Key Points
Ikaros controls cellular proliferation by repressing genes that regulate cell cycle progression and the PI3K pathway in leukemia.
CK2 inhibitor restores Ikaros tumor suppressor function in high-risk B-ALL with IKZF1 deletion and has a strong therapeutic effect in vivo.
Abstract
Ikaros (IKZF1) is a tumor suppressor that binds DNA and regulates expression of its target genes. The mechanism of Ikaros activity as a tumor suppressor and the regulation of Ikaros function in leukemia are unknown. Here, we demonstrate that Ikaros controls cellular proliferation by repressing expression of genes that promote cell cycle progression and the phosphatidylinositol-3 kinase (PI3K) pathway. We show that Ikaros function is impaired by the pro-oncogenic casein kinase II (CK2), and that CK2 is overexpressed in leukemia. CK2 inhibition restores Ikaros function as transcriptional repressor of cell cycle and PI3K pathway genes, resulting in an antileukemia effect. In high-risk leukemia where one IKZF1 allele has been deleted, CK2 inhibition restores the transcriptional repressor function of the remaining wild-type IKZF1 allele. CK2 inhibition demonstrated a potent therapeutic effect in a panel of patient-derived primary high-risk B-cell acute lymphoblastic leukemia xenografts as indicated by prolonged survival and a reduction of leukemia burden. We demonstrate the efficacy of a novel therapeutic approach for high-risk leukemia: restoration of Ikaros tumor suppressor activity via inhibition of CK2. These results provide a rationale for the use of CK2 inhibitors in clinical trials for high-risk leukemia, including cases with deletion of one IKZF1 allele.
Introduction
Ikaros (IKZF1) activity is essential for normal hematopoiesis and immune development.1-4 Ikaros knockout mice have severely impaired hematopoiesis,5-7 whereas mice with the heterozygous loss of Ikaros develop T-cell leukemia.8 In humans, impaired Ikaros activity due to the deletion or inactivating mutation of a single IKZF1 allele results in high-risk B-cell leukemia that is resistant to treatment.9-14 Ikaros regulates transcription of target genes via chromatin remodeling.15-17
Ikaros activity is controlled through multiple mechanisms. Mouse studies suggest that the transcription of IKZF1 during normal hematopoiesis is regulated by a complex network.18 However, Ikaros protein is expressed at high levels in most hematopoietic cells, and posttranslational modifications are hypothesized to play a critical role in regulating Ikaros activity.19 Several groups have shown that phosphorylation,19-24 sumoylation,25 and ubiquitination22 can regulate Ikaros function as a transcriptional repressor. However, the role of posttranslational modification in the regulation of Ikaros tumor suppressor activity in leukemia is unknown.
Despite extensive global analyses of Ikaros DNA binding in normal murine hematopoietic cells,26-28 the molecular mechanisms by which Ikaros exerts its tumor suppressor effects in human leukemia are not well understood. Moreover, the mechanisms that regulate Ikaros activity in leukemia are largely unknown. Here, we show that Ikaros regulates proliferation of leukemia cells by repressing the transcription of genes that promote cell cycle progression and the phosphatidylinositol-3 kinase (PI3K) pathway. We present evidence that the ability of Ikaros to regulate transcription of target genes and function as a tumor suppressor is impaired in B-cell acute lymphoblastic leukemia (B-ALL) due to increased phosphorylation of Ikaros by the pro-oncogenic casein kinase II (CK2). The inhibition of CK2-mediated phosphorylation of Ikaros restores Ikaros tumor suppressor activity in high-risk leukemia including those characterized by the deletion of a single IKZF1 allele. Our findings use a panel of preclinical models to identify CK2 inhibition as a novel therapeutic approach for targeted treatment of high-risk ALL.
Patients, materials, and methods
Cell culture and reagents
The Nalm6 B-ALL cell line was described previously.29 Normal human bone marrow (BM) mononuclear cells (MNCs) were purchased from StemCell Technologies (Vancouver, BC, Canada) or obtained from Loma Linda University. Primary human B-ALL cells were cultured with or without precoating with a mixture of human HS-27 stroma (American Type Culture Collection [ATCC], Rockville, MD) and murine MS-5 stromal cells as described previously.30 Cells were cultured with or without 4,5,6,7-tetrabromobenzotriazole (TBB) or CX-4945 and collected for total RNA isolation.
Plasmid construction and viral gene transfer
Chromatin immunoprecipitation-Seq assays
Chromatin immunoprecipitation (ChIP)-Seq assays for Ikaros were performed as previously reported,33,34 and ChIP-Seq data are accessible on Gene Expression Omnibus with an access number of GSE44218 at the following link: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE44218.
Human leukemia mouse xenograft models
Nalm6 dsRED+Luc+ cells (1 × 107) were injected via tail vein into 4- to 6-week-old NOD.Cg-PrkdcscidIl2rgtm1wjl/SzJ (NSG) mice (The Jackson Laboratory). Starting 7 days after cell injection, mice (n = 17-21/group) received CX-4945 daily via gavage at 75 mg/kg/day for 10 days. The presence of leukemia was assessed and quantified by luminescence using a Xenogen IVIS 50 series system and the Living Image 2.50, respectively (Caliper Life Sciences). At the completion of in vivo studies, mice were killed, tissues were harvested, and single cell suspensions prepared. Total RNA was isolated for assay of target gene expression by quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR) as described in the supplemental Materials, available on the Blood Web site. Wild-type IKZF1 or IKZF1 deletion in the patients’ samples was confirmed by western blot and/or DNA sequencing.
For the primary human B-ALL mouse xenograft model, 2 × 106 cells per mouse were transplanted intravenously into 4-week-old female NOD.Cg-Rag1tm1MomIl2rgtm1Wjl/SzJ (NRG) mice. Following engraftment, mice (n = 10-15/group/per patient sample × 3 patients) received CX-4945 daily via gavage at 100 mg/kg/day for 23 to 24 days. Determination of engraftment for treatment initiation was based on the presence of >25% human leukemia cells in total BM mononuclear cells from killed sentinel animals that were transplanted along with treated animals. Following the treatment period, a single cell suspension was prepared from harvested BM and spleen from killed mice, and red blood cells (RBCs) were lysed using RBC lysis buffer (Biolegend). Resulting cells were used for living cell counts, quantitative ChIP-qPCR (qChIP) assay, qRT-PCR, and flow cytometry analysis. Total living cells in BM and spleen of mice were determined by hemocytometer count using trypan blue exclusion.
CK2 activity assays
Assays were performed in triplicate as previously decsribed.35 CK2 inhibitors were TBB, which was purchased from Sigma (St. Louis, MO), and CX-4945, which was a gift from Cylene Pharmaceuticals (San Diego, CA).
Approval for use of patient samples and animal studies
Anonymous patient samples were provided by the University of Southern California Norris Comprehensive Cancer Center (Los Angeles, CA) and Loma Linda University (Loma Linda, CA) in compliance with Institutional Review Board regulations. The patients’ characteristics are summarized in supplemental Table 1. All animal experiments were conducted under protocols approved by the Institutional Animal Care and Use Committee at Penn State Hershey (Hershey, PA).
Additional methods are provided in the supplemental Materials.
Results
Ikaros suppresses cell cycle progression and the PI3K pathway
We determined the genome-wide occupancy of Ikaros in B-ALL using ChIP-Seq. The analysis of Ikaros occupancy showed that Ikaros binds to the promoter sites of genes that (1) are essential for cell cycle progression and (2) regulate the PI3K pathway. The latter included primarily genes that promote the PI3K pathway, such as the well-known oncogene, PIK3CD,36 but also include a known tumor suppressor, INPP5D (SHIP1).37 Data were obtained from 2 different primary human B-ALL samples (patients 1 and 2; supplemental Table 1) by 6 separate ChIP-Seq experiments using 3 different antibodies for each primary cell sample and from the Nalm6 B-ALL cell line (representative ChIP-Seq signal maps are shown in supplemental Figures 1 and 2). Ikaros binding at the promoters of these genes determined by ChIP-Seq was confirmed by qChIP in Nalm6 (Figures 1A and 2A), and in primary B-ALL cells (Figures 1B and 2B).
Ikaros regulates transcription of genes that control cell cycle. (A-B) qChIP analysis of Ikaros occupancy of target genes that control cell cycle progression identified by ChIP-Seq in (A) Nalm6 B-ALL cells and (B) primary B-ALL cells (representative data from 1 of 4 patients without IKZF1 deletion is shown). (C) Expression of Ikaros target genes that control cell cycle progression in Nalm6 cells transduced with vector containing Ikaros compared with empty vector controls (measured by qRT-PCR). (D) Promoter activity of Ikaros target genes that control cell cycle progression measured by luciferase assay following transfection with Ikaros or control vector. (E) Effect of Ikaros knockdown on the gene expression of Ikaros targets that control cell cycle progression. Gene expression is determined by qRT-PCR using total RNA isolated from Nalm6 cells transfected with scramble shRNA or Ikaros shRNA and cultured for 2 days. (F) Effect of Ikaros knockdown on cell cycle gene expression. Nalm6 cells were transduced with lentivirus containing Ikaros or empty vector then cell cycle analysis was performed by flow cytometry. (G) Quantification of cell cycle data. (H) Model of Ikaros-mediated regulation of cell cycle progression: Ikaros represses transcription of genes that promote cell cycle progression at different stages of the cell cycle, leading to negative regulation of cell cycle progression.
Ikaros regulates transcription of genes that control cell cycle. (A-B) qChIP analysis of Ikaros occupancy of target genes that control cell cycle progression identified by ChIP-Seq in (A) Nalm6 B-ALL cells and (B) primary B-ALL cells (representative data from 1 of 4 patients without IKZF1 deletion is shown). (C) Expression of Ikaros target genes that control cell cycle progression in Nalm6 cells transduced with vector containing Ikaros compared with empty vector controls (measured by qRT-PCR). (D) Promoter activity of Ikaros target genes that control cell cycle progression measured by luciferase assay following transfection with Ikaros or control vector. (E) Effect of Ikaros knockdown on the gene expression of Ikaros targets that control cell cycle progression. Gene expression is determined by qRT-PCR using total RNA isolated from Nalm6 cells transfected with scramble shRNA or Ikaros shRNA and cultured for 2 days. (F) Effect of Ikaros knockdown on cell cycle gene expression. Nalm6 cells were transduced with lentivirus containing Ikaros or empty vector then cell cycle analysis was performed by flow cytometry. (G) Quantification of cell cycle data. (H) Model of Ikaros-mediated regulation of cell cycle progression: Ikaros represses transcription of genes that promote cell cycle progression at different stages of the cell cycle, leading to negative regulation of cell cycle progression.
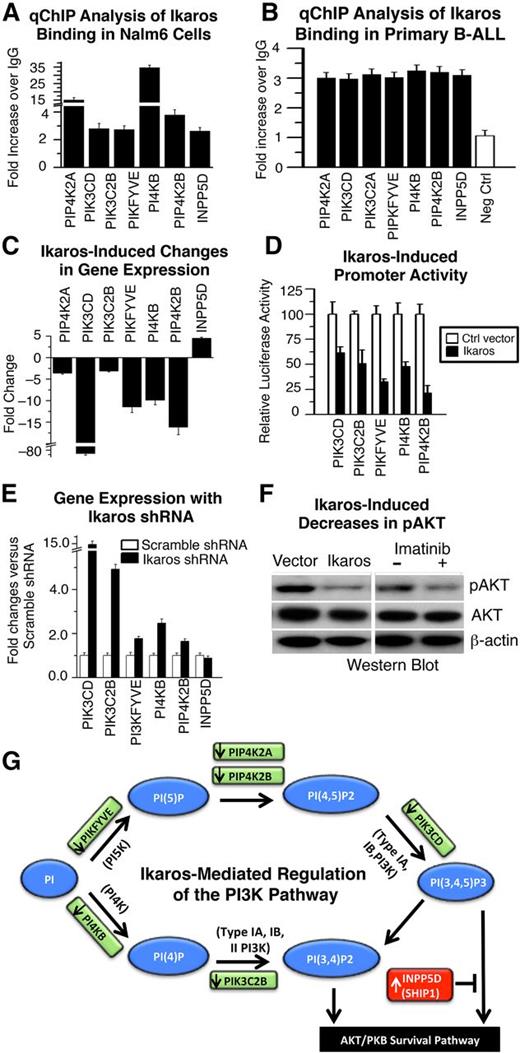
Ikaros regulates transcription of genes that control the PI3K pathway. (A-B) qChIP analysis of Ikaros occupancy of target genes that control the PI3K pathway identified by ChIP-Seq in (A) Nalm6 B-ALL cells and (B) primary B-ALL cells (representative data from 1 of 4 patients without IKZF1 deletion is shown). (C) Expression of Ikaros target genes that control the PI3K pathway in Nalm6 cells transduced with vector containing Ikaros compared with controls (measured by qRT-PCR). (D) Promoter activity of Ikaros target genes that control the PI3K pathway as measured by luciferase assay following transfection with Ikaros or control vector. (E) Effect of Ikaros knockdown on the gene expression of Ikaros targets that control the PI3K pathway. Gene expression was determined by qRT-PCR using total RNA isolated from the Nalm6 cells transfected with scramble shRNA or Ikaros shRNA and cultured for 2 days. (F) Loss of phosphorylation at serine 473 of AKT kinase in cells transduced with vector containing Ikaros compared with control. The loss of phosphorylation of serine 473 of AKT kinase following imatinib treatment was used as positive control (right). (G) Model of Ikaros-mediated regulation of the PI3K pathway: Ikaros represses transcription of genes that promote the PI3K pathway, but induces transcription of a gene that inhibits the PI3K pathway (INPP5D), leading to negative regulation of the PI3K pathway and cellular proliferation.
Ikaros regulates transcription of genes that control the PI3K pathway. (A-B) qChIP analysis of Ikaros occupancy of target genes that control the PI3K pathway identified by ChIP-Seq in (A) Nalm6 B-ALL cells and (B) primary B-ALL cells (representative data from 1 of 4 patients without IKZF1 deletion is shown). (C) Expression of Ikaros target genes that control the PI3K pathway in Nalm6 cells transduced with vector containing Ikaros compared with controls (measured by qRT-PCR). (D) Promoter activity of Ikaros target genes that control the PI3K pathway as measured by luciferase assay following transfection with Ikaros or control vector. (E) Effect of Ikaros knockdown on the gene expression of Ikaros targets that control the PI3K pathway. Gene expression was determined by qRT-PCR using total RNA isolated from the Nalm6 cells transfected with scramble shRNA or Ikaros shRNA and cultured for 2 days. (F) Loss of phosphorylation at serine 473 of AKT kinase in cells transduced with vector containing Ikaros compared with control. The loss of phosphorylation of serine 473 of AKT kinase following imatinib treatment was used as positive control (right). (G) Model of Ikaros-mediated regulation of the PI3K pathway: Ikaros represses transcription of genes that promote the PI3K pathway, but induces transcription of a gene that inhibits the PI3K pathway (INPP5D), leading to negative regulation of the PI3K pathway and cellular proliferation.
We analyzed how a gain or loss of Ikaros function affects transcription of Ikaros target genes. Nalm6 cells were transduced with either an Ikaros-containing retroviral vector or a control retrovirus. Increased Ikaros expression resulted in repressed transcription of genes that regulate cell cycle progression (Figure 1C). The ability of Ikaros to directly transcriptionally repress cell cycle-promoting genes was determined by luciferase reporter assay using their respective promoters (Figure 1D). To assess the effect of Ikaros knockdown on expression of Ikaros target genes that control cell cycle progression, Nalm6 cells were transduced to express Ikaros short hairpin RNA (shRNA) or control scramble shRNA. Reduced Ikaros expression resulted in increased transcription of a majority of the Ikaros target genes that promote cell cycle progression (Figure 1E). Overexpression of Ikaros in Nalm6 cells results in partial arrest of cell cycle progression (Figure 1F-G). The alterations in Ikaros target gene expression and functional effects observed with the gain or loss of Ikaros (Figure 1) provide evidence that Ikaros negatively regulates cell cycle progression. This suggests that Ikaros suppresses leukemia via transcriptional repression of genes that are essential for progression at multiple stages in the cell cycle (Figure 1H).
Gain or loss of function experiments as in Figure 1 revealed that Ikaros represses transcription of genes that promote the PI3K pathway but positively regulates transcription of the (INPP5D/SHIP1) gene that suppresses the PI3K pathway37 (Figure 2C-E). The transcription of the PIK3CD gene was particularly sensitive to the gain or loss of Ikaros function (Figure 2C,E). Increased expression of Ikaros also results in severely reduced phosphorylation of serine 473 of the AKT kinase and repression of the AKT pathway (Figure 2F), which is immediately downstream of PI3K signaling.38
Overall, the above experiments suggest that Ikaros acts to suppress leukemia via transcriptional regulation of genes that control the PI3K pathway (Figure 2G), in addition to genes that promote cell cycle progression. These data provide a mechanistic basis for the development of leukemia in association with Ikaros dysfunction.
CK2 impairs Ikaros tumor suppressor activity
The above data suggest a mechanism for Ikaros tumor suppressor activity in preventing ALL but raise the question of why leukemia cells with normal Ikaros protein expression show impaired cell cycle control and activated oncogenic pathways. A possible explanation is that Ikaros activity is reduced in leukemia and is thus insufficient to regulate the transcription of Ikaros target genes to the extent necessary to halt the proliferation of leukemia cells.
CK2 has been shown to directly phosphorylate Ikaros resulting in reduced Ikaros DNA-binding affinity and loss of Ikaros’ ability to regulate cell cycle progression.20-22 Here we show that CK2 activity in primary B-ALL cells is similar to that in Nalm6 cells and significantly higher than in normal BM cells and normal B-cell precursors (Figure 3A). This is in accordance with increases in CK2 activity previously observed in B-ALL and other types of cancer39-44 and suggests that Ikaros function might be impaired due to CK2 in ALL.
CK2 impairs Ikaros-mediated transcriptional regulation of genes that control cell cycle progression and the PI3K pathway. (A) CK2 kinase activity assay of the Nalm6 B-ALL cell line and primary B-ALL, normal BM and normal B cell precursors. Graphed are means ± standard error of the mean, comparison by Student t test. CK2 activity was similar in B-ALL with or without IKZF1 mutation or deletion (data not shown). (B-C) Effect of molecular inhibition of CK2 by shRNA (gray) or pharmacologic inhibition of CK2 with CX-4945 (black) on expression of Ikaros target genes that regulate (B) cell cycle progression and (C) the PI3K pathway as measured by qRT-PCR. (D) Effect of CX-4945 treatment on Ikaros-mediated transcriptional repression of promoters of cell cycle and PI3K pathway genes as measured by qRT-PCR. (E-F) Effect of Ikaros knockdown on changes in (E) cell cycle and (F) PI3K pathway gene expression induced by CK2 inhibition with CX-4945 as measured by qRT-PCR.
CK2 impairs Ikaros-mediated transcriptional regulation of genes that control cell cycle progression and the PI3K pathway. (A) CK2 kinase activity assay of the Nalm6 B-ALL cell line and primary B-ALL, normal BM and normal B cell precursors. Graphed are means ± standard error of the mean, comparison by Student t test. CK2 activity was similar in B-ALL with or without IKZF1 mutation or deletion (data not shown). (B-C) Effect of molecular inhibition of CK2 by shRNA (gray) or pharmacologic inhibition of CK2 with CX-4945 (black) on expression of Ikaros target genes that regulate (B) cell cycle progression and (C) the PI3K pathway as measured by qRT-PCR. (D) Effect of CX-4945 treatment on Ikaros-mediated transcriptional repression of promoters of cell cycle and PI3K pathway genes as measured by qRT-PCR. (E-F) Effect of Ikaros knockdown on changes in (E) cell cycle and (F) PI3K pathway gene expression induced by CK2 inhibition with CX-4945 as measured by qRT-PCR.
We hypothesized that the inhibition of CK2 would enhance Ikaros tumor suppressor function in leukemia. First, we determined whether the loss of CK2 activity results in the transcriptional repression of Ikaros target genes that are involved in cell cycle progression and the PI3K pathways. Nalm6 cells were transduced to express CK2 shRNA (molecular inhibition of CK2) (supplemental Figure 3) or treated with the CK2-specific inhibitor CX-4945 (pharmacologic inhibition of CK2), and the transcription of Ikaros target genes that promote cell cycle progression and the PI3K pathway was measured by qRT-PCR. Results showed that molecular and pharmacologic inhibition of CK2 act similarly to repress the transcription of Ikaros target genes that promote cell cycle progression or the PI3K pathway (Figure 3B-C, gray and black vs white bars). Transcriptional upregulation was observed for INPP5D/SHIP-1, a gene that represses the PI3K pathway.37 The effect of CK2 inhibition on the transcription of Ikaros target genes was markedly similar to the effect of Ikaros overexpression (Figure 3B-C compared with Figures 1C and 2C), including the positive regulation of INPP5D/SHIP-1 transcription. Inhibition of CK2 by CX-4945 enhances the direct repression of Ikaros target genes as indicated by the luciferase reporter assay (Figure 3D, black vs gray bars). These data support the hypothesis that CK2 inhibition enhances Ikaros activity as a transcriptional regulator.
Next, we determined whether the presence of Ikaros is necessary for the transcriptional repression of genes involved in cell cycle progression and the PI3K pathway during CK2 inhibition. Nalm6 cells were transfected with scramble or Ikaros shRNA. The effect of CK2 inhibition on the transcription of Ikaros target genes was compared in cells with reduced Ikaros expression and in control cells. Expression analysis showed that the ability of the CK2 inhibitor CX-4945 to repress a majority of Ikaros target genes is lost or reduced, in cells with shRNA knockdown of Ikaros, compared with cells with scramble shRNA (Figure 3E-F, black bars vs light gray bars). These results suggest that the repression of genes that promote cell cycle progression and the PI3K pathway following CK2 inhibition requires Ikaros function as a transcriptional repressor.
CK2 inhibition results in an antileukemia effect in vivo
The above data suggest the possibility that inhibition of CK2 could result in an antileukemia effect. In vitro inhibition of CK2 in Nalm6 cells with CX-4945 greatly reduced cellular proliferation and survival (Figure 4A; supplemental Figure 4). Similar results were seen with another CK2 inhibitor, TBB (data not shown). This suggests that elevated CK2 activity is essential for the proliferation of Nalm6 cells (Figure 4A). Knockdown of Ikaros by shRNA in Nalm6 cells significantly blocks the CX-4945–induced arrest of cellular proliferation (Figure 4B, compare black bar with light gray bar). These data suggest that CK2 inhibition by CX-4945 has an antileukemia effect and that Ikaros activity is an important component of the therapeutic action of CK2 inhibition.
CK2 inhibition has an antileukemia effect in vitro and in vivo. (A) In vitro inhibition of cellular proliferation in Nalm6 cells following treatment with the CK2 inhibitor CX-4945. B-ALL cells were treated with increasing doses of CX-4945, and cell proliferation was measured by WST-1 assay over time. (B) Effect of Ikaros knockdown on CX-4945–induced suppression of cell proliferation in Nalm6 cells. Graphed data in A-B are the mean ± standard error of the mean of triplicates from 1 experiment, representative of 3 independent experiments. (C-E) Antileukemia effects of CK2 inhibition in the Nalm6 human-mouse xenograft. (C) Representative images and (D) quantification of leukemia progression measured by in vivo bioluminescence in mice treated with the CK2 inhibitor, CX-4945, and in vehicle controls. (F) Human-mouse xenografts established with Nalm6 B-ALL cells were treated for 7 days with the CK2 inhibitor, CX-4945, or vehicle control, and survival was followed. Survival curves were generated using the Kaplan-Meier method, and differences in survival were analyzed by χ2 test. Comparisons in A-B and D were by Student t test. **P ≤ .01; ***P ≤ .001.
CK2 inhibition has an antileukemia effect in vitro and in vivo. (A) In vitro inhibition of cellular proliferation in Nalm6 cells following treatment with the CK2 inhibitor CX-4945. B-ALL cells were treated with increasing doses of CX-4945, and cell proliferation was measured by WST-1 assay over time. (B) Effect of Ikaros knockdown on CX-4945–induced suppression of cell proliferation in Nalm6 cells. Graphed data in A-B are the mean ± standard error of the mean of triplicates from 1 experiment, representative of 3 independent experiments. (C-E) Antileukemia effects of CK2 inhibition in the Nalm6 human-mouse xenograft. (C) Representative images and (D) quantification of leukemia progression measured by in vivo bioluminescence in mice treated with the CK2 inhibitor, CX-4945, and in vehicle controls. (F) Human-mouse xenografts established with Nalm6 B-ALL cells were treated for 7 days with the CK2 inhibitor, CX-4945, or vehicle control, and survival was followed. Survival curves were generated using the Kaplan-Meier method, and differences in survival were analyzed by χ2 test. Comparisons in A-B and D were by Student t test. **P ≤ .01; ***P ≤ .001.
We tested the in vivo efficacy of CK2 inhibition on leukemia progression using the novel specific CK2 inhibitor CX-494545,46 in a Nalm6 human-mouse xenograft model with established leukemia. A short (7-day) course of CX-4945 (administered once daily) reduced leukemia progression (Figure 4C-D), leading to prolonged survival of treated mice (Figure 4E). No toxic effects were observed with this dose. These results suggest that increased CK2 activity in B-ALL impairs the tumor suppressor activity of Ikaros and that inhibition of CK2 induces an antileukemia effect.
CK2 inhibition enhances Ikaros regulation of the cell cycle and PI3K pathways in vivo
To determine whether the therapeutic activity of in vivo CK2 inhibition involves an alteration in the expression of Ikaros target genes, transcripts of these genes were measured by qRT-PCR and the results are presented using heat maps (Figure 5A-B). Changes in expression of Ikaros target genes were compared among (1) control leukemia cells (wild-type Nalm6, Nalm6 cells transduced with empty vector, and Nalm6 cells expressing scramble shRNA; lanes 1-3); (2) leukemia cells with increased Ikaros activity (Nalm6 cells retrovirally transduced with IKZF1; lane 4); and (3) Nalm6 cells with reduced CK2 activity (Nalm6 cells expressing CK2 shRNA [lane 5], Nalm6 cells treated with TBB [lanes 6-7], or Nalm6 cells from the human-mouse xenograft treated with CX-4945 [lanes 8-9]). These data demonstrate that the inhibition of CK2, both in vitro (lanes 5-7) and in vivo (lanes 8-9), produces changes in the expression of Ikaros target genes that are highly similar to those that result from increased Ikaros expression (lane 4).
CK2 inhibition and Ikaros overexpression similarly affect transcription of genes that control cell cycle progression and the PI3K pathway in vitro and in vivo. (A-B) Heatmap of qRT-PCR analysis of Ikaros target genes that promote (A) cell cycle and (B) the PI3K pathway. Expression in controls: wild-type Nalm6, Nalm6 transduced with empty vector, and Nalm6 transduced with scramble shRNA (lanes 1-3); in Nalm6 with overexpressed Ikaros (lane 4); in Nalm6 treated with CK2 shRNA (lane 5); and Nalm6 following CK2 inhibition, in vitro (with TBB) (lanes 6-7), or in vivo (with CX-4945) (lanes 8-9). (C) Ikaros knockdown partially rescues transcriptional repression following CX-4945 treatment of Nalm6 cells in vivo. Nalm6 cells stably transfected with Ikaros shRNA or scramble shRNA (control) and selected with puromycin were injected into NSG mice and treated with CX-4945 for 3 days. Gene expression was measured by qRT-PCR in cells harvested from engrafted leukemia. (D) Western blots of Nalm6 xenografts showing that CK2 inhibition decreases phosphorylation of AKT (pAKT) (upper), whereas levels of AKT are unchanged (lower). The relative intensity of bands by densitometry scanning are shown on the right.
CK2 inhibition and Ikaros overexpression similarly affect transcription of genes that control cell cycle progression and the PI3K pathway in vitro and in vivo. (A-B) Heatmap of qRT-PCR analysis of Ikaros target genes that promote (A) cell cycle and (B) the PI3K pathway. Expression in controls: wild-type Nalm6, Nalm6 transduced with empty vector, and Nalm6 transduced with scramble shRNA (lanes 1-3); in Nalm6 with overexpressed Ikaros (lane 4); in Nalm6 treated with CK2 shRNA (lane 5); and Nalm6 following CK2 inhibition, in vitro (with TBB) (lanes 6-7), or in vivo (with CX-4945) (lanes 8-9). (C) Ikaros knockdown partially rescues transcriptional repression following CX-4945 treatment of Nalm6 cells in vivo. Nalm6 cells stably transfected with Ikaros shRNA or scramble shRNA (control) and selected with puromycin were injected into NSG mice and treated with CX-4945 for 3 days. Gene expression was measured by qRT-PCR in cells harvested from engrafted leukemia. (D) Western blots of Nalm6 xenografts showing that CK2 inhibition decreases phosphorylation of AKT (pAKT) (upper), whereas levels of AKT are unchanged (lower). The relative intensity of bands by densitometry scanning are shown on the right.
The effect of CX-4945 on Nalm6 cells with reduced Ikaros expression due to the presence of Ikaros shRNA was tested in vivo. Nalm6 cells stably transfection with Ikaros shRNA or scramble shRNA were injected into NSG mice and mice were treated with CX-4945 (Figure 5C) or vehicle control. qRT-PCR analysis of harvested leukemia cells shows that Ikaros knockdown resulted in a complete loss of the ability of CX-4945 to transcriptionally repress some Ikaros target genes (CDC25C and CDC25A) in vivo. For the majority of Ikaros target genes, Ikaros knockdown provided partial rescue from repression following CX-4945 treatment. This effect was less pronounced than that observed in vitro (Figure 5C vs Figure 3E). This is likely due to the lower efficiency of Ikaros knockdown in the stably transfected Nalm6 cells used for in vivo experiments compared with the transient Ikaros knockdown in vitro (supplemental Figure 5) and due to activity of other pathways that regulate transcription of Ikaros target genes in vivo. These results support the idea that the ability of CK2 inhibitors to fully repress genes that promote cell cycle progression and the PI3K pathway is dependent on the presence of Ikaros to act as a transcriptional repressor.
In vivo inhibition of CK2 in Nalm6 xenografts results in suppression of the PI3K pathway as evidenced by the loss of AKT S473 phosphorylation (Figure 5D), similar to what was observed with Ikaros overexpression (Figure 2F). These data suggest that CK2 inhibition enhances Ikaros activity as a tumor suppressor and a regulator of gene expression in vivo and identify CK2 inhibitors as candidate drugs for therapeutically restoring Ikaros function in B-ALL.
CK2 inhibition restores Ikaros function in high-risk leukemia
High-risk B-ALL is frequently characterized by Ikaros haploinsufficiency where 1 IKZF1 allele is either deleted or carries an inactivating mutation. Because the experiments described above suggest that CK2 inhibitors can enhance Ikaros tumor suppressor function, we tested whether CK2 inhibition can enhance the activity of Ikaros produced from the single wild-type allele in high-risk leukemia with Ikaros haploinsufficiency. We used primary cells from 4 patients with high-risk B-ALL that carries a deletion of 1 IKZF1 allele (Figure 6). Treatment of these primary high-risk B-ALL samples with CX-4945 resulted in repression of Ikaros target genes involved in the cell cycle and PI3K pathways (Figure 6A-B; supplemental Figure 6A-B).
CK2 inhibition restores Ikaros-mediated transcriptional regulation in primary high-risk B-ALL with deletion of 1 IKZF1 allele. Primary high-risk ALL cells were cultured on stromal cells with or without CK2 inhibitor CX-4945 (20 μM) for 24 hours. (A-B) Expression (measured by qRT-PCR) of Ikaros target genes involved in cell cycle progression (left) and the PI3K pathway (right) in primary high-risk B-ALL following CK2 inhibitor (CX-4945) treatment compared with untreated cells. (C-D) qChIP analysis of Ikaros occupancy of target genes involved in cell cycle progression (left) and the PI3K pathway (right) in wild-type (white and blue bars) and CX-4945–treated primary high-risk B-ALL (yellow and orange bars).
CK2 inhibition restores Ikaros-mediated transcriptional regulation in primary high-risk B-ALL with deletion of 1 IKZF1 allele. Primary high-risk ALL cells were cultured on stromal cells with or without CK2 inhibitor CX-4945 (20 μM) for 24 hours. (A-B) Expression (measured by qRT-PCR) of Ikaros target genes involved in cell cycle progression (left) and the PI3K pathway (right) in primary high-risk B-ALL following CK2 inhibitor (CX-4945) treatment compared with untreated cells. (C-D) qChIP analysis of Ikaros occupancy of target genes involved in cell cycle progression (left) and the PI3K pathway (right) in wild-type (white and blue bars) and CX-4945–treated primary high-risk B-ALL (yellow and orange bars).
Next, we determined whether CX-4945 treatment enhances Ikaros DNA-binding activity toward the promoters of its target genes in primary high-risk pre-B-ALL cells. qChIP analysis shows that Ikaros binds promoters of its target genes poorly in these cells (Figure 6C-D; supplemental Figure 6C-D, dark blue compared with white bars). The treatment of primary high-risk B-ALL cells with CX-4945 results in strong binding of Ikaros to the promoters of its target genes (Figure 6C-D; supplemental Figure 6C-D, dark orange compared with yellow bars). Thus, the restoration of Ikaros binding to the promoters of its target genes is associated with their transcriptional repression (Figure 6A-B; supplemental Figure 6A-B). These results demonstrate that CK2 inhibition in high-risk B-ALL with Ikaros haploinsufficiency restores Ikaros DNA-binding activity and suggests that repression of the cell cycle and PI3K pathways following CK2 inhibition is the result of restored Ikaros-mediated repression of its target genes.
CK2 inhibition has an antileukemia effect in xenografts of primary high-risk B-ALL with IKZF1 deletion
High-risk pediatric B-ALL is a clinical challenge and is associated with a high relapse rate and a poor prognosis. We tested the efficacy of the CK2 inhibitor CX-4945 on 3 primary samples from patients with high-risk B-ALL. High risk was determined by the presence of 1 or more poor prognostic markers or clinical features (supplemental Table 1). Results show that CX-4945 has a strong cytotoxic effect on primary high-risk B-ALL cells (supplemental Figure 7). Next, we tested the therapeutic efficacy of CX-4945 on primary xenograft models derived from high-risk leukemia. Primary B-ALL cells were injected into mice via the tail vein. Mice were treated with the CK2 inhibitor, CX-4945, or with vehicle control, once engraftment was established. Mice were evaluated for the presence of leukemia following treatment. Results showed that CX-4945 treatment strongly reduced the total number of leukemia cells in BM, as well as disseminated leukemia cells in the spleen, with reduced spleen weight in all 3 xenograft models (Figure 7A-C; supplemental Figures 8 and 9). The antileukemia effect was achieved in primary xenografts of B-ALL that carry deletion of one IKZF1 allele (patient 3), as well as xenografts of other high-risk B-ALL (patients 4 and 5). Flow cytometry demonstrated a reduction in the percentage of leukemia cells in both BM and spleen of CX-4945–treated mice. Treatment with CX-4945 prolonged survival of mice engrafted with primary B-ALL that carries deletion of 1 IKZF1 allele as well as mice engrafted with other high-risk B-ALL (Figure 7D; supplemental Figure 10). These data demonstrate for the first time the efficacy of CK2 inhibitors in the treatment of high-risk B-cell leukemia in preclinical models and identify CX-4945 as a novel therapeutic agent for this disease.
CK2 inhibition has an antileukemia effect on primary xenografts of high-risk B-ALL. NRG mice were injected with primary B-ALL cells with deletion of (A) 1 IKZF1 allele or (B-C) other poor prognostic features (supplemental Table 1). (A-C) Following established engraftment (determined as described in Patients, materials, and methods), mice were treated with the CK2 inhibitor, CX-4945, or with vehicle control. Mice were killed and evaluated for the presence of leukemia in BM and spleen at day 22 (patient 3), day 17 (patient 4), or day 26 (patient 5) following the initiation of treatment. Percentages and numbers of leukemia cells in BM or spleen of xenograft mice are graphed. The effect of drug treatment was assessed by Student t test. (D-F) Patient-derived xenografts established with B-ALL from patient 3 (D), patient 4 (E), and patient 5 (F) were treated for 22 days with CK2 inhibitors, CX-4945, or vehicle control and followed for survival. Survival curves were generated using the Kaplan-Meier method, and differences in survival were analyzed by χ2 test. (G-H) Model for the restoration of Ikaros antileukemia activity following CK2 inhibition. (G) Loss of Ikaros tumor suppressor function can occur by genetic inactivation (deletion or inactivating mutation of IKZF1) and/or by functional inactivation due to increased CK2 activity. (H) Targeted inhibition of CK2 kinase restores Ikaros function as repressor of cell cycle progression and the PI3K pathway, which results in an antileukemia effect.
CK2 inhibition has an antileukemia effect on primary xenografts of high-risk B-ALL. NRG mice were injected with primary B-ALL cells with deletion of (A) 1 IKZF1 allele or (B-C) other poor prognostic features (supplemental Table 1). (A-C) Following established engraftment (determined as described in Patients, materials, and methods), mice were treated with the CK2 inhibitor, CX-4945, or with vehicle control. Mice were killed and evaluated for the presence of leukemia in BM and spleen at day 22 (patient 3), day 17 (patient 4), or day 26 (patient 5) following the initiation of treatment. Percentages and numbers of leukemia cells in BM or spleen of xenograft mice are graphed. The effect of drug treatment was assessed by Student t test. (D-F) Patient-derived xenografts established with B-ALL from patient 3 (D), patient 4 (E), and patient 5 (F) were treated for 22 days with CK2 inhibitors, CX-4945, or vehicle control and followed for survival. Survival curves were generated using the Kaplan-Meier method, and differences in survival were analyzed by χ2 test. (G-H) Model for the restoration of Ikaros antileukemia activity following CK2 inhibition. (G) Loss of Ikaros tumor suppressor function can occur by genetic inactivation (deletion or inactivating mutation of IKZF1) and/or by functional inactivation due to increased CK2 activity. (H) Targeted inhibition of CK2 kinase restores Ikaros function as repressor of cell cycle progression and the PI3K pathway, which results in an antileukemia effect.
Discussion
Gain- and loss-of-function studies established that Ikaros negatively regulates cell cycle progression and the PI3K pathway by transcriptionally regulating a large network of genes that promote cell cycle progression and the PI3K pathway. This led to the hypothesis that Ikaros function in B-ALL is impaired.
CK2 is a pro-oncogenic kinase whose increased expression is associated with poor outcome in acute myelogenous leukemia and that is overexpressed in B-ALL.42,47 The increased activity of CK2 in cancer is most likely due to transcription of the genes that encode its catalytic and regulatory subunits.48,49 CK2 directly phosphorylates Ikaros at multiple serine/threonine residues, resulting in impaired Ikaros function.20-22 Here we show that elevated CK2 activity in B-ALL results in impaired Ikaros function as a transcriptional regulator of cell cycle and PI3K-promoting genes. Molecular and pharmacologic inhibition of CK2 represses transcription of a large number of the cell cycle and PI3K genes, mostly via restoration of Ikaros function as a transcriptional regulator. These data reveal the existence of a CK2-Ikaros signaling axis and identify the role of this pathway in regulating cell cycle and the PI3K pathway in B-ALL. These results demonstrate that Ikaros function in B-ALL can be impaired by 2 distinct mechanisms: (1) genetic inactivation (via deletion or inactivating mutation of IKZF1) and/or (2) functional inactivation of Ikaros by CK2-mediated phosphorylation (Figure 7G-H). Because CK2 activity is highly elevated in B-ALL, this suggests that functional inactivation of Ikaros is a very common event in B-ALL and that restoration of Ikaros function by CK2 inhibition is a therapeutic strategy for this disease. CK2 has been shown to activate the PI3K pathway via phosphorylation of phosphatase and tensin homolog (PTEN)44,50-52 and interaction with AKT.53 CK2 inhibition can suppress the PI3K pathway via restoration of PTEN function.42,44,54-56 Our data demonstrate an additional novel mechanism by which CK2 inhibition suppresses the PI3K pathway: by restoring Ikaros-mediated repression of multiple PI3K-promoting genes. Together, these results show that CK2 inhibition can downregulate the PI3K pathway through 2 mechanisms that are complementary but not exclusive: (1) via restoration of PTEN function and/or (2) via restoration of Ikaros function as a transcriptional regulator of genes that promote the PI3K pathway. These findings do not reduce the significance of other Ikaros-independent mechanisms that contribute to the therapeutic effect of CK2 inhibition, such as inhibition of the Janus kinase-signal transducer and activator of transcription pathway.57
High-risk B-ALL characterized by genetic inactivation of one IKZF1 allele has a very poor prognosis.9,12,14 Ikaros function in high-risk B-ALL is severely impaired; however, it is unclear whether the short form of the deleted allele inhibits the function of the remaining IKZF1 allele (as a dominant-negative mutant) or whether Ikaros produced from the intact IKZF1 allele is still active. Overexpression of small dominant-negative forms of Ikaros can abolish Ikaros activity,58 although it has been suggested that moderate expression of the short isoforms does not affect the DNA-binding function of Ikaros.59 Our results suggest that, in primary high-risk B-ALL cells with 1 intact IKZF1 allele, Ikaros binding to its target genes is abolished or severely impaired (compared with the leukemia cells with both Ikaros wild-type alleles). However, inhibition of CK2 restores Ikaros DNA binding, along with its function as a transcriptional regulator of cell cycle and the PI3K pathway genes. This suggests that in high-risk B-ALL, Ikaros function can be abolished due to genetic and/or functional inactivation of Ikaros (Figure 7A) and that restoration of Ikaros tumor suppressor function via CK2 inhibition is possible even if 1 IKZF1 allele is deleted (Figure 7B). Because therapeutic options for high-risk B-ALL are limited, this makes the use of CK2 inhibitors for the treatment of this type of leukemia a very attractive option.
In summary, we demonstrate that cell cycle progression and the PI3K pathway, along with cellular proliferation, are controlled in B-ALL by the CK2-Ikaros axis and identify the restoration of Ikaros tumor suppressor activity via targeted CK2 inhibition as a novel type of therapy for high-risk leukemia. The therapeutic efficacy of CK2 inhibition in preclinical models of high-risk B-ALL provides a rationale for the use of CK2 inhibitors as a novel treatment of this disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work has been supported by National Institutes of Health (NIH) National Heart Lung and Blood Institute grant R01 HL095120, a St Baldrick’s Foundation Career Development Award, a Hyundai Hope on Wheels Scholar Grant Award, the Four Diamonds Fund of the Pennsylvania State University, College of Medicine, and the John Wawrynovic Leukemia Research Scholar Endowment (to S.D.); a St. Baldrick’s Foundation Fellows Award and Hyundai Hope on Wheels fellowship grant award (to C.G.); by NIH National Cancer Institute (NCI) grants R01 CA1366667 and R01 CA138634 (to G.P.R.); and NSFC grant 81270613 (to Z.G). This work was also supported by NIH NCI grants R21CA162259 and National Institute on Minority Health and Health Disparities grant P20MD006988, a St. Baldrick’s Research Grant, and a Hyundai Hope on Wheels Award (K.J.P.).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Authorship
Contribution: C.S. and C.G. performed a majority of the biological experiments, analyzed the data, and participated in experimental design and writing the manuscript; X.P. provided bioinformatics and biostatistical analysis of the data and participated in manuscript preparation; Y.D., H.W., M.S., Z.G., S.M., K.G., Y.T., B.-H.T., H.S., and R.G. performed experiments, participated in experimental design, and provided conceptual advice; M.M. provided vital reagents and conceptual advice; S.G.A., D.D., and G.P.R. provided vital reagents and conceptual advice and participated in experimental design; K.J.P. provided vital reagents and conceptual advice and participated in experimental design and in writing the manuscript; and S.D. designed the experiments, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sinisa Dovat, Department of Pediatrics, Division of Pediatric Hematology/Oncology, Pennsylvania State University College of Medicine, Hershey, PA 17033; e-mail: sdovat@hmc.psu.edu.
References
Author notes
C.S. and C.G. contributed equally to this work.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal