Key Points
Among adults with SCA, decreased FEV1 percent predicted was associated with earlier death.
Obstructive and restrictive pulmonary function patterns do not predict earlier death in adults with SCA.
Abstract
Pulmonary complications result in mortality in adults with sickle cell anemia (SCA). We tested the hypothesis that abnormal pulmonary function was associated with earlier death. A prospective cohort of adults with SCA, followed in the Cooperative Study for Sickle Cell Disease, was constructed using the first pulmonary function test at >21 years of age. Spirometry measures: forced expiratory volume in 1 second (FEV1), forced vital capacity, and total lung capacity were categorized based on age, gender, height, and race. Pulmonary function patterns were categorized based on the American Thoracic Society guidelines using both spirometry and lung volumes. A cohort of 430 adults with SCA, mean age 32.6 ± 9.5 (range, 21.0-67.8) years at time of first pulmonary function test, and a median follow-up of 5.5 years, was evaluated. A total of 63 deaths occurred. At baseline, 47% had normal, 29% restrictive, 8% obstructive, 2% mixed, and 14% nonspecific lung function patterns. In the final multivariable model, lower FEV1 percent predicted was associated with increased hazard ratio of death (HR per % predicted 1.02; 95% confidence interval [CI] 1.00-1.04; P = .037), as was older age (HR 1.07; 95% CI 1.04-1.10; P < .001), male sex (HR 2.09; 95% CI 1.20-3.65; P = .010), higher lactate dehydrogenase levels (HR per mg/dL 1.002; 95% CI 1.00-1.003; P = .015), and higher acute chest syndrome incidence rate (HR per event/year 10.4; 95% CI 3.11-34.8; P < .001). Presence of obstructive (HR 1.18; 95% CI: 0.44-3.20; P = .740) and restrictive (HR 1.31; 95% CI: 0.64-2.32; P = .557) pulmonary function patterns were not associated with earlier death. Understanding the pathophysiology of a low FEV1 percent predicted in individuals with SCA is warranted, enabling early intervention for those at risk.
Introduction
Sickle cell anemia (SCA) is a life-threatening, monogenic disorder associated with early mortality. In the largest prospective cohort study investigating the natural history of sickle cell disease (SCD) in children and adults, the Cooperative Study of Sickle Cell Disease (CSSCD), Platt et al1 noted an increased risk of earlier death in patients with SCA was associated with acute chest syndrome (ACS), renal failure, seizures, an elevated baseline white cell count, and a low level of fetal hemoglobin (Hb). Since that report, end-stage renal disease requiring dialysis,2 the presence of asthma3-5 and/or wheezing,6 smoking,5 N-terminal (NT)-pro brain natriuretic peptide levels,7 right heart catheter–confirmed pulmonary hypertension along with the physiological severity of the precapillary pulmonary hypertension,8,9 and severity of hemolysis10-12 have all been associated with earlier death among individuals with SCA.
In SCA, no single attribute of pulmonary function test (PFT) results has been associated with earlier death. Powars first described sickle cell chronic lung disease and its association with premature death in 1998.13 The stages of sickle cell chronic lung disease were based on a combination of clinical data (chest pain), laboratory evaluation (arterial blood gas), cardiac evaluation, and PFT, mainly spirometry and lung volumes. There were no outcomes using PFT alone. Unfortunately, the originally proposed sickle cell chronic lung disease classification has neither been validated nor broadly applied as an approach for determining the severity of lung disease in SCA, predictor for premature death, or both. No current PFT spirometric measure has been broadly used to stratify high-risk groups for disease severity in SCA.
For the first time, clinicians now have a universally accepted standard equation with normal reference values for African Americans, allowing for more accurate assessment of abnormal spirometry patterns (the Global Lung Initiative 2012 equations).14 Using this new reference and based on the strong association between forced expiratory volume in 1 second percent (FEV1%) predicted measurements and risk of earlier death in the general15-17 and cystic fibrosis populations,18,19 coupled with the observation that lung disease is associated with mortality in SCA,3-5,13 we tested the hypothesis that FEV1% predicted would be associated with earlier death in adults with SCA after adjustment for known predictors of death in SCA.9
Methods
Patient population
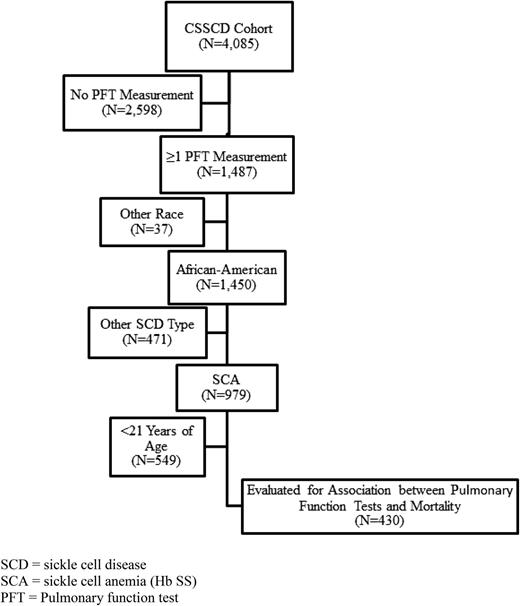
This prospective cohort study was constructed using data from the CSSCD. The design of the CSSCD has been reported elsewhere.20 Briefly, the CSSCD was a prospective cohort study designed to investigate the natural history of SCD. More than 4000 participants were recruited from 23 university-based institutions across the United States in a uniform, standardized fashion. Patients with HbSS, HbSC, and heterozygotes for hemoglobin S and thalassemia (β zero or β plus) were enrolled in the cohort between September 1978 and 1988 and were seen at regular intervals for laboratory evaluations and physical examinations. The dates of PFT evaluation ranged from March 6, 1979, to August 13, 1993. The range of participant’s ages at study entry for this analysis was 21.0 to 67.8 years. Excluded from the analysis were those not self-designated as African American (n = 37) or whose SCD genotype was not HbSS (n = 471) (Figure 1).
Mortality and follow-up time
Death was recorded in the clinical record. Cause of death was not noted for all deaths, and autopsy information was not routinely available. The number of years of patient follow-up was computed from the date of lung function testing until 1 of the following: bone marrow transplant, last CSSCD visit, or death.
Markers of abnormal pulmonary function
Data from the first pulmonary function record at age 21 years and older were used in this study, as predetermined by the investigators for a planned subgroup analysis. All PFTs were reviewed centrally for quality. Percent predicted values were determined for each subject based on their age, sex, height, and race for FEV1, forced vital capacity (FVC), and the ratio of FEV1/FVC using the Global Lung Initiative 2012 equations.14 Percent predicted results for FEV1, FEV1/FVC, and FVC, based on age, sex, race and height, were determined and then compared with the lower limit of normal (LLN), the 5th percentile of the distribution of the values.14 Percent predicted values for total lung capacity (TLC) were obtained using the prediction equations published by Stocks21 and Quanjer14 and adjusted by 12% to account for the effect of race on these values22 ; a value <80% predicted was considered abnormal. Of the 430 participants in our analyses, 90 (21%) were missing diffusing capacity of the lung for carbon monoxide (DLCO) values. Further, for the participants that had DLCO measurement, 185 of 340 (54%) were missing Hb levels on the day of the measurement; moreover, our primary hypothesis, namely that FEV1% predicted was associated with earlier death, was independent of the DLCO. Based on these facts, we elected not to include DLCO in our analysis.
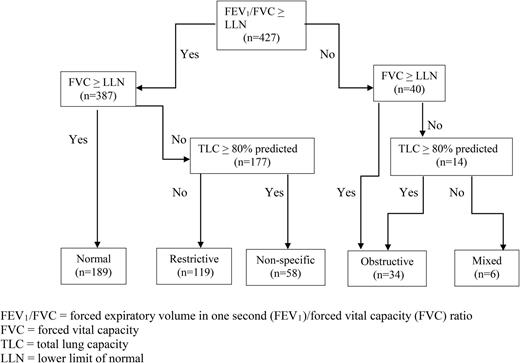
Values of FEV1% predicted, FEV1/FVC, FVC, and TLC were used to categorize pulmonary function patterns as normal, obstructive, restrictive, or mixed based on American Thoracic Society (ATS)/European Respiratory Society guidelines according to a modified algorithm based on Pellegrino et al.23 Specifically, pulmonary function patterns were based on the individual’s percent predicted value and compared with the expected LLN and were defined as follows: normal (FEV1/FVC ≥ LLN and FVC ≥ LLN), obstructive (FEV1/FVC < LLN with FVC ≥ LLN or TLC ≥ 80% predicted), and restrictive (FEV1/FVC ≥ LLN with FVC < LLN and TLC < 80% predicted). The nonspecific pattern, as defined by Hyatt et al24 and Iyer et al25 when FVC was <LLN with FEV1/FVC ≥ LLN and TLC ≥80% predicted, was present after normal, obstructive, restrictive, and mixed patterns defined by the Pellegrino scheme had been applied.23 Details of the classification scheme are given in Figure 2. In addition to the pulmonary function patterns based on LLNs, the FEV1% predicted was determined for each patient using the Global Lung Initiative 2012 equations.14 FEV1% predicted was evaluated as a continuous predictor in the model to use all available information, and it was reverse-coded so that lower values are associated with hazard ratios (HRs) >1. For clinical utility, FEV1% predicted was also categorized >70% predicted (see the supplemental Figure on the Blood Web site).
Classification scheme used to assess lung function in a cohort of adult African-American participants in the CSSCD. (Modified from Pellegrino et al,23 Hyatt el al,24 and Iyer et al.25 ) *The nonspecific pattern was defined based on Hyatt el al24 and Iyer et al25 : FVC% predicted <LLN with FEV1/FVC% predicted ≥LLN and TLC% predicted ≥80%.
Classification scheme used to assess lung function in a cohort of adult African-American participants in the CSSCD. (Modified from Pellegrino et al,23 Hyatt el al,24 and Iyer et al.25 ) *The nonspecific pattern was defined based on Hyatt el al24 and Iyer et al25 : FVC% predicted <LLN with FEV1/FVC% predicted ≥LLN and TLC% predicted ≥80%.
Other predictors of mortality
Data on other potential predictors of mortality were collected. Age was defined in years at the date of pulmonary function testing. Body mass index and laboratory parameters, including white blood cell count, Hb, platelet count, hematocrit, serum lactate dehydrogenase (LDH), and bilirubin levels, were collected at the baseline clinic visit. All covariates were collected as part of the original cohort study design. Definitions of pain and ACS events were previously described in this cohort,1 as was the definition of asthma.3 Data on smoking behavior were collected at multiple time points during the CSSCD. These data were consolidated, and the interview that occurred at the time of PFT or, when not available, as near as possible to the testing date was used to classify smoking status (median absolute time between interview and testing date was 0.46 years). Smoking behavior was classified as “yes” for cigarette, cigar, or pipe smoking. Asthma was not assessed as a predictor for death because a high proportion of participants (13.7%, n = 56) were missing an asthma designation in the record. We could also not determine how many of the participants had ACS episodes before the lung function measurements because the adult cohort was not followed from birth.
Data analysis was performed using SAS, version 9.3 (SAS Institute, Cary, NC) and SPSS, version 22.0 (IBM, Chicago, IL). Differences in demographic, clinical, and pulmonary function covariates between those who did or did not die during follow-up were assessed by χ2 test or t-test for categorical or continuous covariates, respectively, or the Mann-Whitney U test for covariates not normally distributed. The association between markers of pulmonary function and mortality was investigated using Kaplan-Meier product limit estimation and Cox proportional hazards regression. The full regression models were adjusted for factors known to be associated with mortality (age [at spirometry], sex, white blood cell count, fetal Hb level, history of renal insufficiency, history of seizures, acute chest syndrome rate, pain event rate, serum LDH level, and smoking status). A multivariable Cox regression model was constructed, entering all covariates at once. A second reduced model included only those covariates that were nominally significant predictors (P < .20) from the first model.
In the CSSCD cohort, elevated NT-pro brain natriuretic peptide levels were associated with earlier death in adults with SCA7 ; however, this analysis was done in a subgroup of adults who had biological samples available and was subject to sampling bias. Of the 430 participants who had a PFT performed, only 43% (188 of 430) had NT-pro brain natriuretic peptide levels measured. Further, among the individuals who had a PFT and died, only 23% (16 of 63) had NT-pro brain natriuretic peptide levels measured. Among the 16 participants who died with both assessments, none had a single episode of pain or ACS episodes, indicating a significant post hoc sampling bias. Hence, we did not include NT-pro brain natriuretic peptide levels in the multivariable analysis.
Only 6.0% of cases had missing data on a covariate in the Cox regression models, so no adjustment was done for missing data. An analysis of Martingale residuals from the Cox regression indicated that a linear model was appropriate for the continuous covariates. The proportional hazards assumption was assessed with either a log-minus-log plot for the categorical covariates or by plotting partial residuals against time. A review of deviance residuals indicated no outlier cases that had a substantial influence on the models.
Results
Participant characteristics
A total of 430 African-American participants with SCA completed at least 1 PFT and were followed for 2450 patient-years (median of 5.5 years). In the CSSCD study cohort, the baseline characteristics of adult African-American participants with SCA who completed pulmonary function testing (n = 430) compared with those who did not (n = 865) are shown in Table 1.
Characteristics of adult African-American participants in the CSSCD with SCA who completed PFT compared with those who did not
| . | Adult SCA participants with PFT (n = 430) . | Adult SCA participants without PFT (n = 865)* . | P value . |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age at study entry (y) | 27.4 ± 7.4† | 22.6 ± 9.8 | <.001 |
| Age at end of study (y) | 36.0 ± 7.4 | 30.7 ± 9.3 | <.001 |
| Males (%) | 41.2 | 45.2 | .168 |
| ACS rate (events/patient-y), total duration of study | 0.07 ± 0.14 | 0.07 ± 0.29 | .104‡ |
| Pain rate (events/patient-y), total duration of study | 0.98 ± 1.88 | 1.05 ± 2.07 | .424‡ |
| Asthma diagnosis (%) (n = 374) | 4.5 | 4.5 | 1.00 |
| History of chronic transfusion (%) | 12.6 | 11.7 | .645 |
| Laboratory characteristics | |||
| White blood cell count (109/L) (n = 426) | 11.6 ± 2.5 | 12.0 ± 2.8 | .027 |
| Fetal hemoglobin (g/dL) (n = 416) | 5.7 ± 4.1 | 6.1 ± 4.1 | .123 |
| Hemoglobin (g/dL) (n = 427) | 8.6 ± 1.2 | 8.5 ± 1.3 | .258 |
| Platelet count (109/L) (n = 422) | 419.9 ± 114.5 | 440.8 ± 126.6 | .004 |
| Hematocrit (%) (n = 428) | 25.3 ± 3.7 | 24.9 ± 4.0 | .100 |
| History of renal insufficiency (%) | 3.7 | 3.9 | .854 |
| Bilirubin (mg/dL) (n = 427) | 3.3 ± 2.0 | 3.6 ± 2.0 | .025 |
| Lactic dehydrogenase (mg/dL) (n = 417) | 477.4 ± 174.0 | 496.5 ± 202.1 | .101 |
| . | Adult SCA participants with PFT (n = 430) . | Adult SCA participants without PFT (n = 865)* . | P value . |
|---|---|---|---|
| Demographic and clinical characteristics | |||
| Age at study entry (y) | 27.4 ± 7.4† | 22.6 ± 9.8 | <.001 |
| Age at end of study (y) | 36.0 ± 7.4 | 30.7 ± 9.3 | <.001 |
| Males (%) | 41.2 | 45.2 | .168 |
| ACS rate (events/patient-y), total duration of study | 0.07 ± 0.14 | 0.07 ± 0.29 | .104‡ |
| Pain rate (events/patient-y), total duration of study | 0.98 ± 1.88 | 1.05 ± 2.07 | .424‡ |
| Asthma diagnosis (%) (n = 374) | 4.5 | 4.5 | 1.00 |
| History of chronic transfusion (%) | 12.6 | 11.7 | .645 |
| Laboratory characteristics | |||
| White blood cell count (109/L) (n = 426) | 11.6 ± 2.5 | 12.0 ± 2.8 | .027 |
| Fetal hemoglobin (g/dL) (n = 416) | 5.7 ± 4.1 | 6.1 ± 4.1 | .123 |
| Hemoglobin (g/dL) (n = 427) | 8.6 ± 1.2 | 8.5 ± 1.3 | .258 |
| Platelet count (109/L) (n = 422) | 419.9 ± 114.5 | 440.8 ± 126.6 | .004 |
| Hematocrit (%) (n = 428) | 25.3 ± 3.7 | 24.9 ± 4.0 | .100 |
| History of renal insufficiency (%) | 3.7 | 3.9 | .854 |
| Bilirubin (mg/dL) (n = 427) | 3.3 ± 2.0 | 3.6 ± 2.0 | .025 |
| Lactic dehydrogenase (mg/dL) (n = 417) | 477.4 ± 174.0 | 496.5 ± 202.1 | .101 |
Mean ± standard deviation.
The 865 subjects who did not have PFT performed were obtained from the initial 2598 without PFT measurement (Figure 1) after the exclusion criteria of race, other SCD type, and age <21 y were applied.
P value from Mann-Whitney U test.
In the cohort, we could not identify any selection bias for individuals with and without PFT. Most importantly, the mortality rate in those who had a PFT was no different when compared with those without a PFT evaluation, 14.7% vs 16.4% (P = .413). Further evidence of no selection bias was the absence of any differences in baseline Hb level, ACS or pain event rates, history of renal insufficiency (defined based on the CSSCD working definition of renal failure: a 20% increase in the baseline creatinine concentration and a creatinine clearance rate below 100 mL/minute),1 percent receiving regular blood transfusion therapy, HbF levels, or serum LDH levels between groups (Table 1). However, those with a PFT had a slightly lower but clinically irrelevant difference in white blood cell count, platelet count, and bilirubin levels and greater age at study entry and at the end of the study.
Of the 430 eligible participants, 63 (15%) died during follow-up. Those who died during the follow-up period differed from those who did not by being more likely to be male, more likely to have an asthma diagnosis, and having a significantly higher white blood cell count and serum LDH level, but a lower Hb, lower FEV1% predicted, and male sex. There was no difference in FEV1/FVC% predicted or TLC% predicted in those who died during follow-up when compared with those who did not die (Table 2). Among participants who died, age at death was not different by sex (P = .649), smoking status (P = .673), renal insufficiency (P = .458), or FEV1% predicted split at 70% (P = .630). At baseline, 47% of the participants had normal, 29% restrictive, 8% obstructive, 2% mixed, and 14% nonspecific pulmonary function patterns (Table 2; Figure 2).
Characteristics of adult African-American participants in the CSSCD with SCA who completed lung function testing
| . | Adult with SCA (n = 430)* . | Adult with SCA who lived until end of follow-up (n = 367) . | Adult with SCA who died during follow-up (n = 63) . | P (survival vs death)† . |
|---|---|---|---|---|
| Demographic and clinical characteristics | ||||
| Age at PFT (years) | 32.6 ± 9.5‡ | 32.4 ± 9.4 | 33.4 ± 10.4 | .439 |
| Males (%) | 41.2 | 37.9 | 60.3 | .001 |
| Body mass index (kg/m2) | 21.5 ± 3.7 | 21.6 ± 3.7 | 20.9 ± 3.8 | .174 |
| ACS event, post-PFT (%) | 15.1 | 14.2 | 20.6 | .186 |
| ACS rate (events/patient-y), post-PFT | 0.04 ± 0.15 | 0.03 ± 0.10 | 0.11 ± 0.30 | .097§ |
| Pain event, post-PFT (%) | 33.0 | 31.9 | 39.7 | .224 |
| Pain rate (events/patient-y), post-PFT | 0.53 ± 1.34 | 0.43 ± 1.08 | 1.12 ± 2.28 | .078§ |
| Asthma (%) (n = 374) | 4.5 | 3.3 | 13.6 | .009 |
| History of chronic transfusion (%) | 12.6 | 11.4 | 19.0 | .092 |
| Smoking at or near time of PFT (%)(n = 429) | 35.0 | 33.3 | 44.4 | .088 |
| Laboratory characteristics | ||||
| White blood cell count (109/L) (n = 426) | 11.6 ± 2.5 | 11.5 ± 2.4 | 12.4 ± 2.9 | .010 |
| Fetal hemoglobin (g/dL) (n = 416) | 5.7 ± 4.1 | 5.9 ± 4.1 | 4.5 ± 3.7 | .010 |
| Hemoglobin (g/dL) (n = 427) | 8.6 ± 1.2 | 8.7 ± 1.2 | 8.3 ± 1.4 | .013 |
| Platelet count (109/L) (n = 422) | 419.9 ± 114.5 | 419·0 ± 110.4 | 425.2 ± 137.2 | .695 |
| Hematocrit (%) (n = 428) | 25.3 ± 3.7 | 25.4 ± 3.6 | 24.3 ± 4.2 | .037 |
| History of renal insufficiency (%) | 3.7 | 2.7 | 9.5 | .019 |
| Bilirubin (mg/dL) (n = 427) | 3.3 ± 2.0 | 3.3 ± 2.0 | 3.4 ± 2.0 | .699 |
| Lactic dehydrogenase (mg/dL) (n = 417) | 477.4 ± 174.0 | 468.5 ± 169.0 | 529.5 ± 193.9 | .011 |
| Pulmonary function characteristics | ||||
| FEV1% predicted (n = 427) | 77.4 ± 14.1 | 78.3 ± 13.6 | 72.3 ± 16.1 | .002 |
| FEV1% predicted tertile | .017 | |||
| FEV1% predicted <71.1 (%) (n = 140) | 32.8 | 30.1 | 48.4 | |
| 71.1 ≤FEV1% predicted <84.1 (%) (n = 146) | 34.2 | 35.3 | 27.4 | |
| FEV1% predicted ≥84.1 (%) (n = 141) | 33.0 | 34.5 | 24.2 | |
| % predicted FEV1/FVC (n = 427) | 98.1 ± 8.7 | 98.1 ± 8.6 | 97.6 ± 9.2 | .618 |
| % predicted TLC (n = 384) | 83.7 ± 17.5 | 84.0 ± 17.2 | 81.8 ± 19.5 | .373 |
| Ventilatory defect (modified from Pellegrino) (n = 406) | .241 | |||
| Normal pattern (%) (n = 189) | 46.6 | 48.7 | 33.9 | |
| Obstructive pattern (%) (n = 34) | 8.4 | 8.4 | 8.5 | |
| Restrictive pattern (%) (n = 119) | 29.3 | 27.4 | 40.7 | |
| Mixed pattern (%) (n = 6) | 1.5 | 1.4 | 1.7 | |
| Nonspecific pattern (%) (n = 58) | 14.3 | 14.1 | 15.3 |
| . | Adult with SCA (n = 430)* . | Adult with SCA who lived until end of follow-up (n = 367) . | Adult with SCA who died during follow-up (n = 63) . | P (survival vs death)† . |
|---|---|---|---|---|
| Demographic and clinical characteristics | ||||
| Age at PFT (years) | 32.6 ± 9.5‡ | 32.4 ± 9.4 | 33.4 ± 10.4 | .439 |
| Males (%) | 41.2 | 37.9 | 60.3 | .001 |
| Body mass index (kg/m2) | 21.5 ± 3.7 | 21.6 ± 3.7 | 20.9 ± 3.8 | .174 |
| ACS event, post-PFT (%) | 15.1 | 14.2 | 20.6 | .186 |
| ACS rate (events/patient-y), post-PFT | 0.04 ± 0.15 | 0.03 ± 0.10 | 0.11 ± 0.30 | .097§ |
| Pain event, post-PFT (%) | 33.0 | 31.9 | 39.7 | .224 |
| Pain rate (events/patient-y), post-PFT | 0.53 ± 1.34 | 0.43 ± 1.08 | 1.12 ± 2.28 | .078§ |
| Asthma (%) (n = 374) | 4.5 | 3.3 | 13.6 | .009 |
| History of chronic transfusion (%) | 12.6 | 11.4 | 19.0 | .092 |
| Smoking at or near time of PFT (%)(n = 429) | 35.0 | 33.3 | 44.4 | .088 |
| Laboratory characteristics | ||||
| White blood cell count (109/L) (n = 426) | 11.6 ± 2.5 | 11.5 ± 2.4 | 12.4 ± 2.9 | .010 |
| Fetal hemoglobin (g/dL) (n = 416) | 5.7 ± 4.1 | 5.9 ± 4.1 | 4.5 ± 3.7 | .010 |
| Hemoglobin (g/dL) (n = 427) | 8.6 ± 1.2 | 8.7 ± 1.2 | 8.3 ± 1.4 | .013 |
| Platelet count (109/L) (n = 422) | 419.9 ± 114.5 | 419·0 ± 110.4 | 425.2 ± 137.2 | .695 |
| Hematocrit (%) (n = 428) | 25.3 ± 3.7 | 25.4 ± 3.6 | 24.3 ± 4.2 | .037 |
| History of renal insufficiency (%) | 3.7 | 2.7 | 9.5 | .019 |
| Bilirubin (mg/dL) (n = 427) | 3.3 ± 2.0 | 3.3 ± 2.0 | 3.4 ± 2.0 | .699 |
| Lactic dehydrogenase (mg/dL) (n = 417) | 477.4 ± 174.0 | 468.5 ± 169.0 | 529.5 ± 193.9 | .011 |
| Pulmonary function characteristics | ||||
| FEV1% predicted (n = 427) | 77.4 ± 14.1 | 78.3 ± 13.6 | 72.3 ± 16.1 | .002 |
| FEV1% predicted tertile | .017 | |||
| FEV1% predicted <71.1 (%) (n = 140) | 32.8 | 30.1 | 48.4 | |
| 71.1 ≤FEV1% predicted <84.1 (%) (n = 146) | 34.2 | 35.3 | 27.4 | |
| FEV1% predicted ≥84.1 (%) (n = 141) | 33.0 | 34.5 | 24.2 | |
| % predicted FEV1/FVC (n = 427) | 98.1 ± 8.7 | 98.1 ± 8.6 | 97.6 ± 9.2 | .618 |
| % predicted TLC (n = 384) | 83.7 ± 17.5 | 84.0 ± 17.2 | 81.8 ± 19.5 | .373 |
| Ventilatory defect (modified from Pellegrino) (n = 406) | .241 | |||
| Normal pattern (%) (n = 189) | 46.6 | 48.7 | 33.9 | |
| Obstructive pattern (%) (n = 34) | 8.4 | 8.4 | 8.5 | |
| Restrictive pattern (%) (n = 119) | 29.3 | 27.4 | 40.7 | |
| Mixed pattern (%) (n = 6) | 1.5 | 1.4 | 1.7 | |
| Nonspecific pattern (%) (n = 58) | 14.3 | 14.1 | 15.3 |
n = 430 unless other noted otherwise.
P value from independent-samples t-test or χ-square test unless otherwise noted.
Mean ± standard deviation.
P value from Mann-Whitney U test.
FEV1% predicted was associated with earlier death
Decreasing FEV1% predicted values were associated with increasing HRs of death. Clinical and laboratory covariates known to be associated with death in SCA participants, including age (at spirometry), sex, white blood cell count, fetal Hb level, history of renal insufficiency, history of seizures, ACS rate, pain event rate, serum LDH level, and smoking status, were included in a multivariate Cox regression model in addition to FEV1% predicted. Of the 11 variables in the model, 7 met the screening criterion of P < .20, including age, gender, white blood cell count, ACS rate after PFT, pain rate after PFT, FEV1% predicted, and serum LDH level. In the final multivariable model, every 1% decrease of FEV1% predicted was associated with a 2% increased hazard of death (HR per % predicted 1.02; 95% confidence interval [CI] 1.00-1.04; P = .037). Thus, for a 6% decrease in the FEV1% predicted, the difference between survivors and nonsurvivors (78.3 vs 72.3%), there is an expected 11% increase in hazard of death. Other variables in the final model associated with increased hazard of death include: older age (HR 1.07; 95% CI 1.04-1.10; P < .001), male sex (HR 2.09; 95% CI 1.20-3.65; P = .010), higher ACS incidence rate (HR per event/year 10.4; 95% CI 3.11-34.8; P < .001), and higher serum LDH level (HR per mg/dL 1.002; 95% CI 1.00-1.003; P = .015) (Table 3). See the supplemental Figure regarding Kaplan-Meier survival when FEV1% predicted categorized >70% predicted.
Final Cox regression model for death after lung function testing with reduced set of covariates (n = 404)
| Covariate . | B . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|
| Age at PFT | 0.07 | 1.07 (1.04-1.10) | <.001 |
| Male | 0.74 | 2.09 (1.20-3.65) | .010 |
| White blood cell count (109/L) | 0.08 | 1.09 (0.98-1.20) | .096 |
| ACS rate post-PFT (no./y) | 2.34 | 10.39 (3.11-34.78) | <.001 |
| Pain rate post-PFT (no./y) | 0.14 | 1.15 (0.98-1.36) | .095 |
| Lactic dehydrogenase (mg/dL) | 0.002 | 1.002 (1.00-1.003) | .015 |
| FEV1% predicted* | 0.021 | 1.02 (1.00-1.04) | .037 |
| Covariate . | B . | Hazard ratio (95% CI) . | P . |
|---|---|---|---|
| Age at PFT | 0.07 | 1.07 (1.04-1.10) | <.001 |
| Male | 0.74 | 2.09 (1.20-3.65) | .010 |
| White blood cell count (109/L) | 0.08 | 1.09 (0.98-1.20) | .096 |
| ACS rate post-PFT (no./y) | 2.34 | 10.39 (3.11-34.78) | <.001 |
| Pain rate post-PFT (no./y) | 0.14 | 1.15 (0.98-1.36) | .095 |
| Lactic dehydrogenase (mg/dL) | 0.002 | 1.002 (1.00-1.003) | .015 |
| FEV1% predicted* | 0.021 | 1.02 (1.00-1.04) | .037 |
FEV1% predicted is reverse-coded so that lower values are associated with hazard ratios >1.
No relationship between obstructive, restrictive, and nonspecific pulmonary function patterns and earlier death
Three separate multivariable Cox regression models were estimated—the first with obstructive lung pattern vs normal, the second with restrictive lung pattern versus normal, and the third with nonspecific lung pattern vs normal lung pattern—to determine their association with death. For the subsequent regression models, only the 4 covariates (age, male sex, ACS incidence rate, and serum LDH level) that were associated with death in the FEV1% predicted Cox regression were used, with FEV1% predicted excluded. Presence of obstructive (HR 1.18; 95% CI: 0.44-3.20; P = .740), restrictive (HR 1.31; 95% CI: 0.64-2.32; P = .557), or nonspecific lung pattern (HR 1.38; 95% CI: 0.62-3.06; P = .428) were not associated with earlier death.
Discussion
The strong association between lung disease and SCA-related mortality has been well-established3-5,13,26,27 ; however, what is not known is whether pulmonary function results are associated with earlier death. For the first time, we have demonstrated that FEV1% predicted can be used to predict earlier death in individuals with SCA. Prior risk factors associated with earlier death were also identified in our analysis, including the rate of ACS episodes5 and elevated serum LDH level.10-12 Though the FEV1% predicted was only slightly different between survivors and nonsurvivors (78.3 vs 72.3%), this difference was still clinically relevant, because this 6% difference is associated with an 11% increase in the hazard of death in our regression model. Perhaps a useful clinical threshold is a cutoff of FEV1% predicted of <70% that was associated with a statistically significant increase in the HR of earlier death.
The relation between hemolysis, cardiac disease, and lung disease in SCA is complex and poorly understood. Serum LDH level, a nonspecific measure of cell death, including hemolysis, was associated with earlier death, suggesting an independent association between FEV1% predicted values, LDH level, and mortality rate. The imprecision of LDH level, as a maker of hemolysis, makes any cause-and-effect relationship limited and requires further investigation.28
Understanding a possible relationship between lung disease and pulmonary hypertension,8,9 another prominent risk factor for premature death, will also require further study because tricuspid regurgitant jet velocity and right heart catheterization were not measured uniformly in this cohort. NT-pro brain natriuretic peptide levels, another predictor of mortality in SCA, was measured in only a small subsample of the group, as a post hoc analysis, thus could not be analyzed as a risk factor. Only a large prospective study in SCA that includes both pulmonary and cardiac evaluation can tease out the relative contribution of hemolysis markers, lung disease, and heart disease in SCA-related morbidity and mortality.
Data from the CSSCD cohort were also used by Klings et al to investigate the proportion of SCA participants with pulmonary function abnormalities.29 However, several important distinctions exist between the current analysis and that of Klings et al. Our definitions of abnormal spirometry were based on the largest and most rigorously established set of spirometry evaluations ever collected, the Global Lung Function 2012 equations,14 whereas Klings et al29 used standard, but limited, spirometry reference equations for the era. As would be expected, when different spirometry reference equations are used in the same populations, different percentages of pulmonary function patterns will be observed.30 The comparison between the uses of CSSCD data by Klings et al29 and the current studies, as well as comparative algorithms for classification of pulmonary function abnormalities, are presented in supplemental Tables 1 and 2, respectively.
In prospective studies, our group and others have demonstrated that a doctor’s diagnosis of asthma3,5 and the presence of recurrent wheezing6 are both associated with earlier death and airway obstruction. However, neither our group nor others have ever demonstrated that obstructive lung disease, based on ATS criteria (FEV1/FVC% predicted <LLN), was associated with earlier death. Unfortunately, the current lung phenotype in SCA lacks precision and has some overlapping features with asthma and recurrent wheezing. Future work in this area will require better understanding of the lung phenotype in SCA.
Several limitations exist in this prospective cohort study. A single assessment of pulmonary function is not an optimal strategy to ensure accurate classification of patterns of pulmonary function abnormalities, contributing to the misclassification of lung function pattern. Although the inclusion of DLCO may have been interesting, we were unable to include the assessment for several reasons. Twenty-one percent of the cohort with PFT were missing DLCO, and 54% were missing Hb levels on the day of the measurement. Further, the inclusion of DLCO was not required for our primary analysis. Conceivably, young adults in the CSSCD cohort with the most severe lung disease may have died shortly after enrollment or were excluded from PFT evaluation, resulting in selection of a healthier subgroup that completed PFT.
Perhaps the biggest perceived weakness in this analysis is that the cohort was constructed during an era when disease-modifying therapy, such as hydroxyurea, was used infrequently, and we cannot adjust for current risk factors of earlier death, such as elevated tricuspid regurgitant jet velocity.8 Despite this perceived limitation, the size of the cohort (>400 adults), coupled with the length of follow-up (>5 years), and a planned ancillary study provides compelling evidence for the primary hypothesis. Additionally, the adult SCD mortality rate in the current hydroxyurea therapy era is no different than in the immediate period before the start of hydroxyurea therapy.27 Last, our group and others have published data from this cohort initially demonstrating the association between a physician’s diagnosis of asthma, pain, ACS, and earlier death.3 These associations between asthma or recurrent wheezing and SCD morbidity and mortality were later validated in more contemporary prospective cohort studies.5,6 Unfortunately, because of the large amount of missing data, we were unable to include asthma diagnosis into the multivariable analysis. Nevertheless, we believe that the collective body of work from the CSSCD, along with these current results demonstrating the association between lower FEV1% predicted and death, indicate that findings from the CSSCD still have relevance for understanding risk factors for SCD disease severity.
Evidence to support our primary hypothesis that lower FEV1% predicted is associated with earlier death in SCD is also similar to the association between low FEV1% predicted and earlier death in the general15-17 and cystic fibrosis populations.18,19 The consistency of FEV1% predicted being associated with earlier death in other populations provides some biological plausibility for the association observed in adults with SCA. Although we do not understand the rate of decline of FEV1% predicted in the SCA population, our findings provide an opportunity to initiate a new line of investigation to better recognize lung disease in adults with SCA and its progression. As has been the case in individuals with cystic fibrosis, potential therapeutic intervention, such as macrolide therapy, can be evaluated to determine if FEV1% predicted values can be increased.31 Such strategies may enable intervention for those deemed at risk for early death in a population where cardiopulmonary disease is the main cause of death32 and only 1 disease-modifying therapy has been approved by the US Food and Drug Administration, hydroxyurea therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by the National Institutes of Health, National Heart, Lung, and Blood Institute (grant 5R01-HL079937-07) (R.C.S. and M.R.D.) and the Burroughs Wellcome Foundation (M.R.D.). The funding sources provided the financial resources to conduct the research. M.R.D. confirms that he had full access to all of the data in this study and had the final responsibility for the decision to submit for publication.
Authorship
Contribution: R.C.S. and M.R.D. conceived and designed the study, wrote versions of the manuscript, and reviewed the statistical analysis; M.R. performed the statistical analysis and wrote that portion of the manuscript; E.A.M. constructed the data set, reviewed and analyzed the data, and edited the manuscript; A.A.K. and A.B.P. wrote the manuscript and reviewed the analysis; and all authors reviewed the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Michael R. DeBaun, Department of Pediatrics, Vanderbilt – Meharry Center of Excellence in Sickle Cell Disease, Vanderbilt University School of Medicine, 2200 Children’s Way, 11206DOT, Nashville, TN 37232-9000; e-mail: m.debaun@vanderbilt.edu.
References
Author notes
A.A.K. and A.B.P. contributed equally to first authorship.
R.C.S. and M.R.D. contributed equally as senior authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal