Abstract
The treatment of patients with myeloma has dramatically changed over the past decade due in part to the development of new agents and myeloma-specific targets. Despite these advancements, a group for whom the long-term benefit remains less clear are patients with genetically or clinically defined high-risk myeloma. In order to successfully treat these patients, it is important to first identify these patients, treat them with aggressive combination therapy, and employ the use of aggressive long-term maintenance therapy. Future directions include the use of new immune-based treatments (antibodies or cellular-based therapies) as well as target-driven approaches. Until these treatment approaches are better defined, this review will provide a potential treatment approach for standard- and high-risk myeloma that can be followed using agents and strategies that are currently available with the goal of improving progression-free and overall survival for these patients today.
Case report
A 70-year-old woman presented to her internist with progressive back pain. Upon initial evaluation, anemia with a hemoglobin of 9.6 g/dL was noted, and the rest of her complete blood count values were normal. She was referred to a hematologist, who began the workup, noted proteinuria, and began to perform a myeloma workup. Results of laboratory studies demonstrated 8.5 g of κ light chain in the urine with a serum free light chain ratio 40:1. Serum protein electrophoresis showed the presence of an immunoglobulin A κ protein of 2.5 g/dL. The serum albumin was 3.5 g/dL, total protein was 7.9 g/dL, and the β2-microglobulin was 12.9 mg/L. She underwent a bone marrow aspirate and biopsy and was noted to have 30% clonal plasma cells with normal conventional cytogenetics, but fluorescent in situ hybridization (FISH) testing showed that she did have del(17p) (80% of cells) and del(13q). She had a skeletal survey that demonstrated diffuse lytic disease, with several lumbar compression fractures. There was no evidence of cord compression by imaging.
International Staging System (ISS) stage III myeloma was diagnosed and categorized as high-risk myeloma by virtue of the FISH test results and high ISS stage. She received 4 cycles of RVD (lenalidomide, bortezomib, and dexamethasone) induction with prompt achievement of a stringent complete response (CR). This was followed by growth factor mobilization of stem cells, high-dose therapy (HDT), and autologous transplant after conditioning with 200 mg/m2 melphalan. She had an uneventful posttransplant course and was started on RVD maintenance at day +60 posttransplant, which she continued for 3 years.1 She remains in ongoing complete remission and is currently on lenalidomide as single-agent maintenance therapy more than 4 years after her initial presentation. This case demonstrates the importance of early identification of high-risk myeloma patients so that they can be treated with aggressive therapy including planned 3-drug maintenance and, unlike the experience from the Total Therapy regimens, minimization of alkylating agent exposure as is now more commonly used in regimens such as VTD (bortezomib, thalidomide, and dexamethasone), RVD, CTD (cyclophosphamide, thalidomide, and dexamethasone), and CRD (carfilzomib, lenalidomide, and dexamethasone) in the large cooperative group trials of Europe and the United States. Although this represents a novel treatment approach, aggressive planned maintenance for high-risk patients represents an emerging standard of care if we are to improve on outcomes for these patients.
Introduction
New treatment options for myeloma patients in all phases of their disease have helped to enhance the median overall survival (OS) from 3 years in the 1990s to well over 7 to 10 years at the current time. Despite these advances, there remains a subset of patients who do not yet appear to have reaped the benefits of improved treatments, namely those with high-risk myeloma. Although these patients represent only 15% to 20%2 of patients at the time of diagnosis, they represent a significant fraction of patients who experience early death and rapid relapse following or even during initial induction therapy. Critical to success with these patients is early identification and aggressive therapy including the use of HDT and autologous transplant, followed by planned intensive maintenance. This review will distill available clinical and laboratory data to help provide a clear roadmap for successful diagnosis and management, with an eye toward strategies directed at improving OS for these challenging patients.
Defining high-risk myeloma
Before considering treatment options for a high-risk patient, one must first identify which patients are considered to be high risk. Risk in myeloma can be attributed to 4 major factors: disease stage, chromosomal abnormalities, disease biology, and gene expression. As outlined below, a combination of these factors is required to determine if a patient has high-risk myeloma, but for the best long-term outcomes, this must be determined at the time of disease presentation (Table 1).
Summary of determinants of high-risk myeloma
| Risk factor . | Measurement . | High risk . |
|---|---|---|
| Disease burden | β2M, serum albumin | ISS III (β2M ≥5.5 mg/mL) |
| Tumor biology | Extramedullary disease | PCL plasmacytoma |
| Proliferation | Plasma cell labeling index ≥3% | |
| Genetics | Cytogenetics | del(17p), 1q21 amplification |
| FISH | t(4;14), t(14;16), del(17p), 1q21 amplification, 1p deletions | |
| ISS/genetics | β2M, serum albumin cytogenetics, FISH | ISS II/III, at least one genetic abnormality |
| Gene expression | Expression microarray | UAMS 70-gene signature, IFM 15-gene signature, proliferation index, EMC-92-gene signature |
| Risk factor . | Measurement . | High risk . |
|---|---|---|
| Disease burden | β2M, serum albumin | ISS III (β2M ≥5.5 mg/mL) |
| Tumor biology | Extramedullary disease | PCL plasmacytoma |
| Proliferation | Plasma cell labeling index ≥3% | |
| Genetics | Cytogenetics | del(17p), 1q21 amplification |
| FISH | t(4;14), t(14;16), del(17p), 1q21 amplification, 1p deletions | |
| ISS/genetics | β2M, serum albumin cytogenetics, FISH | ISS II/III, at least one genetic abnormality |
| Gene expression | Expression microarray | UAMS 70-gene signature, IFM 15-gene signature, proliferation index, EMC-92-gene signature |
β2M, β2-microglobulin; PCL, plasma cell leukemia.
Disease staging is currently defined using the ISS,3 which uses 2 clinical measurements to assess stage. The ISS classification is based on the serum levels of albumin and β2-microglobulin, with stage III patients (serum β2-microglobulin ≥5.5 mg/mL) at the greatest risk. The median survival of stage III patients was reported as 29 months compared with patients classified with stage I myeloma (serum albumin ≥3.5 mg/mL, serum β2-microglobulin <3.5 mg/mL), who had a median survival of 62 months. Stage II disease is defined as patients who are not stage I or III (either serum albumin <3.5 mg/mL but β2-microglobulin <3.5 mg/mL or β2-microglobulin 3.5 to <5.5 mg/mL) and had a median survival of 44 months. Different from the Durie-Salmon4 staging, which preceded adoption of the ISS, disease burden is not all that is measured by the ISS. The ISS also provides the clinician with a sense of biological impact of the tumor, as lower albumin represents a systemic effect of the disease, and this, coupled with the clear prognostic impact of serum β2-microglobulin, allows the clinician to get a sense of long-term outcomes. The patients who were studied to define ISS criteria were not treated with current approaches using combinations of “novel” agents; therefore, the median survival for each group may no longer be relevant. However, the staging system remains a useful means of defining different patient populations within a heterogeneous group of patients at the time of initial diagnosis.
Chromosomal abnormalities were one of the first genetically based measures of disease heterogeneity in myeloma and also demonstrated prognostic value. Both conventional cytogenetics and FISH-based methods provide information about risk and patient categorization. Cytogenetic abnormalities associated with poor prognosis include 17p deletions,5 1q21 gains6 (although not all studies have confirmed this as high risk7-9 ), and potentially any cytogenetic finding. FISH is required for detection of additional translocations associated with high-risk myeloma: t(4;14), t(14;16), and t(14;20).10-13 Additionally 1p deletions have been established as a potential high-risk marker.14-16 Interestingly, simply the presence of an abnormality by FISH does not relegate all patients to a poor-risk category. The Institut Francophone du Myélome (IFM) has reported that the impact of t(4;14) can be dependent on other clinical factors (hemoglobin or β2-microglobulin) in addition to simply the presence or absence of the FISH-based marker.17 Similarly, the percentage of cells with del(17p) has also been linked with the effect of del(17p) on prognosis.7 The cutoff that is of significance is a source of some debate and has not yet been universally accepted, even by the most recent International Myeloma Working Group consensus paper on risk stratification.11
Although genetics and FISH information have prognostic value, an IFM analysis of the IFM99 trial demonstrated that in a multivariate analysis, cytogenetic features (del(17p) and t(4;14)), and serum β2-microglobulin were statistically independent predictors of both event-free survival and OS, suggesting that combining the most important part of the ISS with genetic markers could provide a more accurate assessment of disease risk.7 This was determined to be the case in 3 independent studies by the International Myeloma Working Group, Medical Research Council, and an analysis of patients seen at Heidelberg.6,18,19 All 3 groups defined high risk as ISS II/III with at least 1 chromosomal abnormality. In 2 groups, the chromosomal abnormality was either del(17p) or t(4;14),18,19 whereas in the third group, the event could be either of these or +1p21, t(14;16), or t(14;20).6
In addition to cytogenetic abnormalities, alterations in disease biology can have prognostic power. Plasma cell proliferation, as measured by the plasma cell labeling index, is an indicator of high-risk disease20 ; however, the assays required to measure this are not routinely used11 and may not have prognostic value in the context of newer agents.21 A second biological indicator of high-risk disease is the presence of extramedullary plasma cells.22 Both circulating plasma cells and extramedullary plasmacytomas are high-risk factors.23 In the case of plasmacytomas, patients with soft tissue disease do worse than those with bone-related extramedullary disease.24
Finally, gene expression profiling has provided a greater understanding of the heterogeneity of myeloma and has also been proposed as a prognostic indicator for patients. Several gene signatures have been developed from microarray studies to define risk, including the University of Arkansas for Medical Sciences (UAMS) 70-gene signature,25 the IFM 15-gene signature,26 a proliferation signature,27 and the EMC-92-gene signature.28 Although there are advantages to using expression signatures (standard methodologies in data acquisition and analysis), there are several reasons that these approaches have not become routine practice. Unfortunately, it remains unclear why these signatures do not completely overlap; therefore, it is not clear if one or more signatures should be used. Additionally, recent analysis of the UAMS 70-gene signature suggests that almost all the prognostic power lies within the expression of 5 genes, suggesting most of the genes in the 70-gene signature may not be needed.29 Moreover, all of these signatures were defined using microarray analysis and will need to be validated as expression analysis by RNA sequencing becomes the standard. Based upon these factors, gene expression profiling is not considered a standard method for risk assessment.
Defining patients as high risk in our practice is exemplified in our recent publication of maintenance and consolidation following early transplant in high-risk patients. High risk was defined at diagnosis by the presence of high-risk genetic abnormalities (del(17p), del(1p), t(4;14), and t(14;16)) or the presence of circulating plasma cells (≥20%). ISS staging was not used; however, 69% of the patients were II/III (16% were unknown).1
Activity of current agents in high-risk myeloma
The utility of conventional chemotherapy in various combinations is not sufficient to overcome or even mitigate the impact of high-risk myeloma, and there are theoretical concerns that in a genomically unstable plasma cell disorder such as high-risk myeloma, the use of genotoxic therapy may contribute to more rapid progression due to its effects on DNA integrity. Furthermore, the initial identification of many of the above-listed high-risk features arose from large trials where the predominate therapy was alkylator based. What is known is that depth of response following induction and consolidation is of critical importance for high-risk patients. Although the complete response (CR) rate may not be different between standard- and high-risk patients treated with novel agents, for long-term progression-free survival (PFS) and OS in high-risk patients, it is essential achieve and maintain a CR.30
Thalidomide
The use of thalidomide has changed the approach to myeloma management in a number of different settings beginning back in the late 1990s and early part of this century, but in most areas, its use has been supplanted by the newer-generation immunomodulatory drugs (IMiDs) lenalidomide and pomalidomide.31 Data evaluating the use of thalidomide-based approaches for the management of high-risk myeloma have generally not demonstrated the ability to mitigate or overcome high-risk disease when defined using FISH-based risk stratification. From the UAMS data set, there were data among patients with karyotype-based high-risk disease suggesting that the addition of thalidomide to the Total Therapy 2 regimen did have an impact on PFS and OS,32 but this was predominately an alkylator-based approach, which is known to have minimal impact on high-risk disease. In a recently published trial from the Medical Research Council, the use of thalidomide as maintenance therapy for high-risk patients actually resulted in a poorer OS.33 Although patients were not stratified into each group based on FISH results, there was sufficient power among the groups to identify a very poor outcome within the high-risk cohort treated with thalidomide as maintenance. As such, most approaches to improve outcomes for high-risk myeloma patients no longer incorporate the routine use of thalidomide-based therapy.
Bortezomib
Data on the use of bortezomib among high-risk cohorts of patients originated in the SUMMIT trial, where Jagannath published a subset analysis that suggested the activity of bortezomib was consistent regardless of the presence or absence of clinical and genetic high-risk features.34 Among newly diagnosed myeloma patients, Harrouseau35 and Avet-Loiseau both evaluated the impact of bortezomib on a high-risk group of patients in the IFM 2005 clinical trial that compared bortezomib/dexamethasone (VD) with the conventional VAD regimen (vincristine, doxorubicin, and dexamethasone RVD: bortezomib, lenalidomide, and dexamethasone) as induction therapy prior to HDT and autologous transplant. Among the high-risk cohorts of patients, Harousseau initially reported no difference in response rates between standard- and high-risk patients when bortezomib was used as part of the induction regimen. However, when separate outcomes for t(4;14) or del(17p) were evaluated, the t(4;14) group appeared to have improved outcomes with bortezomib/dexamethasone as induction, whereas those with del(17p) did not.36 In long-term outcomes, the use of bortezomib did not eliminate the high-risk effects. Bortezomib alone as part of induction simply reduced the impact of high-risk genetics compared with VAD.
A subsequent phase 3 trial from the GIMEMA group evaluated the impact of VTD vs TD (thalidomide and dexamethasone) among newly diagnosed myeloma patients and again evaluated the impact of bortezomib-based induction on outcomes for a high-risk cohort of patients. Cavo and colleagues demonstrated that among those who received VTD induction and bortezomib as part of consolidation, the negative impact of t(4;14) on outcomes was removed.37 Data from this trial are not sufficient to evaluate the impact on other high-risk subsets, but this finding was consistent with data presented by Barlogie and colleagues, who suggest that the use of Total Therapy 3 with bortezomib also appears to eliminate the negative impact of t(4;14) on PFS among patients in the phase 2 randomized clinical trial.38 Finally, the HOVON group also evaluated bortezomib as part of induction and again in maintenance in their large randomized phase 3 clinical trial.39 This trial compared VAD induction, followed by HDT and then thalidomide maintenance vs PAD induction, HDT, and bortezomib maintenance. In this trial, the use of bortezomib as maintenance therapy was able to reduce the negative impact of high-risk genetics on outcomes but did not completely eliminate the impact of high-risk genetics.
These trials, in aggregate, support the notion that the use of bortezomib is able to reduce the negative impact of high-risk genetics on PFS and OS (Table 2) when used both in induction and in the maintenance or consolidation phase, but only in the context of t(4;14) is the use of a single agent able to completely eliminate the negative impact of a high-risk feature.
Data in high-risk cohorts for patients undergoing autologous transplant
| Trial . | Time point . | EFS all patients . | EFS/PFS t(4;14) . | PFS del(17p) . | OS % all patients . | OS % t(4;14) . | OS % del(17p) . |
|---|---|---|---|---|---|---|---|
| VD vs VAD35,36 | 4 y | 36 mo | 28 mo | 14 mo | 77 | 63 | 49 |
| NR | 16 mo | NR | 82 | 32 | 50 | ||
| VTD vs TD37 | 3 y | 74% | 69% | NR | 86 | NR | NR |
| 63% | 37% | NR | 84 | NR | NR | ||
| PAD vs VAD39 | 3 y | 28 mo | 25 mo | 26 mo | 85 | 66 | 69 |
| 35 mo | 21 mo | 12 mo | 80 | 44 | 17 | ||
| RVD→RVD*1 | 3 y | 32 mo | NR | 28 mo | 93% | NR | 94% |
| Trial . | Time point . | EFS all patients . | EFS/PFS t(4;14) . | PFS del(17p) . | OS % all patients . | OS % t(4;14) . | OS % del(17p) . |
|---|---|---|---|---|---|---|---|
| VD vs VAD35,36 | 4 y | 36 mo | 28 mo | 14 mo | 77 | 63 | 49 |
| NR | 16 mo | NR | 82 | 32 | 50 | ||
| VTD vs TD37 | 3 y | 74% | 69% | NR | 86 | NR | NR |
| 63% | 37% | NR | 84 | NR | NR | ||
| PAD vs VAD39 | 3 y | 28 mo | 25 mo | 26 mo | 85 | 66 | 69 |
| 35 mo | 21 mo | 12 mo | 80 | 44 | 17 | ||
| RVD→RVD*1 | 3 y | 32 mo | NR | 28 mo | 93% | NR | 94% |
EFS, event-free survival; NR, not reported; PAD, bortezomib, doxorubicin, and dexamethasone.
Only high-risk patients were enrolled.
Lenalidomide
Data on the use of lenalidomide among high-risk patients are primarily limited to the relapsed setting. In a series of patients from Reece et al using lenalidomide and dexamethasone (len/dex) in relapsed myeloma,40 there is a suggestion that len/dex is able to overcome the impact of t(4;14). In contrast, another series from Avet-Loiseau et al,41 the positive impact of len/dex on PFS for t(4;14) does not appear to be present. In both data sets, there does not appear to be any benefit for patient with del(17p) for the use of len/dex. In the 2 large randomized trials comparing lenalidomide vs placebo for maintenance therapy, the US trial42 did not have sufficient information on genetics to evaluate this question, whereas the IFM trial43 did not comment on PFS and OS for the high-risk cohort in either arm. It was of interest that there were nearly twice as many high-risk patients in the lenalidomide arm of the Avet-Loiseau report, suggesting a relative imbalance in randomization, and this may in part account for a lack of survival benefit for lenalidomide in this trial.
Carfilzomib
Data on the use of carfilzomib for high-risk myeloma are based upon a very small series of patients in highly selected phase 2 trials evaluating the use of CRD among newly diagnosed myeloma patients. In these trials, there appears to be no impact of high-risk genetics on outcomes.44,45 There was a retrospective analysis of outcomes for high-risk patients among carfilzomib-treated patients in the relapsed setting, and although the overall response rate and PFS appear to be similar among the high-risk and standard-risk patients who received carfilzomib,46 the OS was shorter for high-risk patients (9 vs 23 months), suggesting improvement, but not elimination, of the negative impact of high-risk genetics as a single agent (Table 3).
New agents in high-risk myeloma
| Agent . | ORR . | PFS . | OS . |
|---|---|---|---|
| Carfilzomib46 | HR, 25.8%; SR, 24.6% | HR, 3.5 mo; SR, 4.6 mo | HR, 9.3 mo; SR, 19 mo |
| Pomalidomide49 | del(17p), 32%; t(4;14), 22% | HR, 7.3 mo; 2.8m | HR, 12 mo; SR, 9 mo |
| Car/pom/dex51 | HR, 78%; SR, 74% | HR, 9.7 mo; SR, NR | HR, 16 mo; SR, 18 mo |
| Agent . | ORR . | PFS . | OS . |
|---|---|---|---|
| Carfilzomib46 | HR, 25.8%; SR, 24.6% | HR, 3.5 mo; SR, 4.6 mo | HR, 9.3 mo; SR, 19 mo |
| Pomalidomide49 | del(17p), 32%; t(4;14), 22% | HR, 7.3 mo; 2.8m | HR, 12 mo; SR, 9 mo |
| Car/pom/dex51 | HR, 78%; SR, 74% | HR, 9.7 mo; SR, NR | HR, 16 mo; SR, 18 mo |
Car/pom/dex, carfilzomib, pomalidomide, and dexamethasone; NR, not reported; ORR, overall response rate.
Pomalidomide
The use of pomalidomide in the relapsed setting has the potential ability to overcome lenalidomide resistance in a cohort of patients. Early data from European- and US-based groups suggested early activity for pomalidomide among high-risk patients, as did an analysis from the MM-003 investigators.47,48 A subsequent prospective trial from Leleu et al and the IFM evaluated the impact of pomalidomide and dexamethasone (pom/dex) on overall response rate and PFS among a group of del(17p) and t(4;14) patients only.49 This analysis suggested that del(17p) patients did appear to gain benefit from the use of pom/dex, whereas patients who harbored the t(4;14) translocation did not gain benefit from the use of pom/dex (Table 3).
Proteasome inhibitor/IMiD combination
Clinical data on the combination of bortezomib with lenalidomide or thalidomide developed based on preclinical observations using in vitro studies that supported synergy when these 2 different targets were used together. In an early trial from Richardson and colleagues, the RVD combination demonstrated striking overall response and duration of response, even when patients were resistant to either agent or to both agents given separately.50 In addition, RVD was studied as induction therapy for newly diagnosed patients. In this study, the overall response rate was 100%. Based upon these observations, Barlogie and colleagues used RVD as posttransplant maintenance in their Total Therapy 3 clinical trial in order to try to address the issue of high-risk disease posttransplant. The data from their experience suggested that the use of RVD maintenance posttransplant did not improve outcomes compared with historical cohorts treated at their institution.25 However, our group tested a similar combination of RVD as posttransplant maintenance only for patients with high-risk myeloma.1 In our series, we particularly sought to avoid the use of excessive exposure of high-risk patients to alkylator-based therapy with the theory that in a genomically unstable disease such as high-risk myeloma, the addition of alkylators when there is high tumor burden present will likely enhance clonal evolution and hasten the development of more resistant phenotypes. In our experience, the use of RVD-based consolidation and maintenance following HDT specifically for high-risk patients significantly improved PFS and OS for high-risk patients, more so than was observed with either lenalidomide or bortezomib when used alone.
In another recent trial in the relapsed setting, Shah and colleagues reported on the combination of carfilzomib and pom/dex for relapsed/refractory myeloma.51 Their encouraging overall response rate of >70%, with a significant fraction of patients who harbored high-risk genetics, suggests that this approach can also yield positive results (Table 3).The data for newer agents in high-risk myeloma are currently limited to patients in the relapsed setting. Large induction studies and the benefit for the subset of high risk is currently not available from large randomized trials. As such, one can only extrapolate from relapsed trials, as was initially done with bortezomib and lenalidomide when they were initially evaluated. Nonetheless, the activity seen with the newer versions of proteasome inhibitors and immunomodulatory agents suggests that the second- and third-generation agents may add significant benefit for this patient population when given together.
Role of tandem autologous transplant
Although many are questioning the role of HDT in the context of high-risk myeloma, there are groups suggesting that tandem HDT and autologous transplant may offer an improved benefit specifically for high-risk patients. In a pooled analysis presented by Cavo and colleagues, patients who received bortezomib-based induction and were randomized to either 1 or 2 transplants were evaluated from several different large European cooperative group studies.52 Among all patients, there was a benefit in terms of PFS and OS favoring the group randomized to tandem autologous transplant. Interestingly, when multivariate analysis was performed for PFS, the strongest influencers of posttransplant PFS were among patients who failed to achieve a CR following induction, presence of del(17p) and/or t(4;14), and ISS stage II disease. When PFS was evaluated among patients who failed to achieve a CR following induction with bortezomib-based inductions, the tandem autologous transplant group achieved a median PFS of 42 months, whereas those who received a single transplant achieved a PFS of 21 months (P = .006). When OS was evaluated, OS at 4 years was 76% for the tandem group and only 33% for those who received a single transplant. Although these are not randomized data and there were many different maintenance approaches used here, the benefit for tandem autologous transplant is very intriguing, and the fact the greatest benefit appears to be among those who failed to achieve a CR following bortezomib-based induction speaks to the importance of depth of response initially with high-risk patients.
Role of allogeneic transplantation
Many small phase 2 studies have evaluated the role of allogeneic transplantation for the management of either newly diagnosed or relapsed myeloma.53,54 In order to fully appreciate the potential risks and benefits of allogeneic transplantation, it is important to fully appreciate the genetic risk of the patient at the time this treatment option is considered. For standard-risk myeloma patients, the long-term survival is in excess of 7 years with modern myeloma therapy and autologous transplant. As such, these patients have very good outcomes that will in all likelihood only improve with the future drug development. For high-risk myeloma patients, although the median OS may be shorter, it is not clear that these patients have a disease biology compatible with being able to wait for the presumed graft vs tumor effect, and thus, the benefit may also be less clear. In randomized trials evaluating tandem autologous transplant vs autologous transplant followed by reduced-intensity conditioning allogeneic transplant, there was no benefit among the high-risk cohort of patients, supporting the notion that rapid disease relapse remains a major issue limiting the benefit of allogeneic transplant.55,56 Other studies done specifically among high-risk myeloma patients have also failed to demonstrate benefit.57 Thus, although allogeneic cell therapy is an interesting theoretical concept, given the rapid pace of drug development and identification of druggable targets in myeloma, this is not an approach that should be considered a standard treatment of high-risk patients58,59 outside the confines of a well-designed clinical trial focused on clinical and not laboratory end points. Several such studies are currently underway, and in selected patients, it represents a reasonable treatment option in the absence of access to new agents.
New treatment directions in high-risk myeloma
Although the use of proteasome inhibitors and IMiDs with steroids represents a major part of the treatment plan for high-risk myeloma, the development of new targets specific to the proliferation and genomic instability aspect of high-risk myeloma is a major research goal. For patients with 17p deletion, specific drugs targeting MDM2 are being explored in relapsed myeloma with the goal of bringing them forward in the treatment approach if they are effective.60,61 For patients whose disease harbors the t(4;14), efforts to target FGFR3 through small-molecule inhibitors have not made a clinical impact, but newer approaches are focusing on the reciprocal translocation product, MMSET.62,63 Early drug discovery in the area of MMSET inhibitors is currently in progress and will be tested in the near future. Targeting proliferation with agents such as KSP inhibitors represents yet another potential target for patients with high-risk myeloma, and validation of the effects of KSP inhibitors on relapsed myeloma is ongoing.64 Finally, the use of monoclonal antibodies is another way by which to attack myeloma, using a “risk-agnostic” approach that does not depend upon mutated signaling pathways intracellularly but rather focuses on an cell-surface receptor that is then used to target the cell via innate immune mechanisms.65 These targets can include things unique to plasma cells, such as SLAMF7 (CS1) or CD38, but may also include PD-1 or PDL-1. Each of these new directions represents future approaches to treatment of patients with all types of myeloma but may have specific interest among the high-risk cohorts.
General principles of managing high-risk myeloma
Through the course of clinical management of high-risk myeloma, there are a few guiding principles that are useful for clinical management of these patients. First, quick disease response using combination therapy is critical for sustained control. Given what is known about genomic instability for plasma cell disorders such as myeloma,66,67 it should come as no surprise to anyone that if a rapidly changing tumor is treated with minimal therapy, it will find a way to develop drug resistance. As such, aggressive therapy that, if possible, avoids the use of alkylating agents will prevent the emergence of drug resistance and will hopefully suppress clonal evolution. A prime example of this principle has recently been published in Blood, where a patient with t(4;14) myeloma was exposed to a series of low-intensity treatments and finally to low-dose melphalan over the course of several months. The result was rapid development of drug resistance and ultimately plasma cell leukemia.67,68 There may be situations where alkylator-based therapy is required for quick disease control, such as patients who present with plasma cell leukemia. When treated with RVD or VTD-based therapy, these patients often develop drug resistance within the first 4 cycles, and thus, rapid debulking of the tumor mass is more important than concerns about exposure to DNA-damaging agents and clonal evolution. Additionally, plasma cell leukemia patients are prone to the development of extramedullary disease at sites of catheter placement or surgery. It has been suggested that the risk of developing these “opportunistic plasmacytomas” is linked with longer persistence of plasma cells in the blood. As such, speed of response is very important among these patients. In these patients, alkylators can be minimized in the maintenance setting through the use of RVD.
Second, stay on schedule. Treating patients with high-risk myeloma is much like treating leukemia or other aggressive hematologic malignancies. Any opportunity the tumor is given to grow and develop drug resistance represents a potential source for tumor escape. In the posttransplant period, our group minimizes the period with no therapy to 60 days rather than the conventional 100 days for the initiation of maintenance therapy, as is done in the total therapy program from Arkansas. They have demonstrated that a small number of patients relapse after the prolonged break in therapy after HDT. In so doing, we find that our patients are less likely to break through in the posttransplant period, and we are able to truly “maintain” them rather than start over with salvage therapy. The goal here is to achieve a CR and then sustain it over time. Although this may seem elementary on the surface, in no other patient population in myeloma is the achievement and then maintenance of CR more important.30 Among patients not benefiting from RVD maintenance, it was limited to those who did not achieve a CR1 and thus should now be targeted for change in therapy in order to try and improve their outcomes. This early initiation of maintenance is based upon theoretical benefits for early intervention and, like most recommendations among high-risk patients, has not been validated by prospective randomized trials.
Finally, all of this therapy is contingent upon correct identification of patients at the time of diagnosis. In order to correctly categorize patients, you need to have reliable and accurate data on the genetics of their disease. As a clinician, we may not always know how or where testing is performed, but there are some quality-control measures that can be helpful. Given that 50% of myeloma patients have del(13) on FISH analysis,7 one can go back and check on the frequency with which you are seeing this very common genetic finding. If it is much less than 50%, there may be an issue with the methodology for FISH analysis at your reference laboratory, such as lack of selection of myeloma cells through CD138 selection or fluorescent light-chain staining.12 These are additional factors that need to be known if you are to successfully identify and then appropriately treat patients with high-risk myeloma.
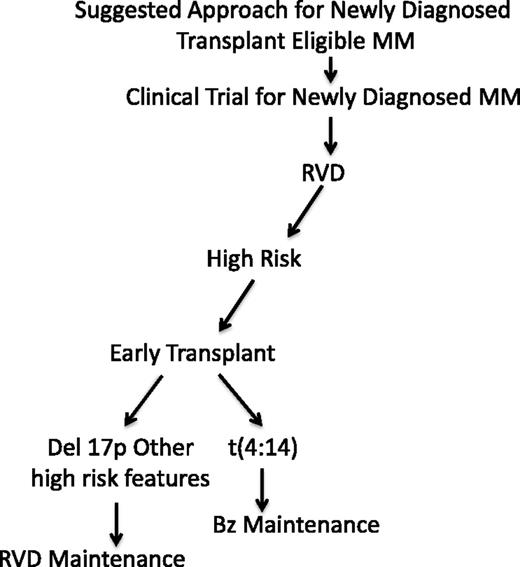
Based upon these principles and data provided above, our treatment approach is described in Figure 1. Although our approach does not have phase 3 data supporting its use, we are discussing a group of patients with generally poor outcomes, and thus, current standard approaches are simply not sufficient. Our treatment is an attempt to adapt the maintenance therapy based upon risk and not treat all patients in a uniform matter, as has been done in nearly all phase 3 trials that evaluate newly diagnosed myeloma patients. Furthermore, this approach of prolonged administration of RVD-based maintenance postinduction therapy forms the basis for the current ongoing Southwest Oncology Group clinical trial for high risk as the control arm (elotuzumab plus RVD vs RVD for high risk only with maintenance until progression in each arm; SWOG S1211, NCT01668719). Initially, we use the most effective induction combining a proteasome inhibitor with an immunomodulatory agent and steroids, followed by early HDT and autologous transplant. This is then followed at day +60 with risk-adapted maintenance therapy. Patients who harbor the t(4;14) translocation receive proteasome-inhibitor–based maintenance, whereas those with high-risk genetics receive RVD-based maintenance. For patients who present with renal failure, our approach changes to include thalidomide as the initial immunomodulatory agent, as it can be given at full dose in the presence of renal issues.69 This is then switched to lenalidomide if needed in the maintenance setting.
Suggested treatment approach for high-risk myeloma. Bz, bortezomib; MM, multiple myeloma.
Suggested treatment approach for high-risk myeloma. Bz, bortezomib; MM, multiple myeloma.
Conclusion
Management of high-risk myeloma includes a complicated set of steps that requires an aggressive treatment approach with prolonged planned maintenance. The short-term goal of therapy for patients is to achieve a rapid and complete response and then use different treatment targets to maintain this disease at a level below detection. Early and accurate identification of high-risk patients so that their maintenance approaches can be successfully targeted remains an important focus of ongoing research. New drugs and targets may help us to differentiate treatment of these patients in the long-term, but currently, combination therapy with standard active agents is the standard of care.
Authorship
Contribution: S.L., L.H.B., and J.K. all contributed to writing the manuscript and shaping the direction of the paper.
Conflict-of-interest disclosure: S.L. is a consultant for Millennium, Celgene, Novartis, BMS, Onyx, Sanofi, and Janssen. L.H.B. is a consultant for Onyx and Novartis. J.K. is a consultant for Millennium, Onyx, Novartis, and Celgene.
Correspondence: Sagar Lonial, Department of Hematology and Medical Oncology, Winship Cancer Institute, Emory University, 1365 Clifton Rd, Building C, Room 4004, Atlanta, GA 30322; e-mail: sloni01@emory.edu.