Key Points
In vitro, SDF-1α/CXCR4 inhibition by LY2510924 is potent and prolonged and inhibits proliferation and stromal chemoprotection of AML cells.
In vivo, LY2510924 mobilizes AML cells, has striking antileukemia effects as monotherapy, and strongly synergizes with chemotherapy.
Abstract
Targeting the stromal cell–derived factor 1α (SDF-1α)/C-X-C chemokine receptor type 4 (CXCR4) axis has been shown to be a promising therapeutic approach to overcome chemoresistance in acute myeloid leukemia (AML). We investigated the antileukemia efficacy of a novel peptidic CXCR4 antagonist, LY2510924, in preclinical models of AML. LY2510924 rapidly and durably blocked surface CXCR4 and inhibited stromal cell–derived factor 1 (SDF-1)α–induced chemotaxis and prosurvival signals of AML cells at nanomolar concentrations more effectively than the small-molecule CXCR4 antagonist AMD3100. In vitro, LY2510924 chiefly inhibited the proliferation of AML cells with little induction of cell death and reduced protection against chemotherapy by stromal cells. In mice with established AML, LY2510924 caused initial mobilization of leukemic cells into the circulation followed by reduction in total tumor burden. LY2510924 had antileukemia effects as monotherapy as well as in combination with chemotherapy. Gene expression profiling of AML cells isolated from LY2510924-treated mice demonstrated changes consistent with loss of SDF-1α/CXCR4 signaling and suggested reduced proliferation and induction of differentiation, which was proved by showing the attenuation of multiple prosurvival pathways such as PI3K/AKT, MAPK, and β-catenin and myeloid differentiation in vivo. Effective disruption of the SDF-1α/CXCR4 axis by LY2510924 may translate into effective antileukemia therapy in future clinical applications.
Introduction
The interaction between acute myeloid leukemia (AML) cells and the bone marrow (BM) microenvironment has been postulated to be important for resistance to chemotherapy and disease relapse in AML.1 The C-X-C chemokine receptor type 4 (CXCR4) and its ligand, stromal cell–derived factor 1α (SDF-1α [CXCL12]), are key mediators of this interaction. Stromal cell–derived factor 1 (SDF-1)α is produced in the BM microenvironment, activates CXCR4 on leukemic cells, facilitates leukemia cell trafficking and homing in the BM microenvironment, and keeps leukemic cells in close contact with the stromal cells and extracellular matrix that constitutively generate growth-promoting and anti-apoptotic signals.2 Indeed, high CXCR4 expression on AML blasts is known to be associated with poor prognosis.3,4
Our group and others have tested small-molecule inhibitors against CXCR4: AMD3100 (Plerixafor), approved by the US Food and Drug Administration, and its analog AMD3465. These agents disrupted the SDF-1α/CXCR4 axis and enhanced the anti-leukemic effects of chemotherapy, markedly reducing leukemic burden and prolonging overall survival in xenograft models.5,6 Disruption of the SDF-1α/CXCR4 axis by CXCR4 antagonists is therefore an attractive investigational therapeutic approach for AML and is being tested in clinical trials. A phase 1/2 study recently reported that adding AMD3100 to cytotoxic chemotherapy increased response rates in patients with relapsed AML.1 However, the mobilization of leukemic blasts induced by AMD3100 is transient, and cell counts return to baseline levels within 12 hours,5 likely because of incomplete inhibition of the SDF-1α/CXCR4 axis and the short in vivo half-life (3-5 hours) of AMD3100.7 Furthermore, AMD3100 and AMD3465 did not show antileukemic effects as single agents in vivo,5,6 although they did have inhibitory effects on multiple cancers of nonhematologic origin.8-16
LY2510924 is a novel and potent selective peptide antagonist of CXCR4.17 A recent phase 1 study in advanced cancers revealed good tolerability with mostly grade 1 to 2 adverse events, favorable pharmacokinetics, and target engagement as indicated by dose-dependent increases in CD34+ cell mobilization.18 Here, we report preclinical studies using LY2510924 to disrupt the SDF-1α/CXCR4 axis in AML cells in vitro and in vivo.
Materials and methods
Please refer to supplemental Methods available on the Blood Web site for detailed descriptions of the methods and reagents used.
Cell lines, primary samples, and cultures
Human AML cell lines OCI-AML3, U937, and MOLM-13 (supplemental Table 1) were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (Gemini Bio-Products, West Sacramento, CA) and 1% penicillin-streptomycin (Gibco Laboratories, Grand Island, NY). Cells were harvested during the log phase of growth and seeded at a density of 0.2 × 106 cells per milliliter. Peripheral blood samples from patients with AML were collected during routine diagnostic procedures after informed consent was obtained in accordance with Institutional Review Board regulations of The University of Texas MD Anderson Cancer Center and the Declaration of Helsinki. Mononuclear cells were separated by Ficoll-Hypaque (Sigma-Aldrich, St. Louis, MO) density gradient centrifugation.
Flow cytometry
The expression of surface CXCR4 protein was analyzed by using a Gallios flow cytometer (Beckman Coulter, Brea, CA). Harvested cells were stained with antibodies against CXCR4-allophycocyanin (APC; 12G5) and CXCR4-phycoerythrin (PE; 1D9). All antibodies were purchased from BD Biosciences (San Jose, CA). The appropriate isotype-matched antibody was used as a negative control. In vivo leukemic cells were isolated by further staining of samples from mice with human CD45-APC for OCI-AML3/Luc/mCherry cells, CD45-PerCP-Cy5.5 for OCI-AML3/Luc/green fluorescent protein (GFP) cells,19 and CD34-PE-Cy7 and CD45-APC-H7 for primary AML cells. Flow cytometric data were analyzed with FlowJo vX.0.6 software.
Chemotaxis studies
OCI-AML3 cells and primary samples from AML patients (supplemental Table 2 and supplemental Figure 1) were subjected to chemotaxis studies in 6.5-mm-diameter Transwell culture inserts (Costar, Corning, NY) with a pore size of 5 µm. All experiments were conducted in triplicate, and results are expressed as the percentage of migrated cells.
Western blot analysis
Cell lysates were separated on 12% polyacrylamide gels, transferred to nitrocellulose membranes, stained with the appropriate antibodies and infrared secondary antibodies (LI-COR Biosciences, Lincoln, NE), and quantified by the Odyssey imaging system (LI-COR Biosciences). Antibodies used were rabbit antihuman phospho-AKT (Ser473), AKT, phospho-glycogen synthase kinase 3 beta (GSK-3β; Ser9), and mouse antihuman phospho-p44/p42 MAPK (Erk1/2)(Thr202/Tyr204) (all four, Cell Signaling Technology, Beverly, MA), mouse antihuman active β-catenin (Millipore, Temecula, CA), Bcl-2 (Dako North America, Inc., Carpenteria, CA), ERK2, and GSK-3β (Santa Cruz Biotechnology, Santa Cruz, CA). Glyceraldehyde-3-phosphate dehydrogenase and β-tubulin were used as the loading control.
Coculture with stromal cells
OCI-AML3 cells were cocultured with MS-5 or human mesenchymal stromal cells. After 72 hours of incubation at 37°C in a humidified atmosphere containing 5% CO2, cocultured cells were harvested, and viable and apoptotic OCI-AML3 cells were quantified by flow cytometry.
AML mouse models
NOD/SCID/IL-2rγnull (NSG) mice were used for in vivo xenograft experiments. Mice were injected with OCI-AML3 cells labeled with Luc/mCherry or Luc/GFP19 or primary AML cells to establish AML. All animal experiments were done in accordance with a protocol approved by the Institutional Animal Care and Use Committee at The University of Texas MD Anderson Cancer Center.
Flow cytometry for detection of intracellular phospho-proteins
Changes in AKT and ERK phosphorylation were measured in the xenograft mouse model of primary AML by multiparametric phospho-flow cytometry in dual-positive cells (human CD34 and CD45) recovered from the BM and spleen of a representative mouse from each treatment group on day 48 after transplantation.
Gene expression profiling
Xenotransplanted OCI-AML3 cells were harvested and isolated on days 41 to 43 and preserved in RNAlater solution. Total RNA was extracted, amplified, and labeled through 2 rounds of in vitro transcription and then hybridized to Illumina HT12 (version 4) human whole-genome arrays as described previously.20 Gene expression data were submitted to Gene Expression Omnibus as GSE64623.
Statistical analysis
In general, results are expressed as the mean ± standard deviation (SD) for triplicate experiments or mean ± standard error of the mean (SEM) for triplicate independent experiments. The Student paired t test was used to compare differences between groups. For in vivo mouse experiments, overall survival and mean group survival times were estimated by the Kaplan-Meier method and compared with the log-rank test. Differences with P values ≤.05 were considered statistically significant.
Results
LY2510924 rapidly and durably blocks surface CXCR4 and inhibits SDF-1α–induced chemotaxis and prosurvival signals of leukemic cells
To determine the effects of CXCR4 blockade by LY2510294, we used AML cell lines OCI-AML3, U937, and MOLM-13, which express high levels of CXCR4 on their cell surface. Flow cytometry results showed that LY2510924 inhibited binding of anti-CXCR4 antibody 12G5 to surface CXCR4 in a concentration-dependent fashion in AML cells (Figure 1A-B and supplemental Figure 2). LY2510924 was much more potent than AMD3100 in causing CXCR4 blockade. CXCR4 occupancy by LY2510924 in OCI-AML3 cells started as early as 1 minute after treatment (Figure 1C) and continued for as long as 72 hours at 10 nM, in contrast to the short duration of blockade by AMD3100 (Figure 1D). Both LY2510924 and AMD3100 induced moderate upregulation of cell surface CXCR4 as measured by the 1D9 antibody, which binds to a different CXCR4 epitope (supplemental Figure 3), suggesting the accumulation of CXCR4 on cell surface due to inhibition of SDF-1α–induced CXCR4 internalization.1 SDF-1α–induced migration of OCI-AML3 (Figure 1E) and primary AML cells (Figure 1F) was abolished by 1 nM LY2510924 but not by 1 nM AMD3100. Western blots showed that LY2510924 inhibited SDF-1α–induced phosphorylation of ERK and AKT (Figure 1G-H).
LY2510924 rapidly and durably blocks surface CXCR4 and inhibits SDF-1α–induced chemotaxis and prosurvival signals of leukemic cells. Both (A) OCI-AML3 and (B) U937 cells were cultured with different concentrations of LY2510924 or AMD3100 for 150 minutes, and surface CXCR4 was measured by flow cytometry with antibody 12G5, which is blocked by receptor occupancy with either agent. Results are expressed as percentage change in the mean fluorescent intensity (MFI) compared with control (untreated) cells. (C) OCI-AML3 cells were cultured with 1 nM LY2510924, and surface CXCR4 12G5 binding was measured by flow cytometry at different time points. (D) OCI-AML3 cells were cultured with different concentrations of LY2510924 or AMD3100 for 72 hours, and surface CXCR4 12G5 binding was measured by flow cytometry. (E) OCI-AML3 (0.5 × 106) or (F) primary AML (1.0 × 106; n = 3) cells were plated onto the upper chamber of Transwell plates and exposed to 50 ng/mL SDF-1α in the lower chamber with or without 1 nM LY2510924 or AMD3100 for (E) 2.5 hours or (F) 12 hours. The results are expressed as percentage of migrating cells relative to the number of input cells. After overnight serum starvation, (G) 1 × 106 OCI-AML3 or (H) U937 cells in RPMI medium containing 0.5% bovine serum albumin were or were not pretreated with 1 µM LY2510924 for 1 hour and were exposed to 100 ng/mL SDF-1α for 10 minutes. Phosphorylation of AKT (pAKT) and ERK (pERK) was detected by western blot analysis, and the intensity of the bands was quantified by densitometry and displayed as the ratio of phosphorylated protein to control phospho-protein. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. All results are expressed as the mean ± SD, with the exception of (F), expressed as the mean ± SEM.
LY2510924 rapidly and durably blocks surface CXCR4 and inhibits SDF-1α–induced chemotaxis and prosurvival signals of leukemic cells. Both (A) OCI-AML3 and (B) U937 cells were cultured with different concentrations of LY2510924 or AMD3100 for 150 minutes, and surface CXCR4 was measured by flow cytometry with antibody 12G5, which is blocked by receptor occupancy with either agent. Results are expressed as percentage change in the mean fluorescent intensity (MFI) compared with control (untreated) cells. (C) OCI-AML3 cells were cultured with 1 nM LY2510924, and surface CXCR4 12G5 binding was measured by flow cytometry at different time points. (D) OCI-AML3 cells were cultured with different concentrations of LY2510924 or AMD3100 for 72 hours, and surface CXCR4 12G5 binding was measured by flow cytometry. (E) OCI-AML3 (0.5 × 106) or (F) primary AML (1.0 × 106; n = 3) cells were plated onto the upper chamber of Transwell plates and exposed to 50 ng/mL SDF-1α in the lower chamber with or without 1 nM LY2510924 or AMD3100 for (E) 2.5 hours or (F) 12 hours. The results are expressed as percentage of migrating cells relative to the number of input cells. After overnight serum starvation, (G) 1 × 106 OCI-AML3 or (H) U937 cells in RPMI medium containing 0.5% bovine serum albumin were or were not pretreated with 1 µM LY2510924 for 1 hour and were exposed to 100 ng/mL SDF-1α for 10 minutes. Phosphorylation of AKT (pAKT) and ERK (pERK) was detected by western blot analysis, and the intensity of the bands was quantified by densitometry and displayed as the ratio of phosphorylated protein to control phospho-protein. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. All results are expressed as the mean ± SD, with the exception of (F), expressed as the mean ± SEM.
LY2510924 inhibits proliferation of AML cells rather than causing cell death and reverses stroma-mediated chemoresistance
OCI-AML3 cells were cultured with LY2510924 to examine its ability to induce cell death and/or inhibit growth in vitro. Measurement of viable cell numbers over an 8-day period demonstrated that SDF-1α significantly enhanced the proliferation of OCI-AML3 cells compared with controls (at day 8, P = .013). Proliferation was inhibited by LY2510924 (day 4, P = .003; day 6, P = .013; day 8, P = .001; Figure 2A) to the same level in the presence or absence of SDF-1α; the effect of LY2510924 alone compared with control was significant at days 4 (P = .005), 6 (P < .001), and 8 (P = .002) (Figure 2A). However, LY2510924 did not induce AML cell death in vitro (supplemental Figure 4).
LY2510924 inhibits proliferation of AML cells and reverses stroma-mediated chemoresistance. (A) OCI-AML3 cells (3 × 104/mL) were grown in 2% fetal bovine serum containing RPMI in the presence or absence of 100 ng/mL SDF-1α with or without daily treatment with 1 µM LY2510924 for up to 8 days. Flow cytometry using annexin V–positive/4′,6 diamidino-2-phenylindole (DAPI) –positive staining and counting beads were used to assess the percentage of apoptotic cells. (B-C) OCI-AML3 cells were cultured alone (monoculture) or cocultured with stromal cells (MS-5) as indicated in “Materials and methods.” Monocultured and cocultured cells were treated for 72 hours with 2.5 µM cytarabine (Ara-C) in the presence or absence of 1 µM LY2510924. The surface CXCR4 (B) 12G5 and (C) 1D9 staining and percentages of (D) apoptotic cells and (E) viable cells were assessed by flow cytometry. All results are expressed as the mean ± SD. *P < .05; **P < .01.
LY2510924 inhibits proliferation of AML cells and reverses stroma-mediated chemoresistance. (A) OCI-AML3 cells (3 × 104/mL) were grown in 2% fetal bovine serum containing RPMI in the presence or absence of 100 ng/mL SDF-1α with or without daily treatment with 1 µM LY2510924 for up to 8 days. Flow cytometry using annexin V–positive/4′,6 diamidino-2-phenylindole (DAPI) –positive staining and counting beads were used to assess the percentage of apoptotic cells. (B-C) OCI-AML3 cells were cultured alone (monoculture) or cocultured with stromal cells (MS-5) as indicated in “Materials and methods.” Monocultured and cocultured cells were treated for 72 hours with 2.5 µM cytarabine (Ara-C) in the presence or absence of 1 µM LY2510924. The surface CXCR4 (B) 12G5 and (C) 1D9 staining and percentages of (D) apoptotic cells and (E) viable cells were assessed by flow cytometry. All results are expressed as the mean ± SD. *P < .05; **P < .01.
We then tested whether CXCR4 inhibition by LY2510924 can overcome stroma-mediated chemoresistance of AML cells in vitro by coculturing the AML cells with MS-5 stromal cells for 3 days. As reported by another group,21 cytarabine significantly upregulated CXCR4 surface expression by OCI-AML3 cells in conditions reflected by measurement of antibody 12G5 (monoculture, 2.6-fold increase vs control, P = .006; with MS-5 cells, 2.7-fold increase vs control, P = .002; Figure 2B) or antibody 1D9 (monoculture, 2.7-fold increase vs control, P < .001; with MS-5 cells, 3.3-fold increase vs control, P < .001; Figure 2C). CXCR4 detection with 12G5 was blocked by LY2510924 (Figure 2B), but 1D9 showed even further upregulation of CXCR4 by cytarabine plus LY2510924 (Figure 2C), especially in cells cocultured with MS-5 cells. This is perhaps expected, because LY2510924 alone upregulates CXCR4 (supplemental Figure 3). Cytarabine-induced apoptosis of AML cells was significantly reduced by stromal cells (70.3% ± 7% vs 43.8% ± 4.2%; P = .005), but this chemoprotective effect of stromal cells was significantly inhibited by LY2510924 (61.9% ± 5.6%; P = .011; Figure 2D-E). Similar chemoprotection by stromal cells and its inhibition by LY2510924 were seen in OCI-AML3 cells treated with doxorubicin in human mesenchymal stromal cell coculture (supplemental Figure 5).
LY2510924 monotherapy has antileukemia activity in OCI-AML3 xenograft models
To test the antileukemia efficacy of LY2510924 in vivo, we injected sublethally irradiated NSG mice with OCI-AML3 cells (OCI-AML3/Luc/mCherry) engineered to allow bioluminescent imaging (BLI) of tumor burden as well as sorting on the basis of fluorescence. In mobilization studies, circulating leukemic cells were increased at 3 hours (3.4- ± 1.4-fold) and further at 24 hours (24.1- ± 15.4-fold) in 3 mice that received the first LY2510924 injection on day 21 after AML cell injection (Figure 3B). In 2 other groups (control and LY2510924) that began treatment on day 12, mice treated daily with LY2510924 had significantly less BLI signal than controls (P = .030 on day 18; P < .001 on day 25; Figure 3C-D). Moreover, in representative mice euthanized on day 22, immunohistochemical staining of tissues for human CD45 demonstrated that LY2510924-treated mice had less leukemic infiltration than controls (Figure 3E). Analysis of multispectral images (see supplemental Data) further confirmed that controls had significantly more leukemia than LY2510924-treated mice in BM (84.6% ± 1.4% vs 40.7% ± 3.2%; P < .001), spleen (85.2% ± 2.2% vs 30.2% ± 2.7%; P < .001), and liver (88.5% ± 0.5% vs 12.7% ± 1.6%; P < .001; Figure 3E). This antileukemia effect translated into significant prolongation of survival in LY2510924-treated mice (median survival, 40 days vs 26 days; P < .001; Figure 3F).
LY2510924 monotherapy has antileukemia activity in OCI-AML3 xenograft models. OCI-AML3/Luc/mCherry cells (1 × 106 per mouse) were intravenously injected into sublethally irradiated (250 cGy) NSG mice. Circulating OCI-AML3 cells were (A) identified by flow cytometry, and (B) percentages were determined among all nucleated cells in mice (n = 3) before and 3 or 24 hours after a single LY2510924 treatment on days 21 and 22. Shown are (C) serial bioluminescence images and (D) intensity quantitation of 5 representative mice from 2 groups, control and LY2510924 (n = 10 each), that began 3 weeks of daily treatment 12 days after cell injection. (E) Three representative mice per group were euthanized on day 22, and tissues were fixed and sectioned for immunohistochemical analysis with antihuman CD45 antibodies to identify human leukemic cells. Analysis of the multispectral images further confirmed that leukemic cell burden was significantly reduced in LY2510924-treated mice. Original magnification, ×200. (F) Overall survival rate in each group was estimated by the Kaplan-Meier method. Results of (D), (E), and (F) are expressed as the mean ± SEM. *P < .05; **P < .01.
LY2510924 monotherapy has antileukemia activity in OCI-AML3 xenograft models. OCI-AML3/Luc/mCherry cells (1 × 106 per mouse) were intravenously injected into sublethally irradiated (250 cGy) NSG mice. Circulating OCI-AML3 cells were (A) identified by flow cytometry, and (B) percentages were determined among all nucleated cells in mice (n = 3) before and 3 or 24 hours after a single LY2510924 treatment on days 21 and 22. Shown are (C) serial bioluminescence images and (D) intensity quantitation of 5 representative mice from 2 groups, control and LY2510924 (n = 10 each), that began 3 weeks of daily treatment 12 days after cell injection. (E) Three representative mice per group were euthanized on day 22, and tissues were fixed and sectioned for immunohistochemical analysis with antihuman CD45 antibodies to identify human leukemic cells. Analysis of the multispectral images further confirmed that leukemic cell burden was significantly reduced in LY2510924-treated mice. Original magnification, ×200. (F) Overall survival rate in each group was estimated by the Kaplan-Meier method. Results of (D), (E), and (F) are expressed as the mean ± SEM. *P < .05; **P < .01.
LY2510924 inhibits progression of primary AML cells in xenograft models
To confirm the antileukemia efficacy of LY2510924, we injected primary AML cells derived from patients with complex karyotype and high CXCR4 surface expression (supplemental Figure 6) into nonirradiated NSG mice. Mice were divided into 2 groups after engraftment was documented in peripheral blood, and 1 group began treatment with LY2510924 on day 25. The proportion of circulating leukemic cells was measured in 5 representative mice from each group after the first LY2510924 administration. Circulating leukemia cells were significantly increased by LY2510924 3 hours after treatment compared with controls (2.1- ± 0.2-fold vs 1.4- ± 0.1-fold increase over baseline; P = .008) and were increased by even more 24 hours after treatment (2.7- ± 0.4-fold vs 1.0- ± 0.1-fold increase over baseline; P = .008; Figure 4A and supplemental Figure 6). Flow cytometry showed sustained inhibition of CXCR4 staining by 12G5 at 3 and 24 hours after the first LY2510924 injection (Figure 4B) and significant blockade after 5 daily treatments compared with controls (2.7- ± 0.1-fold vs 10.2- ± 1.9-fold increase over isotype; P = .017; Figure 4C).
LY2510924 durably blocks surface CXCR4, induces mobilization of leukemia cells, and inhibits AKT and ERK intracellular signaling in vivo, retarding progression of primary AML xenografts. Primary AML cells (0.4 × 106 per mouse) were intravenously injected into NSG mice. Mice were divided into two groups, control (n = 13) and LY2510924 (n = 15), after engraftment was documented in peripheral blood on day 25 and began to receive daily treatment. (A) In 5 representative mice of each group, percentages of circulating primary AML cells before and 3 or 24 hours after the first LY2510924 injection on days 25 and 26 were compared with those of untreated mice. (B) Identification of circulating leukemic cells by flow cytometry and staining for CXCR4 with antibody 12G5 (inversely reflective of receptor occupancy) at points during the initial 2 days of daily LY2510924 administration are shown for a representative mouse. (C) CXCR4 staining with antibody 12G5 after 5 days of LY2510924 treatment was compared in 5 representative mice of each group. (D-E) Three representative mice per group were euthanized on day 45, and cells from BM, spleen, and blood in all mice (control, n = 6; LY2510924, n = 11) on day 45 were analyzed by flow cytometry; dual-positive cells (human CD34 and CD45) were compared between each group in terms of (D) expression of CXCR4 12G5 and (E) proportion of leukemic cells. (F) AKT and ERK phosphorylation in dual-positive cells (human CD34 and CD45) recovered from BM and spleen were measured by multiparametric phospho-flow cytometry in a representative mouse from each group on day 48. The results showed that receptor occupancy by LY2510924 correlates with reduced AKT and ERK phosphorylation. (G) Overall survival rate in each group was estimated by the Kaplan-Meier method. Results of (A), (C-E), and (G) are expressed as the mean ± SEM. *P < .05; **P < .01.
LY2510924 durably blocks surface CXCR4, induces mobilization of leukemia cells, and inhibits AKT and ERK intracellular signaling in vivo, retarding progression of primary AML xenografts. Primary AML cells (0.4 × 106 per mouse) were intravenously injected into NSG mice. Mice were divided into two groups, control (n = 13) and LY2510924 (n = 15), after engraftment was documented in peripheral blood on day 25 and began to receive daily treatment. (A) In 5 representative mice of each group, percentages of circulating primary AML cells before and 3 or 24 hours after the first LY2510924 injection on days 25 and 26 were compared with those of untreated mice. (B) Identification of circulating leukemic cells by flow cytometry and staining for CXCR4 with antibody 12G5 (inversely reflective of receptor occupancy) at points during the initial 2 days of daily LY2510924 administration are shown for a representative mouse. (C) CXCR4 staining with antibody 12G5 after 5 days of LY2510924 treatment was compared in 5 representative mice of each group. (D-E) Three representative mice per group were euthanized on day 45, and cells from BM, spleen, and blood in all mice (control, n = 6; LY2510924, n = 11) on day 45 were analyzed by flow cytometry; dual-positive cells (human CD34 and CD45) were compared between each group in terms of (D) expression of CXCR4 12G5 and (E) proportion of leukemic cells. (F) AKT and ERK phosphorylation in dual-positive cells (human CD34 and CD45) recovered from BM and spleen were measured by multiparametric phospho-flow cytometry in a representative mouse from each group on day 48. The results showed that receptor occupancy by LY2510924 correlates with reduced AKT and ERK phosphorylation. (G) Overall survival rate in each group was estimated by the Kaplan-Meier method. Results of (A), (C-E), and (G) are expressed as the mean ± SEM. *P < .05; **P < .01.
On day 45, after 3 weeks of daily LY2510924 treatment, 3 mice in each group were euthanized, and flow cytometry of leukemic cells in their blood, BM, and spleens revealed significant blockade of CXCR4 12G5 staining by LY2510924 (Figure 4D). This analysis also demonstrated that mice treated with LY2510924 had lower leukemic cell burden in blood (50.5% ± 2.4% vs 86.1% ± 2.9%; P < .001), BM (72.1% ± 3.6% vs 89.7% ± 1.9%; P = .012), and spleen (19.6% ± 0.6% vs 60.1% ± 0.7%; P < .001; Figure 4E). Multiparametric phospho-flow cytometry showed decreases in AKT and/or ERK phosphorylation in leukemic cells recovered from the spleen and BM of an LY2510924-treated mouse on day 48 (Figure 4F), which was consistent with our in vitro data (Figure 1G-H). These antileukemia effects of LY2510924 alone resulted in significant prolongation of survival (median survival, 56 days vs 44 days; overall, P < .001; Figure 4G).
Combination with chemotherapy enhances LY2510924 antileukemia effects in OCI-AML3 xenograft models
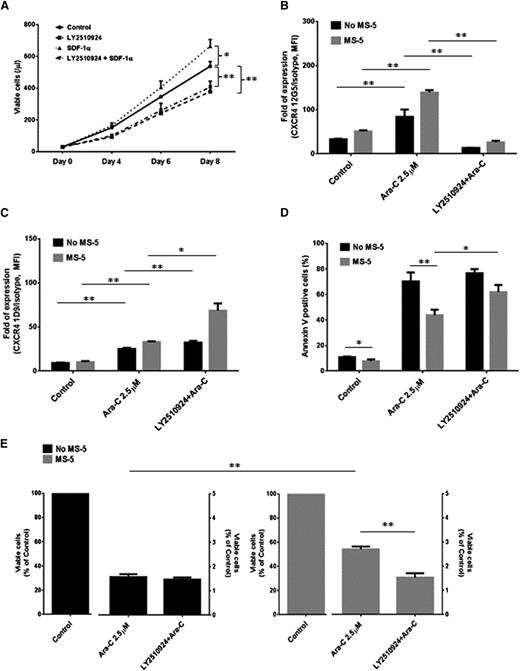
To further explore the antileukemia efficacy of LY2510924 in combination with chemotherapy in vivo, we injected OCI-AML3/Luc/GFP cells19 into nonirradiated NSG mice. Mobilization studies were carried out in 2 groups of 6 mice each; in mice given their first LY2510924 injection on day 25, prolonged mobilization of leukemic cells into the circulation by LY2510924 was confirmed (3.1- ± 0.7-fold vs 1.3- ± 0.3-fold increase over baseline at 3 hours, P = .035; 5.3- ± 1.7-fold vs 1.3 ± 0.2-fold increase over baseline at 24 hours, P = .036; Figure 5A and supplemental Figure 7). Four other groups began treatment on day 8: control, chemotherapy (cytarabine/doxorubicin) only, LY2510924 only, or combination (LY2510924 plus chemotherapy). BLI demonstrated significantly less tumor burden in all treated groups than in controls by day 26 (Figure 5B-C); leukemia progression was reduced equivalently by LY2510924 alone and chemotherapy (P = .249) and was lowest with combination therapy. Immunohistochemical staining for human CD45 from mice euthanized on day 27 (Figure 5D) showed findings consistent with those from BLI.
LY2510924 induces mobilization of OCI-AML3 cells in vivo and enhances antileukemia effects in combination with chemotherapy. OCI-AML3/Luc/GFP cells (1 × 106 per mouse) were intravenously injected into NSG mice. (A) In mobilization studies, percentages of circulating OCI-AML3/Luc/GFP cells before and 3 or 24 hours after the first LY2510924 injection on day 25 in 6 mice were compared with those in untreated mice (n = 6). (B-E) After confirming leukemia engraftment by BLI, mice were divided into 4 groups (10 mice per group) and began to receive treatment on day 8: control (no treatment), chemotherapy (cytarabine [Ara-C]/doxorubicin), LY2510924, or the combination of chemotherapy with LY2510924. Chemotherapeutics were administered 3 hours after LY2510924 administration. Five representative mice from each group were subjected to (B) serial BLI and (C) intensity quantitation on days 7, 26, and 40 after leukemic cell injection. (D) Three representative mice per group were euthanized on day 27 for immunohistochemical analysis for human CD45 to identify human leukemic cells. Analysis of the multispectral images further confirmed the significantly reduced leukemic cell burden in LY2510924-treated mice and even more so in the combination group. Original magnification, ×40. (E) Overall survival rate in each group was estimated by the Kaplan-Meier method. Results of (A) and (C-D) are expressed as the mean ± SEM. *P < .05; **P < .01.
LY2510924 induces mobilization of OCI-AML3 cells in vivo and enhances antileukemia effects in combination with chemotherapy. OCI-AML3/Luc/GFP cells (1 × 106 per mouse) were intravenously injected into NSG mice. (A) In mobilization studies, percentages of circulating OCI-AML3/Luc/GFP cells before and 3 or 24 hours after the first LY2510924 injection on day 25 in 6 mice were compared with those in untreated mice (n = 6). (B-E) After confirming leukemia engraftment by BLI, mice were divided into 4 groups (10 mice per group) and began to receive treatment on day 8: control (no treatment), chemotherapy (cytarabine [Ara-C]/doxorubicin), LY2510924, or the combination of chemotherapy with LY2510924. Chemotherapeutics were administered 3 hours after LY2510924 administration. Five representative mice from each group were subjected to (B) serial BLI and (C) intensity quantitation on days 7, 26, and 40 after leukemic cell injection. (D) Three representative mice per group were euthanized on day 27 for immunohistochemical analysis for human CD45 to identify human leukemic cells. Analysis of the multispectral images further confirmed the significantly reduced leukemic cell burden in LY2510924-treated mice and even more so in the combination group. Original magnification, ×40. (E) Overall survival rate in each group was estimated by the Kaplan-Meier method. Results of (A) and (C-D) are expressed as the mean ± SEM. *P < .05; **P < .01.
Analysis of the multispectral images (see supplemental Data) revealed less leukemic infiltration in all treated groups, lowest infiltration in the combination group, and no significant difference in infiltration between mice treated with cytarabine/doxorubicin and those treated with LY2510924 (BM: 3.0% ± 0.4% vs 2.8% ± 0.3%, P = .738; spleen: 0.7% ± 0.1% vs 0.6% ± 0.1%, P = .785; liver: 0.5% ± 0.2% vs 0.4% ± 0.1%, P = .741). LY2510924-treated mice had prolonged survival compared with controls (median survival, 52 days vs 40 days; P = .006), and combination therapy extended survival even further (median survival, 62 days vs 52 days; P = .004; Figure 5E).
LY2510924 induces gene expression changes in leukemic cells in vivo consistent with loss of SDF-1α/CXCR4 signaling
To elucidate the molecular effects of inhibiting CXCR4-mediated signaling in AML cells in vivo, we again used the OCI-AML3/Luc/GFP xenograft model. Engraftment, assessed as sufficient involvement of the blood by leukemic cells (3.3% ± 1.1%), was reached in 8 mice on day 40. Groups of these mice began daily treatment with LY2510924 on that day and were euthanized 24 hours later (day 41 after 1 treatment; n = 3) or 72 hours later (day 43 after 3 treatments; n = 2). Three untreated mice were euthanized on day 42 as controls. Leukemic cells were isolated from BM, blood, and spleen of all mice by fluorescence-activated cell sorting by human CD45 and GFP (supplemental Figure 8). Samples from each site and time point were also analyzed for CXCR4 occupancy by using flow cytometry with antibody 12G5. CXCR4 blockade by LY2510924 was delayed in BM compared with that in blood and spleen (Figure 6A), but unoccupied CXCR4 in BM was decreased to a level similar to that measured in blood and spleen at 72 hours, suggesting that repeated doses are needed to affect leukemic cells in BM.
LY2510924 induces gene expression changes in leukemic cells in vivo that are consistent with loss of SDF-1α/CXCR4 signaling. In the OCI-AML3/Luc/GFP xenograft model, engraftment was confirmed in 8 mice on day 40 by proportion of leukemic cells in blood (3.3% ± 1.1%). Groups of these mice were then given daily LY2520924 treatment and euthanized after 24 hours (n = 3 on day 41) or 72 hours (n = 2 on day 43). Control mice receiving no treatment (n = 3) were euthanized on day 42. Leukemic cells were sorted and separated from BM, blood, and spleen by fluorescence-activated cell sorting (FACS) using the specific markers human CD45 and GFP. (A) Samples from each site and treatment time point were also analyzed by FACS with CXCR4 antibody 12G5 as an inverse indicator of CXCR4 occupancy by LY2510924. (B) From genome-wide GEP of 24 samples (3 groups, 3 tissue sites, 2 or 3 mice per group), log2 values of each sample’s genes from the treated mice were subtracted by the average for the corresponding gene in the control BM samples. The heat map shows subtracted values converted to fold-change for genes with an absolute subtracted log2 value of at least 1.5 for at least 6 samples, with samples and genes hierarchically clustered for similarity in variation. The color bar indicates fold-change values, and the dendrogram indicates similarity between samples, which are labeled according to duration of LY2510924 treatment. (C) Selected genes whose expression levels are highly correlated with a score of the presumed relative degree of loss of SDF-1α/CXCR4 signaling, based on non-BM localization and/or LY2510924 treatment and its duration. Score values are shown above sample names, and gene expression values are shown by fold-change from the mean, according to the color bar. The upper positively correlated group of genes is related to myelomonocytic differentiation. The lower negatively correlated genes are involved in differentiation, proliferation, or apoptosis. Values for genes shown twice were detected by different probes. (D) Western blot analysis was performed with leukemic cells sorted and separated from spleen and/or BM by FACS in 2 mice given daily LY2520924 treatment and euthanized after 72 hours compared with 2 control mice. The intensity of the bands was quantified by densitometry and displayed as the ratio of phosphorylated protein to control phospho-protein. GAPDH and β-tubulin was used as a loading control. Results of (A) are expressed as the mean ± SEM. BM, bone marrow; Con, control; PB, peripheral blood; SP, spleen.
LY2510924 induces gene expression changes in leukemic cells in vivo that are consistent with loss of SDF-1α/CXCR4 signaling. In the OCI-AML3/Luc/GFP xenograft model, engraftment was confirmed in 8 mice on day 40 by proportion of leukemic cells in blood (3.3% ± 1.1%). Groups of these mice were then given daily LY2520924 treatment and euthanized after 24 hours (n = 3 on day 41) or 72 hours (n = 2 on day 43). Control mice receiving no treatment (n = 3) were euthanized on day 42. Leukemic cells were sorted and separated from BM, blood, and spleen by fluorescence-activated cell sorting (FACS) using the specific markers human CD45 and GFP. (A) Samples from each site and treatment time point were also analyzed by FACS with CXCR4 antibody 12G5 as an inverse indicator of CXCR4 occupancy by LY2510924. (B) From genome-wide GEP of 24 samples (3 groups, 3 tissue sites, 2 or 3 mice per group), log2 values of each sample’s genes from the treated mice were subtracted by the average for the corresponding gene in the control BM samples. The heat map shows subtracted values converted to fold-change for genes with an absolute subtracted log2 value of at least 1.5 for at least 6 samples, with samples and genes hierarchically clustered for similarity in variation. The color bar indicates fold-change values, and the dendrogram indicates similarity between samples, which are labeled according to duration of LY2510924 treatment. (C) Selected genes whose expression levels are highly correlated with a score of the presumed relative degree of loss of SDF-1α/CXCR4 signaling, based on non-BM localization and/or LY2510924 treatment and its duration. Score values are shown above sample names, and gene expression values are shown by fold-change from the mean, according to the color bar. The upper positively correlated group of genes is related to myelomonocytic differentiation. The lower negatively correlated genes are involved in differentiation, proliferation, or apoptosis. Values for genes shown twice were detected by different probes. (D) Western blot analysis was performed with leukemic cells sorted and separated from spleen and/or BM by FACS in 2 mice given daily LY2520924 treatment and euthanized after 72 hours compared with 2 control mice. The intensity of the bands was quantified by densitometry and displayed as the ratio of phosphorylated protein to control phospho-protein. GAPDH and β-tubulin was used as a loading control. Results of (A) are expressed as the mean ± SEM. BM, bone marrow; Con, control; PB, peripheral blood; SP, spleen.
Genome-wide gene expression profiling (GEP) was performed on 24 samples of leukemic cells from various sites and treatment time points. When profiles of all samples were compared with the average profile of untreated BM by displaying fold-change values for altered genes in a subtracted and clustered heat map (Figure 6B), there was remarkable similarity between the changes detected in leukemic cells localized in a site other than BM (ie, blood or spleen) and changes caused by treatment with LY2510924. Furthermore, changes were greatest in samples from non-BM locations in treated mice, especially at 72 hours. GEP findings were also consistent with the finding of less CXCR4 blockade in BM at 24 hours (Figure 6A): at 24 hours, BM samples were relatively unaffected, whereas moderate changes were noted in all 3 spleen samples and 1 of 3 blood samples, and marked changes were noted in 2 of 3 blood samples. Assuming that SDF-1α/CXCR4 signaling is strongest in the BM, the consistency of GEP changes caused by either non-BM localization or LY2510924 treatment and the enhancement and extension of these changes by the combination of these factors, further accentuated by treatment duration, suggest that LY2510924 is indeed a specific inhibitor of SDF-1α/CXCR4 signaling.
Biological effects of SDF-1α/CXCR4 signaling in vivo involve differentiation and proliferation of AML
GEP data were also analyzed to investigate the biological effects of SDF-1α/CXCR4 signaling in leukemic cells. To use GEP data for all 24 samples, we relied on the impression from Figure 6B that there was qualitative similarity in the effects of non-BM localization and/or LY2510924 treatment, assigning to each sample a score for the presumed relative degree of loss of SDF-1α/CXCR4 signaling (see supplemental Data). Genes were then assessed for correlation between their expression levels and this score in all samples by using the Pearson correlation r value in a metric that also considered the variance of gene expression values (supplemental Data). Genes with the highest positive values of the correlation metric (ie, those upregulated by loss of SDF-1α/CXCR4 signaling) were predominantly associated with myelomonocytic differentiation, including CD14, TREM1, CD300a, LILRA5, LILRB3, and S100A12 (Figure 6C). This suggests that although OCI-AML3 is a cell line, it undergoes myelomonocytic differentiation in vivo regulated by SDF-1α/CXCR4 signaling. Genes with the most negative values of the correlation metric (ie, those downregulated by loss of SDF-1α/CXCR4 signaling) include MS4A3, a hematopoietic cell cycle regulator that is downregulated during differentiation.22 The effect of SDF-1α/CXCR4 inhibition on myeloid differentiation was supported by immunohistochemical analysis of tissues from the OCI-AML3-Luc-mCherry xenograft model, which demonstrated increases of CD11c+ cells in an LY2510924-treated mouse compared with an untreated mouse (BM, 60% vs 23%; liver, 45% vs 18%; Figure 7). Tissues from a primary AML xenograft model also showed similar findings (supplemental Figure 9). Other notable negatively correlated genes include negative regulators of apoptosis (BCL2 and RPL23) and genes involved in proliferation (eg, MAD2L1, BUB1, and NCAPG2; Figure 6C). We confirmed decreased Bcl-2 levels by western blot analysis (Figure 6D).
LY2510924 induces myeloid differentiation of AML cells in organs of OCI-AML3 xenograft model. (A) In the OCI-AML3/Luc/mCherry xenograft model, BM and liver samples of representative control and LY2510924-treated mice were fixed and sectioned for immunohistochemical analysis with antihuman CD11c antibodies to investigate myeloid differentiation of leukemic cells. Low magnification of BM sections shows that leukemic cells almost completely efface the BM medullary space in the control mouse whereas clusters of large pale leukemic cells are intermixed with small darker normal hematopoiesis in the LY2510924-treated mouse. Low magnification of liver sections also demonstrates that the periportal sheets of neoplastic cells are significantly larger in the control compared with the LY2510924-treated mouse. At high magnification, the leukemic cells demonstrate similar morphology in BM and liver in both control and treated mice. However, anti-CD11c antibody demonstrates more differentiated cells in BM and liver of a treated mouse compared with BM and liver of a control mouse. (B) The CD11c expression was assessed by manually counting positive and negative signal in 200 leukemic cells, which were discriminated from mouse cells based on size and nuclear chromatin characteristics; the bar graph demonstrates the difference between groups. H&E, hematoxylin and eosin.
LY2510924 induces myeloid differentiation of AML cells in organs of OCI-AML3 xenograft model. (A) In the OCI-AML3/Luc/mCherry xenograft model, BM and liver samples of representative control and LY2510924-treated mice were fixed and sectioned for immunohistochemical analysis with antihuman CD11c antibodies to investigate myeloid differentiation of leukemic cells. Low magnification of BM sections shows that leukemic cells almost completely efface the BM medullary space in the control mouse whereas clusters of large pale leukemic cells are intermixed with small darker normal hematopoiesis in the LY2510924-treated mouse. Low magnification of liver sections also demonstrates that the periportal sheets of neoplastic cells are significantly larger in the control compared with the LY2510924-treated mouse. At high magnification, the leukemic cells demonstrate similar morphology in BM and liver in both control and treated mice. However, anti-CD11c antibody demonstrates more differentiated cells in BM and liver of a treated mouse compared with BM and liver of a control mouse. (B) The CD11c expression was assessed by manually counting positive and negative signal in 200 leukemic cells, which were discriminated from mouse cells based on size and nuclear chromatin characteristics; the bar graph demonstrates the difference between groups. H&E, hematoxylin and eosin.
Gene set enrichment analysis was performed on the basis of ranking genes by the correlation metric to further investigate the biological effects of these changes. Among multiple gene sets that were significantly enriched (supplemental Data), positive enrichment of the gene set JAATINEN_HEMATOPOIETIC_STEM_CELL_DN representing genes downregulated in CD133+ cells (hematopoietic stem cells) compared with CD133– cells23 implies that loss of SDF-1α/CXCR4 signaling promotes differentiation. The possibility that LY2510924-induced differentiation might have therapeutic potential was implied by positive enrichment for MARTENS_TRETINOIN_RESPONSE_UP, which represented genes upregulated in acute promyelocytic leukemia NB4 cells in response to tretinoin.24 Also positively enriched were WHITMAN_AML_FLT3_TKDMUTATION_VS_FLT3_WT_112UPREGULATED_HUMAN genes upregulated in FLT3-mutated de novo cytogenetically normal AML without FLT3 internal tandem duplication vs those without FLT3 abnormality.25 The implication that loss of SDF-1α/CXCR4 signaling retards proliferation was supported by negative enrichment of multiple proliferation-associated gene sets. One of the gene set FEVR_CTNNB1_TARGETS_DN, representing genes downregulated in intestinal crypt cells upon deletion of CTNNB1, implied that loss of SDF-1α/CXCR4 signaling inhibits Wnt/β-catenin signaling.26 Western blot analysis of fluorescence-activated cell sorted leukemic cells showed that the attenuation of phospho-ATK in vivo by LY2510924 resulted in GSK-3β kinase dephosphorylation and decreased activated β-catenin levels (Figure 6D).
Discussion
Here, we investigated the efficacy of LY2510924 in preclinical models of AML and demonstrated that effective disruption of the SDF-1α/CXCR4 axis can induce antileukemia effects on AML cells by this agent as monotherapy and in combination with chemotherapy. These findings indicate that effective CXCR4 antagonists are not simply chemosensitizers but are also effective targeted antileukemia agents.
SDF-1α/CXCR4 inhibition appears to affect leukemia cells in several mechanisms. One is by reducing protection from apoptosis, whether spontaneous or chemotherapy induced, conferred on leukemic cells by interaction with stromal cells. LY2510924 blocked the protection of leukemic cells from chemotherapy-induced apoptosis by MS-5 stromal cells in coculture, even though it did not induce apoptosis in monoculture, as observed by us and others for other CXCR4 antagonists.5,6,27 However, LY2510924 did have a different, measurable effect in monocultures: it inhibited basal or SDF-1α–induced leukemic cell proliferation, which indicates that CXCR4 antagonism may affect proliferation and cell viability through distinct pathways. Inhibition of proliferation by LY2510924 was also implicated in vivo by our GEP studies. Another effect of SDF-1α/CXCR4 inhibition that was observed with other CXCR4 antagonists2,28 and was obviously specific for the in vivo setting is the mobilization of leukemic cells into the circulation. This physical disruption of leukemia/stroma interactions by displacement is potentially significant in the additional context of chemotherapy, in that chemoprotective effects of the BM microenvironment are thereby abrogated. Finally, an additional effect of SDF-1α/CXCR4 inhibition implicated by our GEP studies was the induction of myeloid differentiation, which was supported by tissues stained with CD11c from xenograft models. This is also clearly relevant by the clinical success of differentiation therapy with epigenetic modifiers in AML.29 These findings are supported by those of an earlier study of U937 cells treated in vitro with anti-CXCR4 monoclonal antibody in which microarray data demonstrated effects on proliferation and differentiation of AML cells.30
At a molecular level, both in vitro and in vivo experiments demonstrated that LY2510924 attenuated SDF-1α–induced PI3K/AKT and MAPK prosurvival signaling, consistent with our previous report on AMD3465.6 PI3K/AKT and MAPK pathways are key signaling pathways that promote leukemic cell survival31,32 and are known to be linked to the SDF-1α/CXCR4 axis.33 We have reported the potential of both stromal cells and SDF-1α to activate PI3K/AKT and MAPK prosurvival pathways.6,34 There may be other effects and signaling pathways activated in AML cells by stromal cells; although these need to be identified, especially for rational combination strategies, they may be indirectly abrogated by the mobilizing effect of SDF-1α/CXCR4 inhibition. Our microarray results also suggest that inhibiting SDF-1α/CXCR4 signaling may affect the Wnt/β-catenin pathway, and western blot analysis showed that the attenuation of PI3K/ATK in vivo by LY2510924 results in GSK-3β kinase activation by dephosphorylation, which decreases activated β-catenin levels, consistent with data in peripheral nerve sheath tumors.16 There may be additional signaling pathways and effectors of AKT and β-catenin that require further investigation.
Previous preclinical and clinical studies of SDF-1α/CXCR4 inhibition in AML have shown both promise and limitations. Small-molecule CXCR4 antagonists such as AMD3100 or AMD3465 did not show antileukemia effects as monotherapy in certain studies.5,6 Although this limitation may have been isolated to these particular drugs or to the way in which they were used, our results suggest that this limitation is overcome when the SDF-1α/CXCR4 axis is inhibited more effectively, by LY2510924 for example. Previous studies used CXCR4 antagonists only for very short periods (less than 2 days) or with every-other-day administration and focused on their effectiveness as chemosensitizers. In line with our results, 2 reports demonstrated monotherapeutic antileukemic effects in preclinical models for CXCR4 antagonism by monoclonal antibody MDX-133835 or by peptide inhibitor TN140.27 MDX-1338 (given 5 times over a period of 14 days) inhibited growth of the acute promyelocytic leukemia cell line HL60,35 and TN140 (given once per day by osmotic pump for 7 days) reduced leukemic cell numbers in the BM and prolonged survival in primary AML xenograft models.27 In both studies, AMD3100 had no effect. In future clinical trials with CXCR4 antagonists, the use of more potent agents and extended treatment duration may be essential to fully disrupt the SDF-1α/CXCR4 axis and induce antileukemia effect, in addition to optimal enhancement of chemosensitization. The combination of potential agents to disrupt the interaction of CD44, α-integrins (VLA-4), or osteopontin to their ligands, which are important for adhesion to the stromal niche,36 with CXCR4 antagonist may enhance the efficacy of CXCR4 inhibition.
Using CXCR4 antagonists with chemotherapy might raise concerns about increased toxicity to normal hematopoiesis. However, our in vivo data (Figure 5E) suggested that the toxicity of LY2510924 plus chemotherapy was similar to that of chemotherapy alone. Importantly, recent clinical studies with novel potent CXCR4 antagonists ulocuplumab and BL-8040 demonstrated lack of additive myelosuppression when combined with chemotherapy.37,38
In conclusion, our study shows that LY2510924 at nanomolar concentrations rapidly and durably disrupts the SDF-1α/CXCR4 axis in AML cells and has greater antileukemia efficacy than AMD3100. LY2510924 disrupts the SDF-1α/CXCR4 axis, causes mobilization of leukemic cells into the circulation, inhibits multiple prosurvival signals generated by the SDF-1α/CXCR4 axis, and induces myeloid differentiation, thereby producing antileukemia effects as monotherapy or in combination with chemotherapy. CXCR4 blockade by LY2510924 may translate into effective antileukemia activity in future clinical applications.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Jared K. Burks for kindly helping to quantify multispectral images.
This work was supported by grants P01 CA55164 and P01 CA016672 from the National Cancer Institute of the National Institutes of Health, and by Eli Lilly and Company.
Authorship
Contribution: B.-S.C., M.K., and M.A. conceived of and designed the study; B.-S.C., R.E.D., M.K., and M.A. developed the study methodology; B.-S.C. and Z.W. acquired data; S.K. acquired, analyzed, and interpreted data; B.-S.C., Z.Z., R.E.D., M.K., and M.A. analyzed and interpreted data; B.-S.C., R.E.D., M.K., and M.A. wrote, reviewed, and/or revised the manuscript; Z.Z., H.M., T.M., M.P., J.C., J.R.M., S.-B.P., W.M., and D.E.T. provided administrative, technical, or material support; and M.K. and M.A. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marina Konopleva, Section of Molecular Hematology and Therapy, Department of Leukemia, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0448, Houston, TX 77030; e-mail: mkonople@mdanderson.org.




![Figure 5. LY2510924 induces mobilization of OCI-AML3 cells in vivo and enhances antileukemia effects in combination with chemotherapy. OCI-AML3/Luc/GFP cells (1 × 106 per mouse) were intravenously injected into NSG mice. (A) In mobilization studies, percentages of circulating OCI-AML3/Luc/GFP cells before and 3 or 24 hours after the first LY2510924 injection on day 25 in 6 mice were compared with those in untreated mice (n = 6). (B-E) After confirming leukemia engraftment by BLI, mice were divided into 4 groups (10 mice per group) and began to receive treatment on day 8: control (no treatment), chemotherapy (cytarabine [Ara-C]/doxorubicin), LY2510924, or the combination of chemotherapy with LY2510924. Chemotherapeutics were administered 3 hours after LY2510924 administration. Five representative mice from each group were subjected to (B) serial BLI and (C) intensity quantitation on days 7, 26, and 40 after leukemic cell injection. (D) Three representative mice per group were euthanized on day 27 for immunohistochemical analysis for human CD45 to identify human leukemic cells. Analysis of the multispectral images further confirmed the significantly reduced leukemic cell burden in LY2510924-treated mice and even more so in the combination group. Original magnification, ×40. (E) Overall survival rate in each group was estimated by the Kaplan-Meier method. Results of (A) and (C-D) are expressed as the mean ± SEM. *P < .05; **P < .01.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/126/2/10.1182_blood-2015-02-628677/4/m_222f5.jpeg?Expires=1767715547&Signature=RWvjU2H3HSObDcq79virUa2iXqGrVa~tebA6l2XN0QlVca47jynAoLGohCSoLj~imZHlJjgGUMgey09CBHn~6ArNzbtFqtAg949kn0qlmX2BfKZp7Z5aAuOUQMAHRNsODe6IheAzLmMV75l2Nfza1HsXwAga95M8BILJF-qHfT77AZAT0g2pEi9mb~QNfWt5amPzzyrBzBXFiM-VwBuLZ7uqM5DwG1jMbnCmHeYoxbjCmFFLhYYYE81ikDCvSFWCuQ~B~m28J4N5VjUSDCvMsXQc-fMTpELO5Q4EHSDGbMXp3LgjaF65MElPnToHNGu0uPK-jrjw78pbqUi3ToqqFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

