Abstract
Blastic plasmacytoid dendritic cell neoplasm is a rare and aggressive neoplasm for which there is still no current consensus on the best therapeutic approach. Most patients respond to intensive chemotherapy, but relapses are almost inevitable with median overall survival (OS) in the largest patient series ranging from 8 to 12 months except for patients who could benefit from allogenic hematopoietic stem cell transplantation (allo-HSCT). We present results of the first line treatments used in France between 2000 and 2013 for 86 patients recruited in the French network of BPDCN (abstract ASH 2015 N°78460).
Seventeen patients were treated with acute lymphoid leukemia (ALL)-like therapy (median age : 63 yo) , 19 with acute myeloid leukemia (AML)-like therapy (median age : 40 yo), 16 patients with CHOP-like therapy (median age : 72 yo), 16 patients with NK/T-like therapy (based on high-dose methotrexate and L-asparaginase, ± dexamethasone, median age: 59 yo), and 12 patients received "other treatments" (OT, means variable drugs, median age : 82 yo). Thirty four patients obtained a complete remission (CR) and received HSCT (autologous n=4, or allogeneic n=30). The response rates for CHOP-like and OT groups were 31.3% and 25.0% respectively. For ALL-like, AML-like, and NK/T-like groups, response rates reached 70.6%, 78.9%, and 62.5% respectively (no statistic difference). Relapse rates among responders for CHOP-like and OT groups were 60% and 33.3% whereas there were only 25%, 26.7%, and 20% in ALL-like, AML-like, and NK/T-like groups respectively. For patients who obtained remission, the median of remission duration was 8.0 and 14.0 months for patients who received CHOP-like treatments (n=5) and OT (n=3) respectively and 10.0, 10.0, and 9.0 months for ALL-like (n=11), AML-like (n=14), and NK/T-like groups (n=9) respectively (p = 0.6339).
In preclinical studies, we have shown that BPDCN cells are sensitive in vitro to idarubicine (Angelot Delettre F et al, 2015) so we studied patients receiving idarubicine in first line therapy in our series (n=9). From these 9 patients, 7 obtained CR and only one relapsed after 10 months. The 6 patients in continuous CR without any relapse have received HSCT (allo, n=5 or auto, n=1). Two out of those 6 patients are alive at the time of data collection with a follow-up of 40 and 87 months; the other 4 patients died after the graft, one relapsed after auto-HSCT, and 3 died of infectious complications after allo-HSCT.
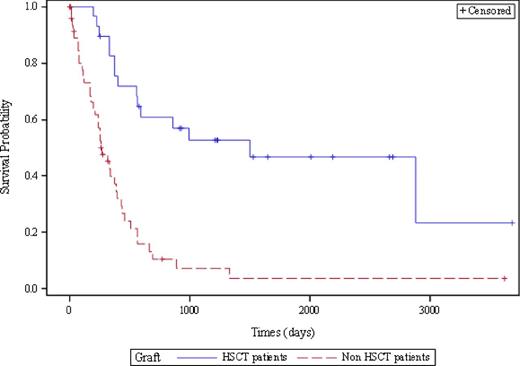
The median OS for patients who received HSCT, auto or allo (n=34) and other patients (n = 52) is respectively 49 and 8 months (p < 0.0001, Figure 1). The beneficial effect of HSCT persists independently of age in multivariate analysis.
These results suggest that NK/T-like, AML-like, and ALL-like groups give better results than CHOP-like and OT groups. However, there is no significant statistical difference between AML-like, ALL-like, and NK/T-like groups. Thus it seems to be wise to combine "lymphoid" drugs like methotrexate, L-asparaginase and dexamethasone with "myeloid" drug such as idarubicine. The importance of allogenic stem cell transplantation to sustain remission is clear in this study and other one (Roos-Weil et al, 2013). We also observed a prolonged CR in one patient after auto-HSCT. Based on our results, we will propose the first prospective, multicentric, phase II trial in BPDCN, testing a combination of 3 cycles of methotrexate, L-asparaginase, idarubicine and dexamethasone followed by an allo-HSCT in first clinical remission for all eligible patients or repeated cycle of these drugs for unfit patients with auto-HSCT if possible.
Kaplan-Meier overall survival curves compared by the Log-Rank test in the cohort of 34 HSCT patients (auto and allo, blue line) and 52 non HSCT patients (red line) (p<0.0001). Censured patients are patient's alive or lost (+). OS of HSCT patients is still statistically significative with adjustment of age in multivariate analysis (Cox multivariate).
Recher:Celgene; Amgen; Chugai: Research Funding; Janssen; Novartis; Amgen: Other: Travel, accommodations, expenses; Sunesis; Celgene: Consultancy. Deconinck:CHUGAI: Other: Travel for international congress; NOVARTIS: Other: Travel for international congress; ALEXION: Other: Travel for international congress; LFB loboratory: Consultancy; JANSSEN: Other: Travel for international congress; PFIZER: Research Funding; ROCHE: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal