Key Points
The JAK2p.V617F mutation leads to constitutive activation of JAK2 and contributes to dysregulated JAK signaling in myelofibrosis.
Long-term ruxolitinib treatment decreased JAK2p.V617F allele burden, with some patients achieving complete or partial molecular remissions.
Abstract
The JAK2 c.1849G>T (p.V617F) mutation leads to constitutive activation of Janus kinase (JAK)2 and contributes to dysregulated JAK signaling in myelofibrosis (MF), polycythemia vera (PV), and essential thrombocythemia (ET). In the phase 3 Controlled Myelofibrosis Study with Oral JAK Inhibitor Treatment-I trial, patients with MF, post-PV MF, or post-ET MF achieved significant reductions in splenomegaly and improvements in symptoms with ruxolitinib vs placebo at week 24. This long-term follow-up analysis was performed to determine whether ruxolitinib therapy altered the JAK2p.V617F allele burden in JAK2p.V617F-positive patients. Assessments at baseline and weeks 24, 48, 120, 144, 168, and 216 demonstrated reductions in allele burden from baseline with ruxolitinib treatment that correlated with spleen volume reductions. Of 236 JAK2p.V617F-positive patients analyzed, 20 achieved partial and 6 achieved complete molecular responses, with median times to response of 22.2 and 27.5 months, respectively. Allele burden reductions were greater in patients with shorter disease duration, which suggests a potential benefit of earlier treatment. This trial was registered at www.clinicaltrials.gov as #NCT00952289.
Introduction
The JAK2 c.1849G>T (p.V617F) mutation is present in a high proportion of patients with BCR-ABL1–negative myeloproliferative neoplasms (MPNs): essential thrombocythemia (ET), polycythemia vera (PV), and myelofibrosis (MF).1 The valine-to-phenylalanine change in the Janus kinase (JAK)2 pseudokinase domain leads to constitutive JAK2 activity, increased signal transducer and activator of transcription (STAT) signaling, and growth factor hypersensitivity/independence.2 Increased signaling through the JAK/STAT pathway is an important contributor to the MPN phenotype. Additional mutations leading to JAK/STAT activation in MPNs include activating MPL mutations,3 JAK2 exon 12 mutations,4 and CALR mutations.5,6
Ruxolitinib, an oral JAK1/JAK2 inhibitor, demonstrated rapid and durable reductions in splenomegaly and improvements in disease-related symptoms in patients with intermediate-2 or high-risk MF in Controlled Myelofibrosis Study with Oral JAK Inhibitor Treatment (COMFORT)-I and COMFORT-II. Findings also suggested an overall survival benefit for ruxolitinib over placebo and best available therapy.7-10 Presence of the JAK2p.V617F mutation was not required for enrollment; responses to ruxolitinib were achieved in patients with and without the mutation. However, JAK2p.V617F allele burden (percentage of mutant allele relative to total [wild type + mutant]) has been associated with disease phenotype; patients with higher leukocyte counts and hemoglobin levels and more profound splenomegaly have higher allele burden.11-14 Ruxolitinib and other MF therapies have demonstrated overall modest changes in JAK2p.V617F allele burden7,11 ; however, those findings were based on relatively short-term follow-up. This analysis was designed to evaluate the effect of ruxolitinib on allele burden in JAK2p.V617F-positive patients in COMFORT-I after long-term follow-up.
Study design
In COMFORT-I, patients with intermediate-2 or high-risk MF were randomized to ruxolitinib (15 or 20 mg twice daily; n = 155) or placebo (n = 154). Patients in the placebo group were allowed to cross over to ruxolitinib for protocol-defined progression of splenomegaly.7 This analysis incorporated the most recent allele burden (October 2014) and efficacy data (September 2013, 3.5-year analysis). Genomic DNA isolation from peripheral blood and JAK2p.V617F assays were performed as described previously15 on samples collected at baseline and weeks 24, 48, 120, 144, 168, and 216. All patients provided written informed consent in accordance with the Declaration of Helsinki. The COMFORT-I study protocol was approved by the institutional review board at each participating site.
The allele burden percent change from baseline was calculated for all patients harboring the JAK2p.V617F mutation at baseline (236 patients) with ≥1 postbaseline measurement (214 patients). Baseline was defined as the measurement prior to the first dose of randomized treatment. For multiple same-day observations, average allele burden was used. For patients who crossed over to ruxolitinib, the percent change from the last allele burden measurement prior to the first ruxolitinib dose to each postcrossover measurement was calculated. Patients originally randomized to receive ruxolitinib with evaluable percent change in allele burden from baseline were classified into 3 tertiles, based on the maximum reduction in allele burden (tertile 1: −100% to ≤−24.66%; tertile 2: >−24.66% to ≤−8%; tertile 3: >−8%). Tertile 3 captured patients with no reduction or increased allele burden. The lower limit of assay quantitation was 2%; values <2% were designated below quantifiable limit. Complete molecular remission (CMR), partial molecular remission (PMR), and relapse of molecular remission were defined based on the International Working Group-Myeloproliferative Neoplasms Research and Treatment/European LeukemiaNet consensus criteria.16 Percent change in spleen volume and spleen response (≥35% reduction from baseline to week 24) was determined as described previously.7
Results and discussion
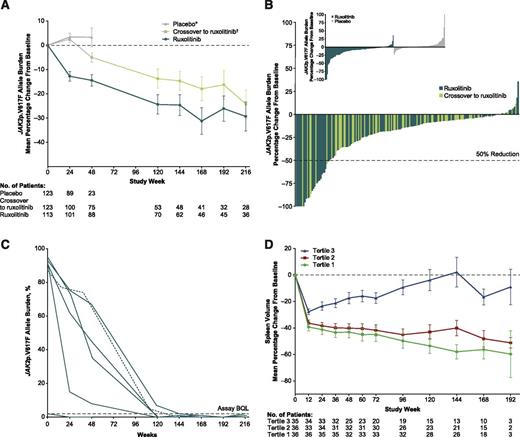
During the randomized phase (through week 24), the mean percent change from baseline in allele burden decreased in the ruxolitinib group and increased in placebo-treated patients (Figure 1A). After crossover (all patients receiving ruxolitinib), the mean percentage change in allele burden for those originally randomized to ruxolitinib and those originally randomized to placebo continued to decrease with continued ruxolitinib treatment. The mean/median (range) of maximal change in the ruxolitinib-randomized arm was −27%/−16% (−100%, 36%) and in patients who crossed over from placebo to ruxolitinib was −19%/−11% (−100%, 12%). Twenty-eight patients (12%) had >50% decreases in JAK2p.V617F allele burden (Figure 1B). Of these, 20 met the criteria for PMR; 1 patient with baseline allele burden of <20% was disqualified from having a PMR, despite a 64% decrease in JAK2p.V167F. Six patients had JAK2p.V617F values below quantifiable limit for 2 successive samples collected 6 months apart, meeting the criteria for CMR (Figure 1C). Median times to PMR and CMR among patients achieving these remissions were 22.2 and 27.5 months, respectively, with all responses ongoing at the time of this analysis.
JAK2p.V617F allele burden reductions and correlations with spleen volume reductions in patients with myelofibrosis. (A) Mean percent change (±SE) in allele burden from baseline. (B) Best molecular response in individual patients randomized to ruxolitinib and in patients receiving ruxolitinib after crossover from placebo. (Inset) Change from baseline at week 24 for individuals randomized to ruxolitinib and placebo. (C) Allele burden change over time for patients achieving CMR (<2% with confirmation). Solid green lines, patients randomized to ruxolitinib; dashed green line, patient initially randomized to placebo who crossed over to ruxolitinib, with time 0 marking the start of ruxolitinib treatment. (D) Mean percent change in spleen volume stratified by tertile of maximum allele burden reduction. SE, standard error. *Patients on placebo who did not cross over to ruxolitinib. †All patients randomized to placebo; shading of the line in this group represents crossover of patients from placebo to ruxolitinib.
JAK2p.V617F allele burden reductions and correlations with spleen volume reductions in patients with myelofibrosis. (A) Mean percent change (±SE) in allele burden from baseline. (B) Best molecular response in individual patients randomized to ruxolitinib and in patients receiving ruxolitinib after crossover from placebo. (Inset) Change from baseline at week 24 for individuals randomized to ruxolitinib and placebo. (C) Allele burden change over time for patients achieving CMR (<2% with confirmation). Solid green lines, patients randomized to ruxolitinib; dashed green line, patient initially randomized to placebo who crossed over to ruxolitinib, with time 0 marking the start of ruxolitinib treatment. (D) Mean percent change in spleen volume stratified by tertile of maximum allele burden reduction. SE, standard error. *Patients on placebo who did not cross over to ruxolitinib. †All patients randomized to placebo; shading of the line in this group represents crossover of patients from placebo to ruxolitinib.
The relative allele burden reduction was independent of baseline allele burden; patients with high and low allele burdens both showed significant reductions. Changes in allele burden are shown in supplemental Figures 1 and 2, available on the Blood Web site. When baseline characteristics were compared among tertiles of best JAK2p.V617F response for patients in the ruxolitinib arm (supplemental Table 1), decreases in JAK2p.V617F allele burden were inversely associated with time from diagnosis (patients with the largest decreases in allele burden had the shortest mean and median times from diagnosis; Table 1). Patients with greater decreases in allele burden also tended to have intermediate-risk MF, post-PV MF, and primary MF. Patients with greater allele burden decreases also had larger percent changes in spleen volume (mean/median changes at week 24 for tertile 1: −41.2%/−45.2%; tertile 2: −38.3%/−37.1%; tertile 3: −23.4%/−22.7%; Figure 1D), and a greater proportion had a spleen response (tertile 1: 63.9%; tertile 2: 55.6%; tertile 3: 31.4%). However, even patients with smaller decreases in JAK2p.V617F allele burden (31.4% of 35 patients in tertile 3) and those who were JAK2p.V617F negative at baseline (27.5%) had spleen responses. For patients without an allele burden response or with a subsequent increase after a decrease while on therapy, there was no clear signal that allele burden either correlated with or predicted spleen response. Ruxolitinib dose intensity leading up to the allele burden nadir was similar across tertile groups (supplemental Table 2). Changes in JAK2p.V617F allele burden did not correlate with changes in other clinical/hematologic parameters, bone marrow morphology, constitutional symptoms, or cytokines.
Baseline disease history in JAK2p.V617F-positive patients by tertile
| . | Tertile 1 (−100% to ≤−24.66%) (n = 36) . | Tertile 2 (>−24.66% to ≤−8%) (n = 36) . | Tertile 3 (>−8%) (n = 35) . |
|---|---|---|---|
| Maximum percent change in allele burden | |||
| Mean | −62.6 | −15.3 | −0.5 |
| Median | −63.4 | −14.8 | −2.2 |
| Baseline allele burden | |||
| Mean | 70.9 | 73.5 | 75.3 |
| Median | 84.5 | 81.5 | 84.0 |
| Duration of disease prior to treatment, months | |||
| Mean | 36.8 | 52.5 | 44.8 |
| Median | 15.2 | 21.7 | 22.1 |
| Tumor type, n (%) | |||
| Post-ET MF | 4 (11.1) | 5 (13.9) | 10 (28.6) |
| Post-PV MF | 17 (47.2) | 17 (47.2) | 15 (42.9) |
| PMF | 15 (41.7) | 14 (38.9) | 10 (28.6) |
| IPSS risk level*at screening, n (%) | |||
| High risk | 15 (41.7) | 21 (60.0) | 27 (77.1) |
| Intermediate-2 risk | 21 (58.3) | 14 (40.0) | 8 (22.9) |
| . | Tertile 1 (−100% to ≤−24.66%) (n = 36) . | Tertile 2 (>−24.66% to ≤−8%) (n = 36) . | Tertile 3 (>−8%) (n = 35) . |
|---|---|---|---|
| Maximum percent change in allele burden | |||
| Mean | −62.6 | −15.3 | −0.5 |
| Median | −63.4 | −14.8 | −2.2 |
| Baseline allele burden | |||
| Mean | 70.9 | 73.5 | 75.3 |
| Median | 84.5 | 81.5 | 84.0 |
| Duration of disease prior to treatment, months | |||
| Mean | 36.8 | 52.5 | 44.8 |
| Median | 15.2 | 21.7 | 22.1 |
| Tumor type, n (%) | |||
| Post-ET MF | 4 (11.1) | 5 (13.9) | 10 (28.6) |
| Post-PV MF | 17 (47.2) | 17 (47.2) | 15 (42.9) |
| PMF | 15 (41.7) | 14 (38.9) | 10 (28.6) |
| IPSS risk level*at screening, n (%) | |||
| High risk | 15 (41.7) | 21 (60.0) | 27 (77.1) |
| Intermediate-2 risk | 21 (58.3) | 14 (40.0) | 8 (22.9) |
IPSS, international prognostic scoring system; PMF, primary myelofibrosis.
Cervantes et al.18
These analyses demonstrate that extended duration of ruxolitinib therapy can decrease JAK2p.V617F allele burden. Although mean changes were modest, a subset of patients achieved molecular remissions. Patients with both high and low initial allele burdens had allele burden responses, indicating that changes were not dependent on starting allele burden. Furthermore, JAK2p.V617F reductions correlated with spleen volume reductions, similar to findings from COMFORT-II.17 Analysis of baseline patient characteristics showed that patients with less severe disease and shorter times from diagnosis had a greater reduction in allele burden. This observation is consistent with the survival and clinical benefits observed with earlier ruxolitinib treatment; eg, in the 2-year follow-up of COMFORT-I, patients originally randomized to ruxolitinib had prolonged survival and greater percentage reductions in spleen volume from the time of randomization vs patients who crossed over from placebo to ruxolitinib (ie, patients with delayed treatment).9 Future research should assess if these findings extend to patients with PV.
Because this study was not designed to determine PMRs and CMRs, allele burden measurements were sparse, which may have led to an underestimate of confirmed PMRs and CMRs. Additionally, these analyses did not evaluate other MPN-associated mutations (MPL, CALR, etc) or concurrent genetic factors that may affect JAK2p.V617F allele responsiveness to ruxolitinib. Given the marked allele burden changes observed in some patients over extended treatment durations, further analyses are warranted to assess treatment-related changes in other JAK-STAT pathway mutants in JAK2p.V617F-negative MPNs and to determine the role for response-modifying background mutations that may impact clonal sensitivity to treatment.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by Incyte Corporation. Writing assistance was provided by Stephanie Leinbach and funded by Incyte Corporation. MD Anderson receives a cancer center support grant from the National Institutes of Health, National Cancer Institute (P30 CA016672).
Authorship
Contribution: M.D. and S.V. contributed to patient enrollment, data collection, and data interpretation; J.R. contributed to the interpretation of data; R.H. and T.C.B. performed the research and contributed to the data analyses; D.P. conducted the statistical analyses, and all authors drafted and/or critically reviewed the manuscript and approved the final version of the manuscript for submission.
Conflict-of-interest disclosure: M.D. receives research funding from Bristol Myers Squibb, Novartis, Celgene, and Gilead and is a consultant for and sits on Advisory Boards for Bristol Myers Squibb, Ariad, Novartis, Incyte Corporation, and Pfizer; J.R. serves as an Advisory Board member for Incyte Corporation; T.C.B., R.H., and D.P. are employees of Incyte Corporation; and S.V. receives research funding from Incyte Corporation.
Correspondence: Michael Deininger, Division of Hematology and Hematologic Malignancies, Huntsman Cancer Institute, 200 Circle of Hope, Salt Lake City, UT 84112; e-mail: michael.deininger@hci.utah.edu.