In this issue of Blood, Song et al show that tumor suppressor activity of Ikaros is achieved though repression of cell cycle and phosphatidylinositol-3 (PI3) kinase pathway genes and can be reactivated through pharmacologic inhibition of casein kinase 2 (CK2) to eradicate disease in high-risk B-cell acute lymphoblastic leukemia (B-ALL).1
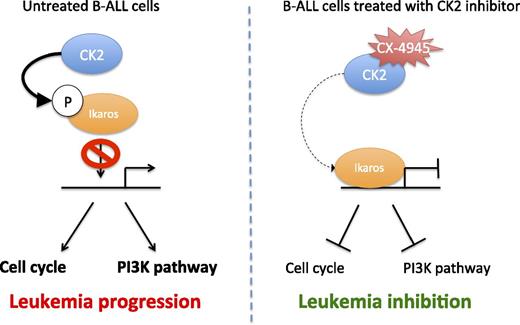
The two “wings” of Ikaros in B-ALL suppression. (Right) In B-ALL cells, CK2 activity is increased and results in Ikaros phosphorylation that prevents its binding to DNA and regulation of cell cycle and PI3K pathway genes. (Left) On treatment with the specific CK2 inhibitor CX-4945, Ikaros is no longer phosphorylated and can actively repress its target genes, leading to leukemia inhibition.
The two “wings” of Ikaros in B-ALL suppression. (Right) In B-ALL cells, CK2 activity is increased and results in Ikaros phosphorylation that prevents its binding to DNA and regulation of cell cycle and PI3K pathway genes. (Left) On treatment with the specific CK2 inhibitor CX-4945, Ikaros is no longer phosphorylated and can actively repress its target genes, leading to leukemia inhibition.
B-ALL is the most common leukemia diagnosed during childhood. Although frontline risk-adapted chemotherapies have improved overall survival, nearly 20% of children and >50% of adults relapse.2 Therefore, this disease remains one of the leading causes of leukemia death. In recent years, extensive genomic profiling has led to better classification and understanding of high-risk B-ALL.3 Elucidation of the mechanisms involving these genetic alterations in the pathogenesis of B-ALL could lead to the design of specific therapies for the most refractory subgroups.
Among these genetic alterations, the IKZF1 gene encoding the Ikaros transcription factor was found to be affected in ∼15% of all B-ALL patients. Strikingly, IKZF1 mutations were found in 70% to 80% of Philadelphia chromosome-positive B-ALL, where it is associated with poor outcome.4 Ikaros is a zinc finger transcription factor characterized in the early 1990s that plays an essential role in the development of lymphoid lineages.5 Ikaros was identified as a tumor suppressor in lymphoid malignancies, as deletion of 1 Ikzf1 allele in mice resulted in T-cell leukemia.6 IKZF1 alterations include deletions or point mutations of a single allele, resulting in loss of function, haplo-insufficiency, or expression of a dominant negative form. Previous attempts to identify direct transcriptional targets of Ikaros focused on normal lymphoid development in mice7 and not in human leukemia. To determine genome-wide occupancy of Ikaros in human B-ALL, Song et al1 performed chromatin immunoprecipitation followed by massively parallel sequencing (ChIP-seq) in a B-ALL cell line and primary B-ALL samples. They identified Ikaros binding on promoters of genes involved in cell cycle regulation such as cyclins (CCND3, CCNE2) or cyclin-dependent kinases (CDK2, CDK6) and genes involved in the phosphatidyl inositol pathway such as PIK3CD or PIK3C2B, among others. Using gain-of-function experiments overexpressing Ikaros or loss-of-function experiments with short hairpin RNA (shRNA) directed against Ikaros mRNA, they demonstrated that Ikaros directly represses expression of these genes. These 2 pathways are essential for proliferation of leukemic cells, and these experiments may define the tumor suppressor activity of Ikaros in B-ALL (see figure). However, progression and development of B-ALL occur even in the presence of 2 normal alleles of Ikaros, suggesting that other posttranscriptional or posttranslational regulatory mechanisms are involved.
Previous studies have shown that phosphorylation of Ikaros by CK2 inhibits its DNA binding8 and that CK2 activity is commonly upregulated in B-ALL.9 Therefore, the authors tested whether CK2 could directly affect Ikaros activity in B-ALL. They first showed that CK2 activity is indeed increased in primary B-ALL cells compared with normal precursor B cells. Inhibition of CK2 with anti-CK2 shRNA or a pharmacologic inhibitor of CK2, CX-4945, resulted in downregulation of Ikaros target genes. Moreover, they demonstrated that this transcriptional repression required Ikaros activity because knockdown of Ikaros reverted CK2 inhibitor-induced gene repression. Using a NALM6 cell line xenograft model, they found that treatment with CX-4945 induced repression of Ikaros target genes in vivo and significantly increased survival of the animals. These findings paralleled earlier studies addressing the efficiency of this inhibitor in B-ALL.9 The most novel and striking finding of the work by Song et al is the efficacy of CX-4945 in high-risk B-ALL where 1 IKZF1 allele is deleted. In primary patient samples, treatment with the CK2 inhibitor in vitro resulted in increased Ikaros binding to and repression of its target genes. The authors went on to validate these findings in patient-derived xenograft (PDX) models of high-risk B-ALL using 3 different patient samples presenting with different characteristics: CRLF2 overexpression, high blood count with 95% blasts, or IKZF1 deletion. After disease establishment, animals were treated for 22 days with CX-4945 inhibitor or vehicle. Cohorts treated with CK2 inhibitor had increased leukemic cell death, decreased infiltration of the bone marrow and spleen, and significantly increased overall survival. Taken together, the experiments by Song et al reveal that the tumor suppressor activity of Ikaros is reduced in high-risk B-ALL due to phosphorylation by CK2 and highlight the therapeutic potential of CK2 inhibitors in this lethal disease.
Recently, CK2 inhibitors have been extended from solid tumors to hematologic malignancies, including chronic lymphocytic leukemia, Hodgkin and non-Hodgkin lymphomas, ALL, and acute myeloid leukemia.10 This work suggests that CK2 inhibition may be an effective strategy in the treatment of high-risk B-ALL, particularly subsets presenting with IKZF1 deletion. However, several questions remain. Although the authors find that CX-4945 treatment is well tolerated in their preclinical model, it was only administered for a limited time with a modest gain in overall survival. As CK2 plays numerous essential functions in other tissues, the tolerability and consequences of extended treatment with this inhibitor remain to be seen. Using PDX models, it would be interesting to examine a possible synergy between moderate doses of CX-4945 and conventional chemotherapies no longer effective on high-risk B-ALL. Finally, as the loss of one IKZF1 allele is encountered in treatment-refractory high-risk B-ALL, would forced reactivation of the remaining IKZF1 allele trigger either loss of this allele or selection of minor clones already deleted for both alleles? Nonetheless, results of these studies by Song et al suggest that CK2 inhibition represents a promising therapeutic strategy for the management of high-risk B-ALL and warrants further evaluation of its clinical potential.
Conflict-of-interest disclosure: The author declares no competing financial interests.