BM, lymph nodes, and spleen was harvested on day +7, utilizing CD45 isoforms to track CD45.1+ CAR T cells in CD45.2+ recipients of CD45.2+ BM. Livers of allogeneic B6 → C3h.sw CAR recipients demonstrated lymphocytic infiltration in the periductal regions, not seen in syngeneic B6 → B6 controls consistent with GVHD (Figure 2B). There was no evidence for leukemic infiltration of liver, marrow, or spleen in allogeneic CAR recipients, confirming the CD19 CAR T-cell activity. Furthermore, CD45.1+ T cells expressing CD19 CAR were present in the BM, lymph node, spleen, and liver of recipients (Figure 2C). Early expansion of the CD45.1+ adoptively transferred cells was markedly greater in the marrow and spleen of B6 → C3h.sw recipients compared with syngeneic recipients, with significant reduction of CD45.2+ recipient/donor marrow-derived cells. This was accompanied by splenic atrophy and hypocellularity (Figure 2D) as further evidence for GVHD.20 Taken together, these results demonstrate that donor-derived CAR T cells are active in allogeneic recipients but have the capacity to induce lethal GVHD in a minor mismatch allo-BMT model.
Early mortality results from acute GVHD. (A) Representative flow cytometry plots of CD19 and B220 representing pre–B-ALL on day 16 in peripheral blood (PB) of recipients of experiments described in Figure 1. (B) Hematoxylin and eosin stain of liver from recipients on day 7 post-BMT: allogeneic C3h.sw recipients of B6 BMT + CAR T cells (right) and syngeneic B6 recipients (left). (C-D) BM, liver, lymph nodes, and spleens were harvested in post-BMT recipients, and counted and analyzed for adoptively transferred cells using CD45 isotypes. (C) Representative flow cytometry plots for BM, lymph nodes, spleen, and liver gated on T cells. (D) Enumeration of splenocytes on day 7 post-BMT: total splenocytes, with white boxes indicating recipient or donor marrow-derived cells, and black boxes representing adoptively transferred cells, based on CD45 isoform expression (left). CAR+ T cells in spleens on day +7 (right). *P < .05; ***P < .001. NS, nonsignificant.
Early mortality results from acute GVHD. (A) Representative flow cytometry plots of CD19 and B220 representing pre–B-ALL on day 16 in peripheral blood (PB) of recipients of experiments described in Figure 1. (B) Hematoxylin and eosin stain of liver from recipients on day 7 post-BMT: allogeneic C3h.sw recipients of B6 BMT + CAR T cells (right) and syngeneic B6 recipients (left). (C-D) BM, liver, lymph nodes, and spleens were harvested in post-BMT recipients, and counted and analyzed for adoptively transferred cells using CD45 isotypes. (C) Representative flow cytometry plots for BM, lymph nodes, spleen, and liver gated on T cells. (D) Enumeration of splenocytes on day 7 post-BMT: total splenocytes, with white boxes indicating recipient or donor marrow-derived cells, and black boxes representing adoptively transferred cells, based on CD45 isoform expression (left). CAR+ T cells in spleens on day +7 (right). *P < .05; ***P < .001. NS, nonsignificant.
We next used C3h.sw mice transplanted with B6 CD45.1+ TCD BM as donors for CAR production. T cells generated from these chimeras are selected in the thymus of C3h.sw mice and thus have markedly reduced alloreactive capacity to mediate GVHD. At the time of collection (day 60 following transplantation), hematopoietic-derived cells were ∼95% donor, with 80% CD45.1+ B6 donor-derived T cells (supplemental Figure 2A). Interestingly, although transplanted B6 BM-derived T cells expanded equivalently to B6 T cells from nontransplanted donors in culture, a significantly lower transduction efficiency was observed (20% vs 67%; supplemental Figure 2B). Remarkably, lethality was still observed in 60% of recipients of CAR T cells generated from transplanted B6 BM-derived T cells without detectable leukemia, and the surviving mice had signs of GVHD (supplemental Figure 2C-D), suggesting that CAR-expressing T cells can partially overcome relative tolerization to alloantigens.
Allogeneic CAR T cells demonstrate comparable early expansion to syngeneic CAR T cells but shorter persistence
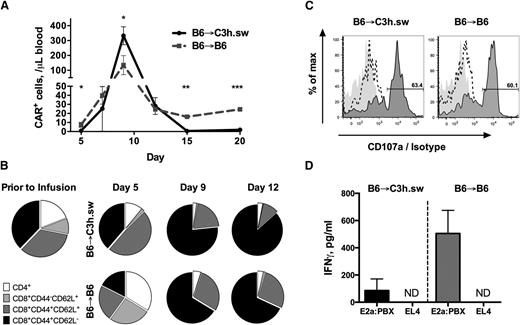
Next, we evaluated the persistence and phenotype of CAR T cells in allogeneic and syngeneic recipients. Initial in vivo expansion of cells was comparable between the two groups, reaching peak numbers on day +9, but with allogeneic cells reaching a significantly higher peak in both peripheral blood (Figure 3A) and tissues (Figure 2C-D). CAR T-cell numbers declined in syngeneic recipients after the day 9 peak but plateaued at persistently detectable numbers through day 30. However, following day 15, the number of allogeneic CAR T cells were significantly diminished compared with syngeneic recipients, consistent with rapid deletion of T cells observed in the setting of severe GVHD.29-32 Early CAR T-cell expansion was associated with an effector memory CD8+ cell (CD44+CD62L−) phenotype in both allogeneic and syngeneic recipients, increasing from 37% prior to transfer to 76% and 66%, respectively (Figure 3B and supplemental Figure 3). However, in allogeneic B6 → C3h.sw recipients, the proportion of CD8+ effector-memory cells continued to increase beyond day 9, whereas in syngeneic recipients there was an emergence of a central memory T-cell population (CD44+CD62L+) (Figure 3B). Thus, in the allogeneic environment, donor-derived CAR T cells demonstrate increased early expansion but fail to persist and do not generate a memory pool.
CAR cells maintain potency despite the allogeneic environment. (A) Absolute number of donor CAR T cells in 1 μL of peripheral blood, following B6 → C3h.sw allo-BMT (black solid) or B6 → B6 syngeneic BMT (gray dotted), gated on CD45.1+ CAR+ T cells. Data shown was generated from 5 mice per group and are representative of 2 separate experiments. (B) T-cell subsets in the CAR T-cell product prior to infusion and in allogeneic vs syngeneic recipients as in (A). (C-D) Mice were euthanized on day 7 post-BMT and CD45.1+ sorted splenocytes were incubated for 4 hours with target. Data are representative of 3 separate experiments. (C) CD107a expression on CD8+CD45.1+ cells in culture. CD45.1+ cells co-cultured with E2a:PBX CD19+ leukemia (dark gray plot); CD45.1+ cells co-cultured with EL4 CD19− cells (dashed line); CD45.1+ cells co-cultured with E2a:PBX1 and stained with isotype control (gray shadow). (D) IFN-γ production by ELISA following the same co-cultures. *P < .05, **P < .01, ***P < .001. ND, not detected.
CAR cells maintain potency despite the allogeneic environment. (A) Absolute number of donor CAR T cells in 1 μL of peripheral blood, following B6 → C3h.sw allo-BMT (black solid) or B6 → B6 syngeneic BMT (gray dotted), gated on CD45.1+ CAR+ T cells. Data shown was generated from 5 mice per group and are representative of 2 separate experiments. (B) T-cell subsets in the CAR T-cell product prior to infusion and in allogeneic vs syngeneic recipients as in (A). (C-D) Mice were euthanized on day 7 post-BMT and CD45.1+ sorted splenocytes were incubated for 4 hours with target. Data are representative of 3 separate experiments. (C) CD107a expression on CD8+CD45.1+ cells in culture. CD45.1+ cells co-cultured with E2a:PBX CD19+ leukemia (dark gray plot); CD45.1+ cells co-cultured with EL4 CD19− cells (dashed line); CD45.1+ cells co-cultured with E2a:PBX1 and stained with isotype control (gray shadow). (D) IFN-γ production by ELISA following the same co-cultures. *P < .05, **P < .01, ***P < .001. ND, not detected.
We next assessed the impact of the allogeneic environment on the in vitro functionality of CAR T cells. Splenocytes of allogeneic B6 → C3h.sw and syngeneic B6 → B6 CAR cells were harvested on day 7 and adoptively transferred T cells were isolated based on expression of CD45 isoforms. The expression of CAR on the sorted cells was >85%, and comparable in allogeneic and syngeneic recipients (supplemental Figure 4). Sorted CAR T cells from both allogeneic and syngeneic recipients demonstrated comparable degranulation when co-cultured with CD19+ leukemia (Figure 3C). Analysis of cytokines in co-culture supernatant demonstrated a trend toward increased IFN-γ production by CAR T cells from syngeneic recipients compared with those recovered from allogeneic recipients, but this did not reach statistical significance (Figure 3D). Taken together, these results indicate that CAR T cells expand to a greater degree in allogeneic recipients and retain functionality, but become increasingly activated and fail to persist as GVHD develops.
Allogeneic CAR T-cell–induced GVHD was seen only in the presence of CD19+ leukemia
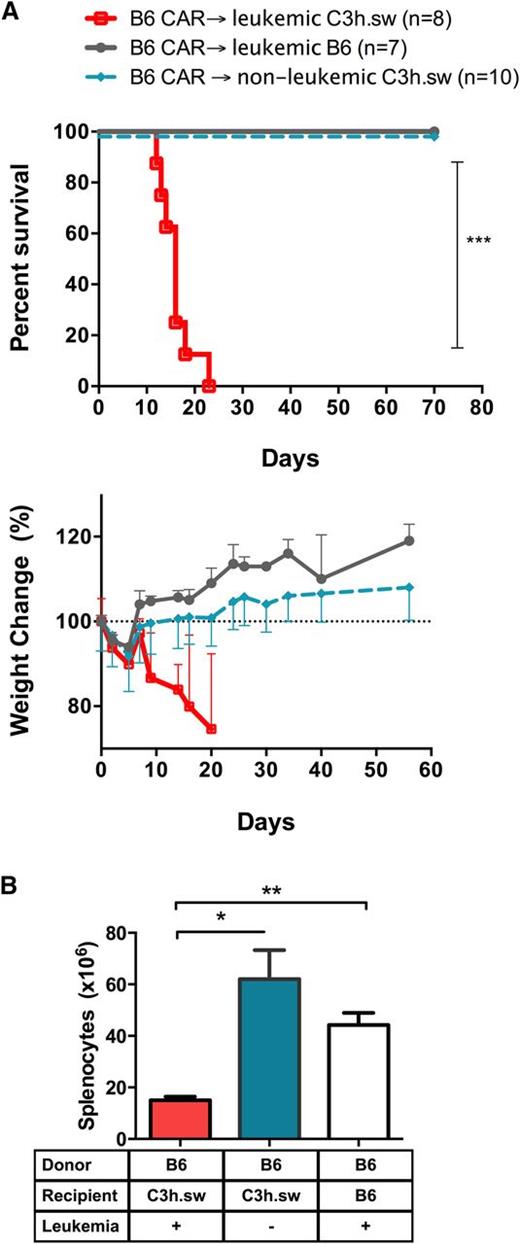
The rapid GVHD mortality was unexpected in this HLA-matched minor-mismatched model at the T-cell doses used.33-35 Thus, we next assessed which components of the model were necessary for the induction of GVHD. Donor-derived CAR T cells did not induce lethality or GVHD-associated weight loss in allogeneic recipients when administered to recipients without leukemia (Figure 4A). Furthermore, spleens harvested on day +7 showed similar cell numbers in non–leukemia-bearing allogeneic CAR T-cell recipients compared to syngeneic controls (Figure 4B), consistent with the absence of GVHD. Thus, the ability for allogeneic CAR T cells to cause GVHD is dependent on the presence of CD19+ leukemia.
Absence of leukemia abrogates CAR-induced GVHD. (A) Survival curves (top) and percent weight change (bottom) of leukemia-bearing B6 mice (gray), C3h.sw mice (red), and nonleukemic C3h.sw mice (cyan) receiving an allogeneic TCD BMT followed by CAR on day 2. Survival graphs indicate pooled data from 2 replicate experiments. (B) Splenocytes of C3h.sw leukemic mice (red), C3h.sw nonleukemic mice (cyan), and B6 leukemic mice (white) recipients of B6 TCD-BMT and CAR T cells were harvested on day 7 and enumerated. *P < .05; **P < .01.
Absence of leukemia abrogates CAR-induced GVHD. (A) Survival curves (top) and percent weight change (bottom) of leukemia-bearing B6 mice (gray), C3h.sw mice (red), and nonleukemic C3h.sw mice (cyan) receiving an allogeneic TCD BMT followed by CAR on day 2. Survival graphs indicate pooled data from 2 replicate experiments. (B) Splenocytes of C3h.sw leukemic mice (red), C3h.sw nonleukemic mice (cyan), and B6 leukemic mice (white) recipients of B6 TCD-BMT and CAR T cells were harvested on day 7 and enumerated. *P < .05; **P < .01.
CAR-induced GVHD is associated with elevated IL-6
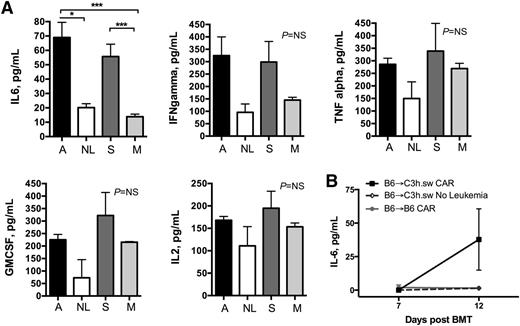
Given the requirements for both allogeneic CAR cells and CD19+ leukemia to generate lethal GVHD in this model, we tested whether cytokine production induced by in vivo CAR T-cell activation by target antigen drove the GVHD induced by adoptively transferred alloreactive T cells. Serum cytokine levels were measured on day +7, just prior to peak expansion of T cells, when peak cytokine production has been shown in similar GHVD models34 and recipients were well appearing. There was a significant increase in serum IL-6 in leukemia-bearing CAR recipients compared with mock controls and non–leukemia-bearing allogeneic CAR recipients (Figure 5). Although there was a trend toward an increase in serum levels of other pro-inflammatory cytokines (IFN-γ, TNF-α, IL-2, and granulocyte macrophage colony-stimulating factor), these did not reach statistical significance (Figure 5A). To confirm that the production of IL-6 is the result of the CAR activation, we delayed CAR infusion to day 7 to minimize the contribution of the post-irradiation inflammatory milieu. Cytokines were measured pre-CAR T-cell injection (day 7) and 5 days post-CAR (day 12). Again, a statistically significant increase in IL-6 was seen only in the leukemia-bearing allogeneic CAR-treated recipients and only 5 days after CAR infusion (Figure 5B).
IL-6 levels are increased following B6 → C3h.sw allogeneic CAR only in the presence of leukemia. (A) Serum was harvested on day 7 from mice in the following groups: allogeneic B6 CAR + BM → leukemia-bearing C3h.sw recipients (A, black bars), allogeneic B6 CAR + BM → nonleukemia bearing C3h.sw recipients (NL, white bars), syngeneic B6 CAR + BM → leukemia-bearing B6 recipients (S, dark gray bars), and syngeneic B6 mock T cells + BM → leukemia-bearing B6 recipients (M, light gray bars). Serum was analyzed for inflammatory cytokines in a multiplex panel. Data are representative of 2 separate experiments. (B) CAR DLI was delayed from day +2 until day +7, serum IL-6 was measured by ELISA pre-CAR (day 7), and 5 days later (day 12). *P < .05; ***P < .001. GMCSF, granulocyte macrophage colony-stimulating factor; NS, nonsignificant.
IL-6 levels are increased following B6 → C3h.sw allogeneic CAR only in the presence of leukemia. (A) Serum was harvested on day 7 from mice in the following groups: allogeneic B6 CAR + BM → leukemia-bearing C3h.sw recipients (A, black bars), allogeneic B6 CAR + BM → nonleukemia bearing C3h.sw recipients (NL, white bars), syngeneic B6 CAR + BM → leukemia-bearing B6 recipients (S, dark gray bars), and syngeneic B6 mock T cells + BM → leukemia-bearing B6 recipients (M, light gray bars). Serum was analyzed for inflammatory cytokines in a multiplex panel. Data are representative of 2 separate experiments. (B) CAR DLI was delayed from day +2 until day +7, serum IL-6 was measured by ELISA pre-CAR (day 7), and 5 days later (day 12). *P < .05; ***P < .001. GMCSF, granulocyte macrophage colony-stimulating factor; NS, nonsignificant.
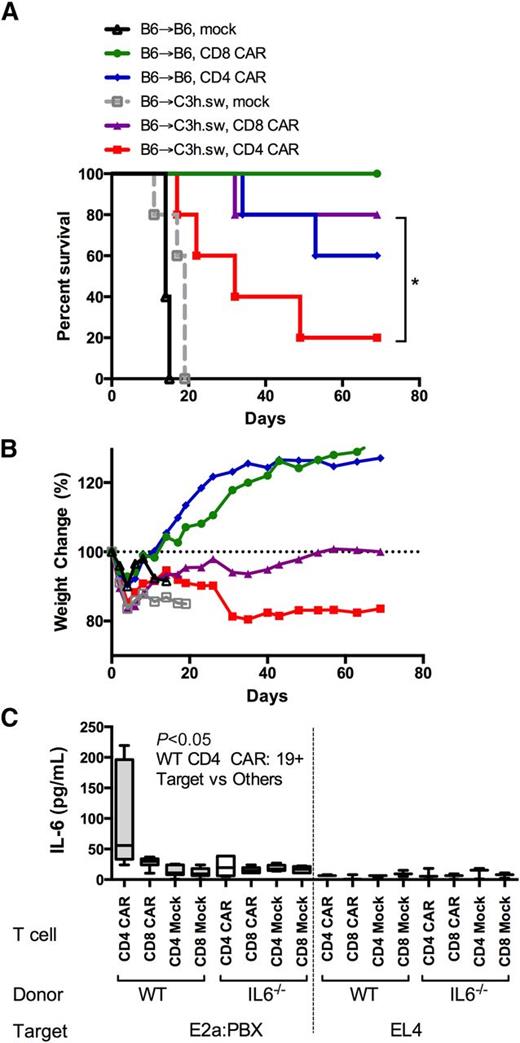
Only allogeneic CD4+ T cells are responsible for CAR-induced lethality and are associated with marked IL-6 elevations
We next evaluated the relative contribution of CD4+ or CD8+ CAR T cells to the induction of GVHD in the allogeneic environment. Purified allogeneic CD8+ CAR-expressing T cells did not cause GVHD, whereas all recipients of CD4+ CAR-expressing T cells developed severe GVHD early after infusion (Figure 6A-B). Interestingly, during co-culture of CD4+ and CD8+ CAR T cells with leukemia, a marked IL-6 production was seen only with CD4+ CAR and CD19+ leukemia (Figure 6C). Thus, CD4+ allogeneic CAR T cells are sufficient for the induction of GVHD, and IL-6 production by CD4+ CAR T cells may initiate or participate in this effect.
CD4+ CAR T cells are responsible for CAR-associated GVHD. (A) Survival curves and (B) percent weight change of mice receiving DLI on day 2 following syngeneic or allo-BMT into leukemia-bearing mice. DLI consisted of sorted CD4 or CD8 CAR T cells, with the following groups: B6 → C3h.sw CD4+ CAR (red), B6 → C3h.sw CD8+ CAR (purple), B6 → C3h.sw mock (gray), B6 → B6 CD4+ CAR (blue), B6 → B6 CD8+ CAR (green), and B6 → B6 mock (black). Data are representative from 2 separate experiments. (C) CD4+ and CD8+ T cells were isolated from wild-type (WT) B6 or IL-6−/− donors. Cells were expanded (mock) or expanded and CD19-CAR transduced (CAR), and co-cultured for 16 hours with CD19+ tumor (E2a:PBX) or CD19− tumor (EL4). IL-6 levels were measured on supernatant by IL-6 Quantikine ELISA. *P < .05.
CD4+ CAR T cells are responsible for CAR-associated GVHD. (A) Survival curves and (B) percent weight change of mice receiving DLI on day 2 following syngeneic or allo-BMT into leukemia-bearing mice. DLI consisted of sorted CD4 or CD8 CAR T cells, with the following groups: B6 → C3h.sw CD4+ CAR (red), B6 → C3h.sw CD8+ CAR (purple), B6 → C3h.sw mock (gray), B6 → B6 CD4+ CAR (blue), B6 → B6 CD8+ CAR (green), and B6 → B6 mock (black). Data are representative from 2 separate experiments. (C) CD4+ and CD8+ T cells were isolated from wild-type (WT) B6 or IL-6−/− donors. Cells were expanded (mock) or expanded and CD19-CAR transduced (CAR), and co-cultured for 16 hours with CD19+ tumor (E2a:PBX) or CD19− tumor (EL4). IL-6 levels were measured on supernatant by IL-6 Quantikine ELISA. *P < .05.
CAR-target induction of IL-6 contributes to the GVHD process
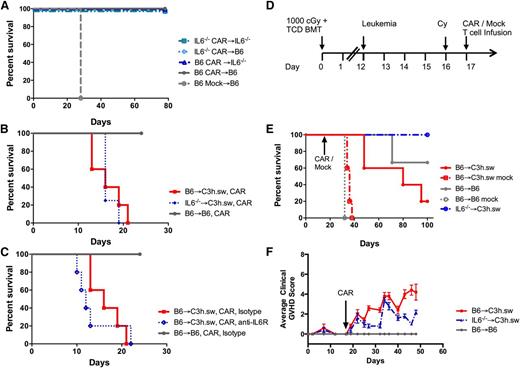
IL-6 has been shown to be elevated following CAR treatment, is associated with the development of cytokine release syndrome (CRS),15-17,36 and contributes to GVHD in murine models.34 We tested the importance of IL-6 to the in vivo function of CAR T cells in a syngeneic adoptive T-cell transfer model by using IL-6−/− mice as recipients, T-cell donors, or both. All combinations of IL-6−/− cells resulted in long-term survival confirming that IL-6 was not essential for the CAR-mediated eradication of leukemia (Figure 7A). Next, the contribution of IL-6 to GVHD induction by CAR T cells in an allogeneic model was evaluated. GVHD-related mortality still occurred when either BM cells or CAR cells lacked IL-6 producing capability (Figure 7B). Neutralization of IL-6 using a murine IL-6 receptor antibody also did not change GVHD-related mortality compared with IgG control-treated allogeneic recipients (Figure 7C).
GVHD can be reversed using an IL-6−/− donor in a delayed DLI model. (A) Survival curve of WT B6 or IL-6−/− leukemia-bearing mice treated with syngeneic CAR (B6 or IL-6−/−) following nonlethal radiation on day −1, without a BMT. (B) Survival curve of leukemia-bearing CAR T recipients with either B6 → C3h.sw setup (red) or using IL-6−/− mice as marrow and T cell donors (blue), with syngeneic B6 → B6 controls (gray). (C) Survival curve for leukemia-bearing CAR T recipients of syngeneic (gray), allogeneic B6 → C3h.sw treated with anti–IL-6 receptor antibody (blue), or isotype control IgG (red). (D) Delayed CAR-DLI model: at 12 days post-TCD–BMT mice were challenged with leukemia (106 cells/mouse), followed by cyclophosphamide 4 mg/mouse on day 16 and 106 CAR/mock T cells on day 17. WT B6 or IL-6−/− were used as marrow donors and all T cells were B6-CD45.1 derived, as indicated in graphs. (E) Survival curve with this delayed CAR model (P = .012; B6 vs IL-6−/− → C3h.sw CAR). (F) Clinical GVHD score in delayed CAR model. All in vivo experiments conducted with 5 mice per group and plots shown are representative of at least 2 separate experiments.
GVHD can be reversed using an IL-6−/− donor in a delayed DLI model. (A) Survival curve of WT B6 or IL-6−/− leukemia-bearing mice treated with syngeneic CAR (B6 or IL-6−/−) following nonlethal radiation on day −1, without a BMT. (B) Survival curve of leukemia-bearing CAR T recipients with either B6 → C3h.sw setup (red) or using IL-6−/− mice as marrow and T cell donors (blue), with syngeneic B6 → B6 controls (gray). (C) Survival curve for leukemia-bearing CAR T recipients of syngeneic (gray), allogeneic B6 → C3h.sw treated with anti–IL-6 receptor antibody (blue), or isotype control IgG (red). (D) Delayed CAR-DLI model: at 12 days post-TCD–BMT mice were challenged with leukemia (106 cells/mouse), followed by cyclophosphamide 4 mg/mouse on day 16 and 106 CAR/mock T cells on day 17. WT B6 or IL-6−/− were used as marrow donors and all T cells were B6-CD45.1 derived, as indicated in graphs. (E) Survival curve with this delayed CAR model (P = .012; B6 vs IL-6−/− → C3h.sw CAR). (F) Clinical GVHD score in delayed CAR model. All in vivo experiments conducted with 5 mice per group and plots shown are representative of at least 2 separate experiments.
In this model, allogeneic CAR T cells were given on day +2, during a significant multi-cytokine inflammatory response34,37 following myeloablative irradiation that can contribute to acute GVHD beyond the contribution of CAR-associated IL-6. Furthermore, at this early time-point, full engraftment of IL-6−/− cells was yet to be established. Thus, we next studied the contribution of IL-6 to GVHD induction by CAR T cells in a delayed GVHD model in IL-6–deficient BM chimeras. Mice were injected with leukemia on day +12 after BMT, followed by cyclophosphamide on day +16 and 106 B6-CD45.1 CAR T cells on day +17 (Figure 7D). Recipients of IL-6−/− BM were completely protected from the lethality induced by CAR T cells (Figure 7E-F), demonstrating the requirement for BM cell-derived IL-6 for the induction of GVHD by CAR T cells.
Discussion
In this study, we established a murine model of donor-derived allogeneic CAR T cells administered to prevent relapse in the immediate post–allo-BMT environment. We demonstrated that, despite allogeneic environment-induced immune dysfunction observed with TCR-based immune responses,21,23 allogeneic CAR T cells retain effectiveness at clearing leukemia. We also demonstrated that C3h.sw → B6 allogeneic minor-mismatched CAR T cells did not lead to clinical GVHD at the T-cell dose required to clear leukemia, but in the presence of leukemia, B6 → C3h.sw CAR T cells drive a lethal acute GVHD. We found that the CAR-target interaction generates an increase in pro-inflammatory cytokines, most notably IL-6, that may drive the proliferation of T cells and induction of GVHD.
Prior studies of T cells following allo-BMT showed significantly reduced T-cell function, as demonstrated by diminished vaccine responses and reduced T-cell proliferation.20-22 Our data demonstrates that, at early time points and despite ongoing GVHD-induced immune-suppression, CAR T cells are still functional in generating a specific response toward their antigen, as evidenced by both in vitro assays of CAR T cells harvested post-adoptive transfer and in vivo clearance of leukemia. Despite this early preservation of allogeneic CAR T-cell functionality, the persistence of CAR T cells is significantly lower in the presence of acute GVHD than in syngeneic recipients. We propose that this is due to robust contraction of allogeneic T cells that are activated by the CAR in the presence of an inflammatory milieu. This explains the in vivo activity of the CAR T cells, and is similar to murine models using transgenic-TCR T cells, in which deletion was demonstrated in allo-BMT models through supraphysiologic TCR stimuli.29-32,38 Thus, our data suggest the expression of a CAR does not protect against the deletion of T cells in the allogeneic setting.
We also demonstrate the potential risk of allogeneic donor-derived CAR T cells to generate GVHD. Much of the CAR pre-clinical literature involves immunodeficient murine models with human CAR T cells.39,40 These are limited by the nonphysiologic xenogeneic setting, in which clearance of tumor is associated with the development of xenogeneic GVHD thus preventing prolonged experiments or clear assessment of the contribution of the endogenous TCR to CAR–T-cell function.41,42 We chose to address this issue in a model that reflects MHC-matched minor-mismatched allo-BMT, similar to the most common clinical transplant setting. This minor-mismatch model is well established, and with early DLI at T-cell doses similar to ours, causes GVHD symptoms but does not typically cause early GVHD-associated lethality. Surprisingly, in this model, CAR T cells were able to mediate severe, lethal GVHD when CD19+ leukemia was present. Importantly, because CAR activation occurs in a non-MHC manner, the expansion of the allogeneic cells is independent of either donor or host APCs, potentially providing a partial explanation for the unusual severity of GVHD. Furthermore, we show here that CD4+ CAR T cells are necessary and sufficient to cause GVHD, a finding not typically seen with minor-mismatch models of GVHD.
Finally, we were able to implicate IL-6 as a significant cytokine contributing to CD4+ CAR-induced GVHD. High levels of IL-6 were observed in recipients of CAR T cells, prior to maximum in vivo cell expansion, correlating with published clinical data regarding IL-6 in CAR-related CRS.15-17,36 Indeed, IL-6 receptor blockade has been shown to control GVHD in preclinical models34,38 as well as early clinical trials,43 and is used to treat CAR-related severe CRS.36 In our models with early CAR T-cell DLI, we were not able to abort GVHD by blocking or genetically removing IL-6, suggesting that other cytokines are also involved early post-transplant. Furthermore, at this early time point, the use of IL-6−/− mice as marrow and T-cell donors may be incomplete, resulting in IL-6 production by surviving recipient cells.34 By delaying the DLI and allowing full engraftment of BM-derived IL-6−/− cells following allo-BMT, we were able to significantly decrease CAR-associated GVHD. Consistent with the complexities of IL-6 production in tumor immunology,44 our finding that loss of IL-6–producing capacity in BM-derived cells was sufficient to prevent GVHD provides insights into the potential source of IL-6 in the setting of CRS and suggests that, although CAR T cells can produce IL-6, they may not be the primary source. Finally, complete absence of IL-6 from BM, recipient, and CAR T cells did not prevent leukemic control in both syngeneic and allogeneic settings, suggesting that IL-6 may not be essential for CAR T-cell efficacy. This finding supports early or pre-emptive use of IL-6 blocking agents following CAR treatment, especially when using donor-derived cells.
In conclusion, we demonstrate that, in certain murine donor-recipient allo-BMT combinations, donor-derived CAR T cells have the potential to cause GVHD. Furthermore, alloreactivity can impair the persistence of CAR T cells, which has implications for the durability of CAR T-cell responses. As with CRS occurring after CAR T-cell infusions in humans, this GVHD is driven by IL-6 produced by a BM-derived cell. Interestingly, CD4+ T cells seem to be the major mediator of CAR T-cell–induced GVHD and a producer of IL-6 that, although not required, may contribute to the severity of GVHD. Finally, the lack of IL-6 production by CAR T cells or host (or neutralization using IL-6R antibodies) did not prevent the activity of CAR T cells. This study has important potential implications for the development of CAR T-cell therapy in the allogeneic setting, particularly when donor-derived T cells are used, an approach that is appealing as it circumvents the negative impact of cytotoxic therapy on T cells collected from a patient with leukemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank John Buckley for assistance with mouse experiments.
Authorship
Contribution: E.J. and T.J.F. conceived and designed research; E.J., Y.Y., H.Q., and C.D.C. performed experiments, collected, and analyzed data; J.N.K. designed and constructed the murine CD19 CAR; E.J. and T.J.F. wrote the manuscript; and all authors revised and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Correspondence: Terry J. Fry, Pediatric Oncology Branch, Center for Cancer Research, National Cancer Institute, National Institutes of Health, Building 10, Room 1-3750, 10 Center Dr, Bethesda, MD 20892; e-mail: fryt@mail.nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal