Key Points
IL-21 activates IL-21R–dependent singling to mediate direct cytotoxicity of MCL cells.
Indirect effects of IL-21 on immune effector cells also contribute to antitumor effects against MCL.
Abstract
Mantle cell lymphoma (MCL) is a distinct subtype of non-Hodgkin lymphoma characterized by overexpression of cyclin D1 in 95% of patients. MCL patients experience frequent relapses resulting in median survival of 3 to 5 years, requiring more efficient therapeutic regimens. Interleukin (IL)-21, a member of the IL-2 cytokine family, possesses potent antitumor activity against a variety of cancers not expressing the IL-21 receptor (IL-21R) through immune activation. Previously, we established that IL-21 exerts direct cytotoxicity on IL-21R–expressing diffuse large B-cell lymphoma cells. Herein, we demonstrate that IL-21 possesses potent cytotoxicity against MCL cell lines and primary tumors. We identify that IL-21–induced direct cytotoxicity is mediated through signal transducer and activator of transcription 3-dependent cMyc upregulation, resulting in activation of Bax and inhibition of Bcl-2 and Bcl-XL. IL-21–mediated cMyc upregulation is only observed in IL-21–sensitive cells. Further, we demonstrate that IL-21 leads to natural killer (NK)-cell–dependent lysis of MCL cell lines that were resistant to direct cytotoxicity. In vivo treatment with IL-21 results in complete FC-muMCL1 tumor regression in syngeneic mice via NK- and T-cell–dependent mechanisms. Together, these data indicate that IL-21 has potent antitumor activity against MCL cells via direct cytotoxic and indirect, immune-mediated effects.
Introduction
Mantle cell lymphoma (MCL) is a morphologically distinct subtype of lymphoma that accounts for 6% to 8% of non-Hodgkin lymphomas. MCL is characterized by the chromosomal translocation (11;14)(q13;q32) that juxtaposes CCND1 to the immunoglobulin (Ig) heavy chain gene enhancer region.1 This translocation leads to constitutive overexpression of cyclin D1, resulting in early expansion of neoplastic B cells in the lymphoid follicle mantle zone, contributing to increased cell proliferation.
MCL is a highly aggressive disease with a median overall survival of 3 to 5 years.2-4 Although treatment with conventional chemotherapy results in an overall response of 60% to 80%, the majority of patients relapse and succumb to MCL.5 The addition of the anti-CD20 antibody rituximab to first line therapy led to improved complete remission rates, but did not prolong progression-free and overall survival.6 Consolidation with autologous stem cell transplantation improved response rates and duration, but did not result in lasting remissions.7 Studies incorporating cytarabine (ie, ARA-C) as part of the initial regimens led to marked increases in complete response rates and prolonged survival, yet failed to lead to cure of patients.8 Consequently, there is an urgent need to develop newer therapeutic approaches for MCL.
Interleukin (IL)-21, a member of the IL-2 cytokine family, is mainly secreted by CD4+ T, natural killer (NK), and Th17 cells.9 Upon binding of IL-21 to the IL-21 receptor (IL-21R), which contains the common cytokine receptor γ chain, Janus kinase-1 and Janus kinase-3 are activated, leading to signal transducer and activator of transcription (STAT)1, STAT3, and STAT5 phosphorylation. Dimerization of phosphorylated STAT proteins results in nuclear translocation and transcription of target genes. IL-21 exerts diverse regulatory effects on NK, and NK T and B cells.10 IL-21 induces B-cell proliferation, differentiation, or apoptosis depending on the cellular context and type of stimulus.10-14 Surprisingly, unlike other γ chain family members, IL-21 exhibits proapoptotic effects on activated and naïve B cells.15
IL-21 antitumor activity was demonstrated in multiple preclinical studies as single agent or in combination with chemotherapy16-24 and was evaluated in clinical trials for renal cell carcinoma and metastatic melanoma.24-26 In solid tumors not expressing IL-21R, the antitumor effects of IL-21 are mediated via NK and/or CD8+ T-cell activation.21,27,28
We have previously demonstrated that IL-21 exerts direct cytotoxicity on IL-21R–expressing diffuse large B-cell lymphoma (DLBCL) cell lines and primary tumors in vitro and in vivo.18 In our study, IL-21–induced cell death was mediated via STAT3-dependent cMyc upregulation, resulting in activation of the intrinsic apoptosis pathway. In vitro studies also demonstrated that IL-21 exerts direct cytotoxicity in chronic lymphocytic leukemias (CLL) and MCL cell lines, but via different mechanisms: caspase-8 activation leading to Bid cleavage followed by caspase-3 activation17,19 and STAT1 activation, respectively.22 The distinct cellular mechanisms of IL-21–mediated cytotoxicity in different B-cell tumors were unexpected and surprising. However, in the case of MCL, the report was based on in vitro studies in only 2 cell lines. To reconcile these findings and to more carefully examine the potential role of IL-21 for MCL therapy, we analyzed the direct IL-21–mediated effects on survival, proliferation, and apoptosis in a large set of MCL cell lines and primary tumors. Further, we also examined the indirect immunostimulatory effects of IL-21 signaling on NK and T cells, and their contribution to its antitumor activity in MCL.
Materials and methods
Reagents, antibodies, cell lines, primary tumors, and in vitro studies
Reagents, acquisition of primary tumors, and statistical methods are described in the supplemental information on the Blood Web site.
MCL cell lines: Mino, HBL-2, SP-53, Jeko-1, IRM-2, G-519, L-128, Z-138, and DLBCL cell line RC-K8 were cultured in RPMI 1640 medium (Gibco BRL) supplemented with 10% heat inactivated fetal bovine serum (FBS) (Mediatech), 2 mM glutamine (Gibco BRL), and 100 U/L penicillin/streptomycin (Gibco BRL). HeLa and 293T cells were cultured in Dulbecco’s modified Eagle medium (Mediatech) supplemented with 10% FBS, 2 mM glutamine, and penicillin/streptomycin. The FC-muMCL1 mouse lymphoma cell line, generated from the splenocytes of a 1-year-old male Eμ-cyclin D1 transgenic C57BL/6 mouse injected with pristane, was a kind gift of Dr M. R. Smith (Cleveland Clinic, OH).29 These cells were maintained in RPMI 1640 medium supplemented with 15% FBS, 1 mM non-essential amino acid, 1 mM Na-pyruvate, 2 mM glutamine, and penicillin/streptomycin.
Mice studies
To assess the in vivo efficacy of IL-21 in a murine MCL model, 4-week-old female C57BL/6 mice (Charles River) were subcutaneously (SC) inoculated with FC-muMCL1 cells (10 × 106 cells/100 μL RPMI) into the right flank. All mice were housed according to Institutional Animal Care and Use Committee guidelines in accordance with an approved protocol and monitored on a daily basis for tumor growth. Once tumor volume reached 100 mm3, mice were divided into 2 experimental groups (n = 5 per group) and treated intratumorally with phosphate-buffered saline (PBS) (50 μL) or IL-21 (10 μg) twice a day in the first week, and once a day for the following week. Tumor-bearing mice were assessed every 2 days for weight loss and tumor volume by standard calipers. Mice were euthanized once the tumor volume exceeded 1500 mm3 or if weight loss >10% total body weight was observed. For toxicity studies, tumor-bearing C57BL/6 mice were treated IV with IL-21 or PBS. Starting at day 4 after the first injection, liver, kidney, lungs, heart, brain, and spleen were extracted at different time-intervals, stained with hematoxylin and eosin, and examined by histology. Complete blood cell count and serum chemistry analyses were performed during follow-up.
In vivo lymphocyte subsets depletion
For in vivo CD4, CD8, and NK cells depletion, C57BL/6 mice were treated IV with anti-mouse CD4 (GK1.5), CD8 (2.43) antibodies (BioXCell), and anti-asialo GM1 (Wako Chemicals) on days −7, −5, and 0 (where day 0 is the day of tumor inoculation). Animals treated with control Ig with irrelevant specificity were used as nondepleted controls. The antibody injections were repeated IV every 6 days until the end of study. Depletion of lymphocyte subsets was confirmed by flow cytometry. Control or NK, CD4, and CD8 depleted mice were implanted with FC-muMCL1 cells and treated as described above.
Results
MCL cell lines and primary tumors express IL-21R on cell surface
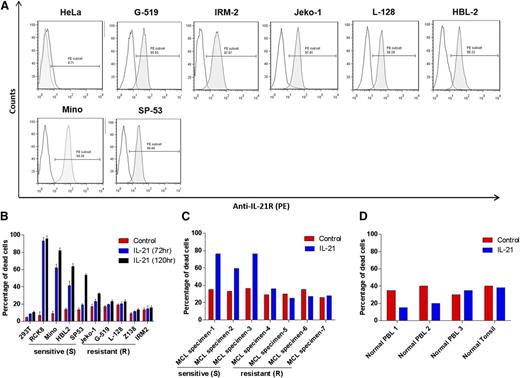
Analysis of cell-surface IL-21R expression in a panel of 8 MCL cell lines demonstrated heterogeneous expression levels, with the highest levels on Mino cells (Figure 1A). All cell lines were IL-21R positive. MCL cells isolated from primary tumors also expressed IL-21R at different levels and proportions of MCL cells (4 MCL tumors had more than 90% cells expressing IL-21R and 3 tumors had IL-21R expression in 18% to 56% of cells; supplemental Figure 1A-B).
Flow cytometric analysis of IL-21R expression in MCL cell lines. (A) The indicated MCL cell lines were stained for IL-21R and analyzed by flow cytometry. Gray histograms represent IL-21R–stained population and white histograms represent background fluorescence observed with matched isotype control. HeLa cells were used as a negative control. Data are representative of 3 independent experiments. (B) MCL cell lines (Mino, HBL-2, SP-53, IRM-2, Z-138, L-128, G-519, and Jeko-1), DLBCL cell line (RCK8; positive control), and 293T cell line (negative control), (C) neoplastic B cells isolated from de novo primary MCL tumors, and (D) normal B cells isolated from peripheral blood or lymph node (tonsil) were treated with IL-21 (100 ng/mL) for 72 hours. The cell death was measured by YO-PRO and PI staining followed by flow cytometry analysis. IL-21–responsive and non-responsive cell lines, and primary tumors are indicated as sensitive (S) and resistant (R), respectively. In panels A-B, data are representative of 3 independent experiments and error bars correspond to standard deviation (SD) between triplicate samples from a single experiment.
Flow cytometric analysis of IL-21R expression in MCL cell lines. (A) The indicated MCL cell lines were stained for IL-21R and analyzed by flow cytometry. Gray histograms represent IL-21R–stained population and white histograms represent background fluorescence observed with matched isotype control. HeLa cells were used as a negative control. Data are representative of 3 independent experiments. (B) MCL cell lines (Mino, HBL-2, SP-53, IRM-2, Z-138, L-128, G-519, and Jeko-1), DLBCL cell line (RCK8; positive control), and 293T cell line (negative control), (C) neoplastic B cells isolated from de novo primary MCL tumors, and (D) normal B cells isolated from peripheral blood or lymph node (tonsil) were treated with IL-21 (100 ng/mL) for 72 hours. The cell death was measured by YO-PRO and PI staining followed by flow cytometry analysis. IL-21–responsive and non-responsive cell lines, and primary tumors are indicated as sensitive (S) and resistant (R), respectively. In panels A-B, data are representative of 3 independent experiments and error bars correspond to standard deviation (SD) between triplicate samples from a single experiment.
IL-21 triggers cell death of MCL cell lines and primary tumors
We next assessed the effects of IL-21 on apoptosis and cell death. It has been previously reported that IL-21 directly induces growth arrest and apoptosis in the MCL cell lines, Mino and SP-53.22 To expand these studies, we initially analyzed IL-21 dose response by treating Mino cells with increasing cytokine concentrations (supplemental Figure 2). IL-21–induced cell death reached a plateau at 100 ng/mL, similar to our previous study in DLBCL.18 The 100 ng/mL concentration of IL-21 was previously commonly used in multiple studies and can be achieved in patients’ serum using IL-21 doses evaluated in clinical trials.17,30-32 Consequently, we selected a 100 ng/mL IL-21 dose and analyzed its cytotoxic effects in additional MCL cell lines by flow cytometry using YO-PRO and propidium iodide (PI) staining assay. Cell lines and primary tumors were defined as “sensitive/responsive” to IL-21 if the IL-21 treatment induced >15% increase in cell death compared with control cells (Figure 1B). IL-21 led to a marked increase in cell death of Mino, HBL-2, and SP-53 cells compared with untreated counterparts. In contrast, Jeko-1, IRM-2, L-128, Z-138, and G-519 cells were resistant to IL-21 treatment (percentage increase in cell death <15% upon IL-21 treatment; Figure 1B). Time-course analysis over 5 days revealed that IL-21–induced apoptosis was time-dependent with progressive transition from early apoptotic cells (YO-PRO+/PI) to late apoptotic cells (YO-PRO+/PI+) (supplemental Figure 3). Noticeably, IL-21–induced cell death was specific to MCL cell lines as non–B cells (293T) were not affected (Figure 1B).
We next evaluated the effects of IL-21 on 7 de novo, untreated primary human MCL specimens. IL-21 induced cell death in 3 of 7 primary tumors, with all susceptible tumors expressing IL-21R in more than 90% of cells (Figure 1C and supplemental Figure 1A-B). The remaining 4 primary tumors were resistant to IL-21 treatment irrespective of the percentage of cells expressing IL-21R (supplemental Figure 1A-B). These findings implied that IL-21R expression is not sufficient for IL-21–induced cell death, because not every cell line and primary tumor with surface IL-21R expression exhibited cell death upon IL-21 treatment (supplemental Figure 1C). Noticeably, none of the normal IL-21R–expressing B cells obtained from blood or lymph nodes of healthy volunteers were killed by IL-21, suggesting specificity of cytotoxic effects to the tumor cells (Figure 1D). Overall, our data demonstrate a non-uniform response of MCL cell lines and primary tumors to the direct cytotoxicity of IL-21.
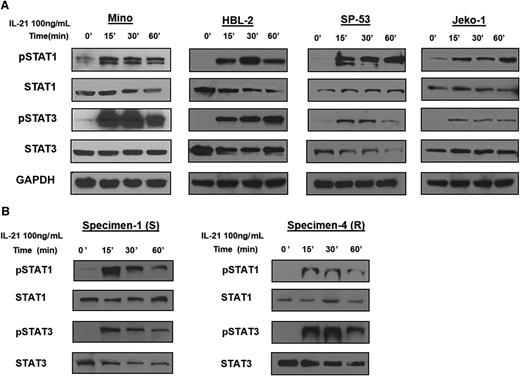
IL-21 induces signaling via tyrosine phosphorylation of STATs in MCL cell lines and primary tumors
Previous reports have indicated that IL-21 can activate STAT family members STAT1 and STAT318,19,22 and to a lesser extent, STAT5.18 To this end, we stimulated MCL cell lines with IL-21 (100 ng/mL) and analyzed tyrosine phosphorylation of STAT proteins by immunoblotting. IL-21 stimulation resulted in phosphorylation of STAT1 (pSTAT1) and STAT3 (pSTAT3) in IL-21–responsive and resistant MCL cell lines. Noticeably, pSTAT1 and pSTAT3 levels were not significantly different between the resistant and responsive cell lines (Figure 2A). Similar to cell lines, IL-21 induced pSTAT1 and pSTAT3 in both responsive and resistant primary MCL specimens (Figure 2B). IL-21 also induced phosphorylation of STAT5 in some of the analyzed cell lines and primary tumors, irrespective of IL-21–induced apoptosis (supplemental Figure 4).
IL-21 induces activation of STAT1 and STAT3 in MCL cell lines and primary tumors. For (A-B), MCL cell lines (Mino, HBL-2, SP-53, and Jeko-1) and primary tumors (specimens 1 and 4) were treated with IL-21 (100 ng/mL) for the indicated time periods. Whole cell lysates were prepared and subjected to immunoblotting to measure the levels of phosphorylated STAT1 and STAT3. Immunoblotting for nonphosphorylated STATs and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as loading controls. Results in panels A-B are representative of 3 independent experiments.
IL-21 induces activation of STAT1 and STAT3 in MCL cell lines and primary tumors. For (A-B), MCL cell lines (Mino, HBL-2, SP-53, and Jeko-1) and primary tumors (specimens 1 and 4) were treated with IL-21 (100 ng/mL) for the indicated time periods. Whole cell lysates were prepared and subjected to immunoblotting to measure the levels of phosphorylated STAT1 and STAT3. Immunoblotting for nonphosphorylated STATs and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as loading controls. Results in panels A-B are representative of 3 independent experiments.
IL-21–induced apoptosis of MCL cells is cMyc dependent
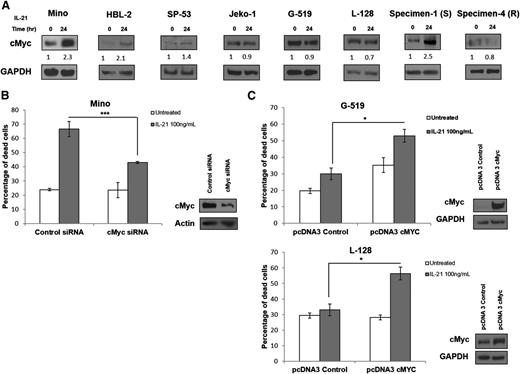
Because STATs activation was observed in both IL-21–responsive and resistant cell lines, we analyzed pathways downstream of these proteins to elucidate key players mediating IL-21–induced apoptosis. In DLBCL, we have previously demonstrated that IL-21 induces cell death by STAT3-mediated induction of cMyc.18 To examine for a similar intracellular signaling mechanism in MCL, we analyzed cMyc protein levels following IL-21 treatment in responsive and resistant MCL cell lines and primary tumors. Sensitive MCL cell lines (Mino, HBL-2, and SP-53) and a primary tumor (specimen 1) exhibited significant increases in cMyc protein levels at 24 hours post–IL-21 treatment (Figure 3A). In contrast, IL-21 did not induce an increase in cMyc protein levels in resistant cell lines (Jeko-1, L-128, and G-519) and primary cells (specimen 4), suggesting that cMyc may play a critical role in IL-21–induced cell death in MCL (Figure 3A).
Upregulation of cMyc correlates with IL-21–induced direct cell death in MCL cells. (A) Immunoblotting for cMyc in IL-21–sensitive (Mino, HBL-2, SP-53, and specimen 1) and resistant (Jeko-1, G-519, L-128, and specimen 4) MCL cell lines and primary tumors treated with 100 ng/mL IL-21 for 24 hours. cMyc protein levels quantified by densitometric analysis are displayed below each panel. (B) Mino cells transfected with cMyc siRNA, and (C) L-128 and G-519 cells transfected with pcDNA3-cMyc plasmid were treated at 24 hours posttransfections with IL-21 (100 ng/mL). Percentage of cell death was quantified using flow cytometry at 48 hours posttreatment. Immunoblotting for cMyc at 24 hours posttransfection were performed to confirm knockdown or overexpression of cMyc. Results in panels A-C are representative of 3 independent experiments. GAPDH and actin served as loading controls. *P < .05; ***P < .005.
Upregulation of cMyc correlates with IL-21–induced direct cell death in MCL cells. (A) Immunoblotting for cMyc in IL-21–sensitive (Mino, HBL-2, SP-53, and specimen 1) and resistant (Jeko-1, G-519, L-128, and specimen 4) MCL cell lines and primary tumors treated with 100 ng/mL IL-21 for 24 hours. cMyc protein levels quantified by densitometric analysis are displayed below each panel. (B) Mino cells transfected with cMyc siRNA, and (C) L-128 and G-519 cells transfected with pcDNA3-cMyc plasmid were treated at 24 hours posttransfections with IL-21 (100 ng/mL). Percentage of cell death was quantified using flow cytometry at 48 hours posttreatment. Immunoblotting for cMyc at 24 hours posttransfection were performed to confirm knockdown or overexpression of cMyc. Results in panels A-C are representative of 3 independent experiments. GAPDH and actin served as loading controls. *P < .05; ***P < .005.
To determine whether cMyc upregulation is required for apoptotic effects of IL-21, we knocked down cMyc using specific small interfering RNA (siRNA). cMyc knockdown prevented cMyc upregulation following IL-21 treatment and blocked IL-21–induced cell death as opposed to nontargeting control siRNA (P < .005; Figure 3B). To further confirm the role of cMyc in mediating the cytotoxic effects of IL-21, we transfected IL-21–resistant MCL cell lines with a cMyc-expressing vector. Transient cMyc overexpression mildly (not statistically significantly) increased the rate of spontaneous cell death in the G-519 but not L-128 MCL cell lines. Importantly, cMyc overexpression markedly increased IL-21–induced cytotoxicity in both of these previously IL-21–resistant MCL cell lines (P < .05; Figure 3C). Overall, these data suggest that cMyc upregulation is imperative for direct IL-21 cytotoxicity in MCL, similarly to DLBCL.18
IL-21 induces MCL apoptosis via STAT3 and Bax
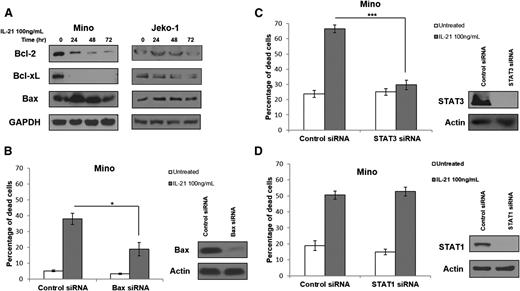
cMyc is known to induce apoptosis via upregulation of proapoptotic Bax and downregulation of antiapoptotic Bcl-2 and Bcl-XL.33-35 Thus, we analyzed the expression of these proteins in MCL cell lines and primary tumors following IL-21 treatment. IL-21 treatment resulted in Bax upregulation and downregulation of Bcl-2 and Bcl-XL in the IL-21–sensitive cell line Mino, but not in the IL-21–resistant cell line Jeko-1 (Figure 4A). Moreover, increased Bax expression was observed in sensitive but not resistant primary tumors (supplemental Figure 4). These findings suggest that similar to DLBCL, IL-21–induced cMyc upregulation modulates expression of Bcl-2 family proteins to trigger cell death.18
IL-21 induces cell death via STAT3 and modulation of expression of Bcl-2 family genes but is independent of STAT1. (A) Immunoblotting for Bcl-2 family genes in IL-21–responsive (Mino) and resistant (Jeko-1) MCL cell lines following treatment with IL-21 at indicated time points. (B-D) Mino cells were first transfected with siRNA targeting STAT3 (B), Bax (C), and STAT1 (D), or control siRNA followed by treatment with IL-21 (100 ng/mL) at 24 hours after transfection. (B-D) Indicate cell death analyzed by YO-PRO/PI staining at 48 hours posttreatment. Immunoblotting for STAT3, Bax, and STAT1 was carried out at 24 hours posttransfection to confirm protein knockdown (B-D). Actin and GAPDH served as a loading control. Data are representative of 3 independent experiments. Error bars represent SD between 3 experiments. *P < .05; ***P < .005.
IL-21 induces cell death via STAT3 and modulation of expression of Bcl-2 family genes but is independent of STAT1. (A) Immunoblotting for Bcl-2 family genes in IL-21–responsive (Mino) and resistant (Jeko-1) MCL cell lines following treatment with IL-21 at indicated time points. (B-D) Mino cells were first transfected with siRNA targeting STAT3 (B), Bax (C), and STAT1 (D), or control siRNA followed by treatment with IL-21 (100 ng/mL) at 24 hours after transfection. (B-D) Indicate cell death analyzed by YO-PRO/PI staining at 48 hours posttreatment. Immunoblotting for STAT3, Bax, and STAT1 was carried out at 24 hours posttransfection to confirm protein knockdown (B-D). Actin and GAPDH served as a loading control. Data are representative of 3 independent experiments. Error bars represent SD between 3 experiments. *P < .05; ***P < .005.
Bax is reported to be essential for cMyc-induced apoptosis.33,34,36 To demonstrate that observed changes in Bax expression might contribute to IL-21–induced apoptosis in MCL, siRNAs were used to knockdown Bax. Bax knockdown markedly decreased apoptosis after IL-21 treatment (Figure 4B). These findings suggest that Bax is a downstream effector of cMyc-induced apoptosis, as we reported in DLBCL.18
We next interrogated the upstream signaling cascade mediating the IL-21–induced increase in cMyc expression. cMyc is a known target of STAT3,37-39 which was shown to be activated in MCL cell lines and primary tumors upon IL-21 stimulation (Figure 2A). We knocked down STAT3 expression using specific siRNAs to analyze the role of STAT3 in regulating cMyc expression following IL-21 stimulation. STAT3 knockdown completely blocked apoptosis after IL-21 stimulation (P < .001; Figure 4C), similarly to our results in DLBCL.18 Taken together, these findings confirm the role of STAT3 in mediating IL-21–induced apoptosis in MCL.
However, previously published data suggested that IL-21–induced apoptosis of MCL cells is mediated via STAT1-dependent signaling.22 To interrogate the potential contribution of STAT1 to IL-21–induced apoptosis in MCL, we knocked down STAT1 using specific siRNAs. In contrast to the previous report, complete STAT1 knockdown did not affect IL-21–induced apoptosis in the Mino cell line (Figure 4D). siRNA-induced STAT3 and STAT1 knockdown was specific (supplemental Figure 5A), excluding the possibility of concomitant knockdown of STAT1 by the STAT3 siRNAs. Gelebart et al22 previously used IL-21 at doses of 20 and 50 ng/mL, whereas we used IL-21 at a dose of 100 ng/mL. Repetition of experiments using IL-21 at doses of 20 and 50 ng/mL also demonstrated that IL-21–induced MCL cell death was not mediated by STAT1 (supplemental Figure 5B). We further validated that IL-21 induces MCL cell death by activation of STAT3 and not STAT1 by overexpressing dominant negative (DN) STAT1 and STAT3 proteins in Mino cells before treatment with IL-21. In absolute concordance with our STAT3 and STAT1 siRNA-mediated knockdown studies, DN STAT3 but not DN STAT1 protein ameliorated IL-21–induced apoptosis (P = .007; supplemental Figure 6). Taken together, our findings demonstrate that IL-21–induced STAT3 activation causes a prohibitively high induction in cMyc expression, leading to Bax upregulation and Bcl-2 and Bcl-XL downregulation, resulting in apoptosis in IL-21–sensitive cells. Furthermore, based on our findings, it is potentially possible to predict MCL sensitivity to direct IL-21 cytotoxicity by evaluating the change in cMyc expression following in vitro stimulation with IL-21.
Baseline mitochondrial priming does not correlate with IL-21 sensitivity in MCL cell lines
To further exclude the possibility of nonspecific IL-21–induced destruction, we examined baseline mitochondrial priming in IL-21–sensitive and resistant cell lines. It was previously shown that baseline cell mitochondrial priming to apoptosis may determine sensitivity of cancer cells to different cytotoxic agents.40,41 Mitochondrial priming is controlled by the BCL-2 family of proapoptotic and antiapoptotic proteins.42,43 We adopted the BH3 profiling technique to determine the magnitude of response of mitochondria to peptides derived from the BH3 domains of proapoptotic proteins via measuring cytochrome C release. The greater the loss of cytochrome C caused by the BH3-only peptides, the more the cells are primed for death. We BH3 profiled MCL cell lines to determine their priming status using different concentrations of BIM BH3 peptide (that promiscuously inhibits all antiapoptotic members)43 and PUMA BH3 peptide (that inhibits response to sensitizers such as BAD, NOXA, and HRK).43 The peptides gain access by diffusion through a plasma membrane that has been permeabilized with low concentrations of digitonin. We found that Mino cells showed the highest level of priming, as evident by the lowest IC50 for both BIM and PUMA peptides compared with Jeko-1, Z138, or L-128 cell lines (supplemental Figure 7). Indeed, this data correlates with cytotoxicity data of IL-21, where the Mino cell line showed the highest cell death in response to IL-21. However, IL-21–responsive cell line HBL-2 showed lower priming compared with resistant Jeko-1 cells. These findings suggest that mitochondrial priming alone may not serve as a predictive biomarker to determine the response of IL-21 in MCL cells. Further, these finding suggest that IL-21 is inducing apoptosis not only in MCL cells that are primed to die.
IL-21 induces indirect cytotoxicity through NK-cell–mediated lysis
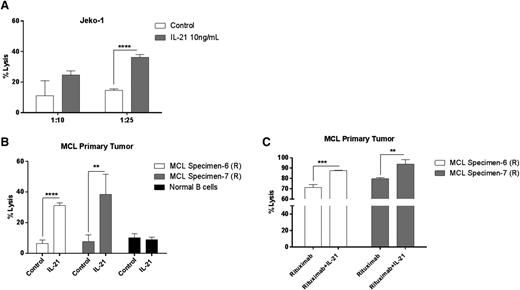
Apart from IL-21R–dependent direct cytotoxicity, IL-21–mediated immune cell activation is also known to contribute to its antitumor effects against a variety of cancers.21,28,44,45 To clarify the contribution of IL-21–induced immunomodulatory effects to its anti-MCL activity, we performed cell cytotoxicity assays using NK cells isolated from peripheral blood. As opposed to the direct cytotoxicity, immune-mediated effects of IL-21 are commonly induced by lower IL-21 concentrations (eg, 10 ng/mL).45 Consequently, human NK cells were stimulated overnight with IL-21 (10 ng/mL) or PBS. IL-21 significantly enhanced NK-cell cytolytic activity against Jeko-1 (Figure 5A) and MCL primary tumors (Figure 5B), whereas B cells isolated from healthy volunteers were unaffected (Figure 5B). Consequently, primary MCLs and MCL cell lines that are resistant to direct IL-21–mediated cytotoxicity may be sensitive to IL-21–activated NK-cell–mediated cell lysis.
IL-21 increases NK-cell–mediated lysis of MCL cells. Enriched human NK cells from peripheral blood were incubated with 51Cr-labeled (A) Jeko-1 and (B) MCL primary tumor cells coated with IL-21 at various effector/target ratios as described in “Materials and methods.” In (C), 51Cr-labeled MCL primary tumor cells were coated with rituximab alone or in the presence of IL-21, followed by incubation with NK cells at variable effector/target ratios. Percentage of specific lysis was derived from 51Cr released by target cells. Data are representative of 3 independent experiments. Error bars represent SD between triplicate wells. *P < .05; **P < .01; ***P < .0005; ****P < .0001.
IL-21 increases NK-cell–mediated lysis of MCL cells. Enriched human NK cells from peripheral blood were incubated with 51Cr-labeled (A) Jeko-1 and (B) MCL primary tumor cells coated with IL-21 at various effector/target ratios as described in “Materials and methods.” In (C), 51Cr-labeled MCL primary tumor cells were coated with rituximab alone or in the presence of IL-21, followed by incubation with NK cells at variable effector/target ratios. Percentage of specific lysis was derived from 51Cr released by target cells. Data are representative of 3 independent experiments. Error bars represent SD between triplicate wells. *P < .05; **P < .01; ***P < .0005; ****P < .0001.
Preclinical studies have demonstrated that IL-21 augments the antibody-dependent cell-mediated cytotoxicity (ADCC) of antibody coated target cells by enhanced NK-cell activation in CLL primary tumors and animal B-cell lymphoma and breast cancer models.17,45 However, this phenomenon was not explored in MCL tumors. To extend these findings, we tested the ability of IL-21 to enhance ADCC against MCL primary tumors coated with an anti-CD20 monoclonal antibody (rituximab). IL-21–activated NK cells exhibited significantly increased rituximab-specific ADCC activity against tested primary tumors (P < .005 and P < .01; Figure 5C).
In vivo IL-21 treatment induces tumor regression in a syngeneic murine model of MCL
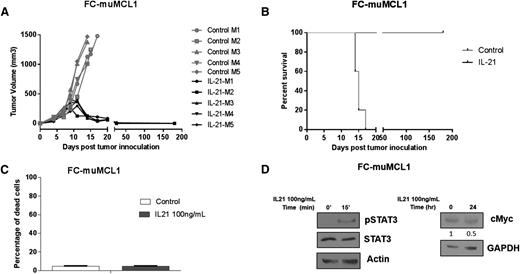
The proapoptotic activity of IL-21 in MCL cell lines and primary tumors suggest that IL-21 may be useful to treat MCL patients. Consequently, we examined the in vivo efficacy of IL-21 against a syngeneic, immunocompetent MCL mouse model, FC-muMCL1 derived from splenocytes of an Eμ-cyclin D1 transgenic C57BL/6 mouse.29 Impressively, IL-21 treatment resulted in complete MCL regression in all animals (Figure 6A), resulting in significantly longer survival compared with control mice (P < .0001; Figure 6B). IL-21–treated mice remained disease free for more than 6 months and no tumors were detected upon extensive postmortem analyses of these mice. Similar to previous reports in animals and humans,20,24,30 the IL-21 treatment was well tolerated, the mice continued to gain weight during treatment, and showed no signs of toxicity. We did not detect any changes in blood cell counts, electrolytes, kidney, and liver functions (not shown). Further, extensive pathological studies of normal organs did not reveal any pathological findings (supplemental Figure 8A-L).
In vivo IL-21 inhibits tumor growth and prolongs survival of MCL-bearing mice. (A-B) C57BL/6 mice (n = 5 per group) were SC injected with FC-muMCL1 cells and treated with IL-21 or PBS as described in “Materials and methods.” (A) Depicts tumor volume of lymphoma-bearing mice and (B) illustrates Kaplan–Meier survival curves. (C) FC-muMCL1 cells were treated with 100 ng/mL IL-21 for 72 hours, and cell death was measured by YO-PRO and PI staining followed by flow cytometry. (D) FC-muMCL1 cells were stimulated with 100 ng/mL IL-21 in vitro for 15 minutes or 24 hours followed by immunoblotting for phosphorylated STAT3 and cMyc, respectively. For panels A-D, data are representative of 2 independent experiments. Error bars represent standard error of the mean.
In vivo IL-21 inhibits tumor growth and prolongs survival of MCL-bearing mice. (A-B) C57BL/6 mice (n = 5 per group) were SC injected with FC-muMCL1 cells and treated with IL-21 or PBS as described in “Materials and methods.” (A) Depicts tumor volume of lymphoma-bearing mice and (B) illustrates Kaplan–Meier survival curves. (C) FC-muMCL1 cells were treated with 100 ng/mL IL-21 for 72 hours, and cell death was measured by YO-PRO and PI staining followed by flow cytometry. (D) FC-muMCL1 cells were stimulated with 100 ng/mL IL-21 in vitro for 15 minutes or 24 hours followed by immunoblotting for phosphorylated STAT3 and cMyc, respectively. For panels A-D, data are representative of 2 independent experiments. Error bars represent standard error of the mean.
To determine whether the observed IL-21 activity on the FC-muMCL1 tumors and immune microenvironment was direct or indirect, we examined the effects of in vitro IL-21 treatment on FC-muMCL1 cell death. Despite expression of IL-21R and pSTAT3 activation upon IL-21 treatment, we did not observe cMyc upregulation and hence FC-muMCL1 cells remained unaffected by in vitro IL-21 treatment (Figure 6C-D). Overall, these results indicate that IL-21 does not exhibit direct cytotoxic effects on FC-muMCL1 cells, suggesting that the observed in vivo IL-21 antitumor effects in the syngeneic mouse model were immune-mediated, as reported in other solid tumors types.21
In vivo antitumor effects of IL-21 are dependent on NK and T cells
Earlier studies demonstrated that IL-21 may induce antitumor immunity through NK cells and/or T-cell (CD4+ or CD8+) activation, depending on the tumor model.21,28 To elucidate the direct contribution of distinct effectors to IL-21 therapeutic efficacy, we carried out cell-subset depletion experiments using specific monoclonal antibodies. Mice were first depleted of lymphocytes (NK, CD4+, and CD8+ T cells), followed by SC tumor implantation with FC-muMCL1 cells. Each depletion was cell-subset specific and did not affect other cellular subsets (supplemental Figure 9A-C). Depletion of NK cells, CD4+, or CD8+ cells did not significantly alter the rate of tumor engraftment and control animal survival (supplemental Figure 9D). IL-21–treated NK-cell–depleted mice showed significantly shorter survival compared with nondepleted controls (P < .01), with 80% of animals dying from the lymphoma, suggesting that the IL-21 antitumor effect is NK-cell dependent (Figure 7A). CD4+ depletion also abrogated the IL-21 antitumor effects, resulting in shorter animal survival compared with nondepleted IL-21–treated animals (P < .05; Figure 7B). Furthermore, NK and CD4+ cell-depleted animals treated with IL-21 exhibited significantly shorter survival compared with IL-21–treated NK-cell–depleted mice (P = .002). The survival of these animals was similar to the survival of animals not receiving IL-21 treatment. CD8+ T-cell depletion only minimally decreased survival in IL-21–treated animals (Figure 7C). There was no statistically significant difference in the survival between animals depleted of NK cells only vs animals concomitantly depleted of both NK and CD8+ T cells (Figure 7C). Overall, these depletion studies indicate that IL-21–mediated anti-MCL effects on the FC-muMCL1 tumors require both NK and CD4+ T cells with minimal contribution from CD8+ T cells.
In vivo antitumor effect of IL-21 is NK- and T-cell dependent. In (A-B), C57BL/6 mice (n = 5 per group) depleted of NK or treated with irrelevant Ig (A), CD4+ T and/or NK cells (B), and CD8+ T and/or NK cells (C) were SC inoculated with FC-muMCL1 cells. Depicted are Kaplan–Meier survival curves of mice treated with IL-21 or PBS (control) as described in “Materials and methods.” (D-E) FC-muMCL1 cells or (F) normal human B cells from PBL were labeled with 51Cr and incubated with purified mice NK cells (D), or mice CD4+ T cells (E), or human CD4+ T cells (F) at the indicated T:E ratios. Percentage of specific lysis was measured based on the 51Cr release. All samples were run in triplicates (n = 3). Data in panels A-E are representative of 2 independent experiments. Error bars represent standard error of the mean. **P < .01; ***P < .0005.
In vivo antitumor effect of IL-21 is NK- and T-cell dependent. In (A-B), C57BL/6 mice (n = 5 per group) depleted of NK or treated with irrelevant Ig (A), CD4+ T and/or NK cells (B), and CD8+ T and/or NK cells (C) were SC inoculated with FC-muMCL1 cells. Depicted are Kaplan–Meier survival curves of mice treated with IL-21 or PBS (control) as described in “Materials and methods.” (D-E) FC-muMCL1 cells or (F) normal human B cells from PBL were labeled with 51Cr and incubated with purified mice NK cells (D), or mice CD4+ T cells (E), or human CD4+ T cells (F) at the indicated T:E ratios. Percentage of specific lysis was measured based on the 51Cr release. All samples were run in triplicates (n = 3). Data in panels A-E are representative of 2 independent experiments. Error bars represent standard error of the mean. **P < .01; ***P < .0005.
IL-21 promotes NK-cell and CD4+ T-cell activation and cytotoxic function
Because NK and CD4+ T cells accounted for tumor inhibition by IL-21, we next investigated the effects of IL-21 on cytotoxic function of these cell subsets. IL-21 markedly activated NK cells, as measured by activation marker CD69 on C57BL/6 mice-derived NK cells treated with IL-21 both in vitro and in vivo (supplemental Figure 10A-B). Furthermore, NK cells enriched from spleens of IL-21–treated C57BL/6 mice demonstrated significantly increased cytolytic activity against FC-muMCL1 cells at various target:effector (T:E) ratios compared with PBS-treated mice (P < .001, Figure 7D). Similarly, CD4+ T cells stimulated with IL-21 demonstrated enhanced in vitro lysis of FC-muMCL1 cells compared with unstimulated controls. This enhanced lysis was observed at T:E ratio of 1:10 (P < .001; Figure 7E) with no further increase at increasing T:E ratio (data not shown). Moreover, IL-21 mediated effector activities of NK cells and CD4+ T cells were found to be restricted to the lymphoma cells since both cell types did not affect the lysis of normal B cells isolated from peripheral blood of healthy volunteers (Figures 5B and 7F). Overall, these results suggest that the observed antitumor activity of IL-21 in the FC-muMCL1 syngeneic mouse model was mediated by stimulation of immune effectors and not by direct cytotoxicity. In contrast to animal studies, IL-21 enhanced NK but not CD4+ T-cell cytolytic activity in human MCL cell line, Jeko-1 (Figure 5A and not shown).
Discussion
MCL is characterized by a relatively short survival. Development of novel targeted therapeutic modalities is urgently needed to improve MCL patient survival. Herein, we demonstrate that IL-21 can induce cell death of MCL cell lines and primary tumors, and extend the survival of mice harboring syngeneic MCL. The anti-MCL activities of IL-21 were mediated via direct (IL-21R–STAT3–cMyc-dependent) and indirect (immune-mediated NK–T-cell–dependent) cytotoxic effects. Further, our studies demonstrate that IL-21R was necessary, but not sufficient, for direct cytotoxic effects of IL-21 on MCL cells, since a subset of IL-21R expressing MCL cell lines and primary tumors were unresponsive to IL-21 therapy. Nevertheless, MCL cells resistant to the direct cytotoxic effects of IL-21 may still be destroyed in vivo via indirect immune-mediated mechanisms. Contrary to our earlier study in DLBCL, where most cell lines and primary tumors were responsive to IL-21, MCL cells showed a heterogeneous response. Such varied response to IL-21 was also observed in CLL cells.19
We demonstrate that IL-21–induced apoptosis of MCL cells is mediated through activation of the STAT3-cMyc pathway, leading to upregulation of proapoptotic (Bax) and downregulation of antiapoptotic (Bcl-2 and Bcl-XL) proteins. In agreement with our study in DLBCL,18 IL-21–induced direct cytotoxic effects in MCL were STAT3 but not STAT1-dependent, since STAT1 knockdown by several siRNAs and use of DN STAT1 failed to rescue IL-21–induced cell death. Our report is consistent with a previous report22 showing that MCL cell lines are sensitive to IL-21 treatment, except that we found the cytotoxic effects to be dependent on STAT3 instead of STAT1. Although the reasons for this difference is unclear, it is possible that the siRNAs used by Gelebart et al may nonspecifically target STAT3, because in contrast to our study, Gelebart et al22 did not evaluate the potential role of STAT3 in mediating the direct cytotoxicity of IL-21.
Apart from our study in DLBCL, STAT3 has been implicated in the induction of apoptosis in additional studies. STAT3 promoted cell death upon triggering terminal differentiation and apoptosis in myeloid leukemia M1 cells or during involution of mammary glands.46 Molecular factors determining whether STAT3 activation would result in cell survival or cell death still remain poorly understood. Sutherland et al showed that loss of SOCS3 is associated with the conversion of STAT3 into a proapoptotic transcription factor.47 However, in our previous study in DLBCL, we demonstrated that SOCS3 expression was induced upon IL-21 treatment in both resistant and sensitive DLBCL cell lines18 and thus could not explain the mechanism behind proapoptotic effects of STAT3. It is likely that the strength and duration of STAT3 phosphorylation may contribute to the final phenotypic outcome, and studies exploring this possibility are needed.
Although STAT3 knockdown prevented IL-21–induced cell death, pSTAT3 levels were not significantly different between resistant and responsive MCL cell lines/primary tumors. This observation implies that STAT3 activation alone is not sufficient to mediate direct cytotoxic effects of IL-21, and hence cannot be used to predict antitumor responses to IL-21 in MCL cells. This prompted us to elucidate the underlying mechanisms of IL-21–induced direct cytotoxicity in MCL cell lines. In concordance with our findings in DLBCL,18 IL-21–induced cMyc upregulation was observed only in cell lines and primary tumors responsive to IL-21, whereas resistant cell lines did not upregulate cMyc. cMyc is a known target of STAT3 and has been implicated in regulating cell growth, differentiation, and apoptosis.38 cMyc expression levels determine cell fate; low cMyc levels promote cell proliferation, whereas high cMyc levels induce cell death.48,49 Knockdown of cMyc prevented IL-21–induced increases in cMyc expression, thus reducing MCL cell apoptosis. Correspondingly, cMyc overexpression in resistant MCL cell lines facilitated IL-21–induced cytotoxicity. Overall, these results are in agreement with our previous study that cMyc upregulation is indispensable for IL-21–induced direct cytotoxicity. Moreover, our data corresponds to studies from other groups that showed a link between STAT3 and cMyc activation.37 Based on these findings, we propose that IL-21–mediated cMyc upregulation may be used as a predictor of IL-21–induced direct cytotoxicity in MCL cell lines and primary tumors, and should be evaluated in clinical settings.
In this study, we have used a syngeneic MCL mouse model to determine IL-21 activity in vivo. This allowed us to effectively examine the contribution of immune effector cells toward IL-21–induced antitumor activity. Using the FC-muMCL1 lymphoma model, we demonstrate that IL-21 completely inhibits tumor growth, and the observed in vivo antitumor effect was indirect and likely attributed to both NK-cell and CD4+ T-cell subsets, because depletion of these cells ameliorated IL-21 antitumor effects. Moreover, IL-21 also enhanced NK and CD4+ T-cell cytolytic activity as demonstrated by increased lysis of 51Cr-labeled target tumor cells. NK cells were implicated in IL-21–mediated antitumor activity in solid tumor models of melanoma. Wang et al reported that NK-cell depletion completely abrogated the antitumor activity of IL-21.21 CD8+ but not CD4+ T cells also contributed to the IL-21 antitumor effects in this model.21 In contrast, CD8+ T cells were the major mediators of the IL-21 antitumor effects in a syngeneic mouse model of breast adenocarcinoma. Independent studies in syngeneic thymoma, B16 melanoma, and renal carcinoma models showed increased CD8+ T-cell infiltration upon IL-21 treatment.28 In these studies, depletion of CD8+ but not CD4+ T cells, or NK and NK T cells, ameliorated IL-21–mediated antitumor responses.21,27,28 Collectively, as emphasized by the earlier studies in solid tumors, the contribution of individual cell subset to in vivo antitumor effects of IL-21 are tumor model and host microenvironment specific. Our study provides the first comprehensive evidence for IL-21–induced immune effectors-mediated antilymphoma activity.
In conclusion, we demonstrate that IL-21 exerts direct and indirect immune-mediated cytotoxicity against MCL. A recent clinical trial of IL-21 in combination with rituximab for relapsed or refractory B-cell lymphomas showed clinical responses in 8 out of 19 patients.50 However, patients with MCL were not included in this study. Since our data demonstrated that IL-21 enhances the ADCC activity of rituximab in MCL primary tumors and cell lines by stimulatory effects on NK cells, clinical studies evaluating the activity and immune mechanisms of IL-21 in MCL patients are warranted.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by the Lymphoma Research Foundation (I.S.L.). I.S.L. was also supported by a grant from the National Institutes of Health National Cancer Institute (CA109335) and the Dwoskin Family, Recio Family, and Antony Rizzo Foundations.
Authorship
Contribution: S.B. performed most of the laboratory work, analyzed the data, and wrote the paper; J.M., S.P., K.A.S., D.Z., E.I., and X.J. performed laboratory experiments; A.L. supervised experiments; and I.S.L. conceptualized the idea of the study, supervised the experiments, analyzed the data, provided funding, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Izidore S. Lossos, University of Miami, Sylvester Comprehensive Cancer Center, Division of Hematology and Oncology, 1475 NW 12th Ave (D8-4), Miami, FL 33136; e-mail: ilossos@med.miami.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal