In this issue of Blood, Bhatt et al describe direct cytotoxic and indirect immune cell-mediated effects of interleukin-21 (IL-21) in mantle cell lymphoma (MCL), providing a preclinical rationale for IL-21 therapy in this aggressive disease.1
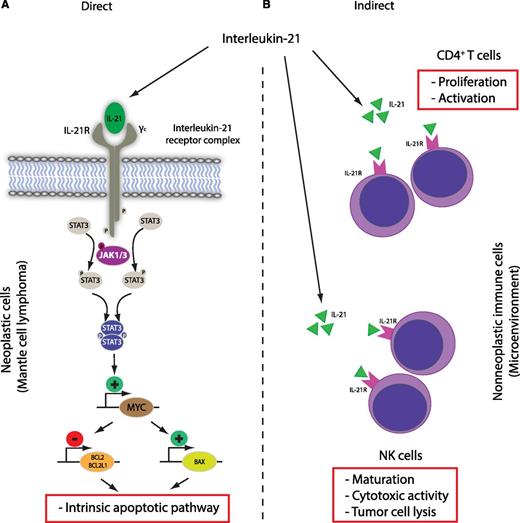
Antitumor activity of IL-21 in mantle cell lymphoma. The main (A) direct and (B) indirect effects are shown. Direct effect: IL-21 receptor engagement leads to activation of the intrinsic apoptotic pathway in neoplastic cells in a STAT3- and MYC-dependent manner. Indirect effects: IL-21 increases NK and CD4+ T-cell activity in the tumor microenvironment and leads to enhanced lysis of tumor cells.
Antitumor activity of IL-21 in mantle cell lymphoma. The main (A) direct and (B) indirect effects are shown. Direct effect: IL-21 receptor engagement leads to activation of the intrinsic apoptotic pathway in neoplastic cells in a STAT3- and MYC-dependent manner. Indirect effects: IL-21 increases NK and CD4+ T-cell activity in the tumor microenvironment and leads to enhanced lysis of tumor cells.
MCL is a virtually incurable aggressive non-Hodgkin lymphoma with short median overall survival. The addition of anti-CD20 immunotherapy (rituximab), intensified frontline therapy, and consolidative autologous transplantation regimens has made some impact on improved response rates, duration of remissions, and overall survival, but, despite these improvements, MCL continues to have one of the worst outcomes of all B-cell lymphomas.2 It can be anticipated that improved biomarker and associated risk stratification approaches, in conjunction with these standard therapies, may somewhat improve outcome, but the task at hand appears abundantly clear: entirely novel treatment approaches have to be explored. Over the last decade, a number of targeted agents have already been approved for MCL therapy, including bortezomib (proteasome inhibitor), lenalidomide (immunomodulatory drug), ibrutinib (Bruton tyrosine kinase [BTK] inhibitor), and temsirolimus (mechanistic target of rapamycin [mTOR] inhibitor). Some of these new drugs, BTK inhibitors in particular,3 might change the entire treatment landscape of MCL in the near future and also the clinical value of related predictive biomarkers.
However, there is another aspect of the most recent additions to the armamentarium of available drugs in MCL that fuels hope for significantly improved survival, namely, drug effects on the nonmalignant immune cells. Although microenvironment biology, niche formation, and host-specific factors are still somewhat underappreciated, the pathogenic importance of nonmalignant cell populations in current disease models is increasingly recognized.4 Most excitingly, strong clinical activity is documented for a number of drugs with molecular targets outside of the malignant cell population, of which immune checkpoint inhibitors are probably the most widely known class of drugs. For example, programmed cell death-1 blockade (nivolumab) has shown convincing activity in Hodgkin lymphoma, a disease that is characterized by immune privilege and extensive cross talk between the malignant and nonmalignant cells.5 Ibrutinib and idelalisib (a phosphatidylinositol 3-kinase δ inhibitor) exert significant effects on natural killer (NK) cells, T cells, macrophages, and osteoclasts that appear to augment therapeutic effects, although some mechanisms might also lead to antagonistic effects with concurrent rituximab therapy.6 Moreover, the activity of lenalidomide has long been associated with direct cytotoxic and indirect immunomodulatory effects via T- and NK-cell activation and a change in cytokine secretion profiles.7 Another example is the treatment of Hodgkin lymphoma with the bispecific tetravalent antibody AFM13 (anti-CD30/CD16a), which recruits and engages NK cells in the direct vicinity of CD30+ neoplastic cells.8 Although the paradigm of simultaneous treatment of cancer cells and host cells is established in principle, more insight into the biological consequences of these dual treatments is needed for drug development and future clinical trial design.
Bhatt et al provide another example of a two-pronged attack on both the malignant cells and host immune cells in the form of preclinical studies of IL-21 in in vitro and in vivo models (see figure). Using established MCL-derived cell lines, the authors convincingly dissect the molecular pathway of IL-21–induced cell death via IL-21 receptor–dependent signaling and STAT3-dependent MYC upregulation (direct cytotoxicity). Similar dependencies have been demonstrated by the same group in diffuse large B-cell lymphoma.9 Overall, the cytotoxic effects of IL-21 and related MYC expression are now well documented in two B-cell lymphoma entities and are in agreement with proapoptotic effects of IL-21 on activated and naïve B cells. However, a number of MCL-derived cell lines are resistant to IL-21 in vitro, and the exact resistance mechanisms need to be determined in future studies. Interestingly, the authors provide some evidence that the level of MYC induction after IL-21 stimulation might be linked to IL-21 sensitivity, suggesting that (dynamic) MYC expression could be evaluated as a biomarker. The indirect effects of IL-21 treatment are related to NK cell-dependent lysis of tumor cells, and the presented in vivo data in a syngeneic mouse transplantation model strongly suggest that antitumor effects are dominated by enhanced activity of CD4+ T and NK cells. In this proof-of-concept study, the authors chose to treat the mice via intratumoral injections, and it remains to be determined whether the observed direct and indirect therapeutic effects of IL-21, their relative contribution to tumor reduction, and the composition of the tumor microenvironment by histologic examination will be dependent on the route of administration (intravenous vs intratumoral).
The presented data provide a solid preclinical rationale to consider recombinant IL-21 in MCL therapy, in particular because the indirect effects on immune effector cells in the tumor microenvironment or in the circulation might maintain activity in tumors that harbor primary resistance to the proapoptotic effects. But how exactly should this approach move forward? Recombinant IL-21 therapy has now been tested in renal cell carcinoma, metastatic melanoma, and, most recently, indolent B-cell lymphomas with clearly demonstrated clinical activity.10 In the last phase 1 trial, including patients with relapsed small lymphocytic lymphoma/chronic lymphocytic leukemia, follicular lymphoma, and marginal zone lymphoma, IL-21 was combined with rituximab via intravenous bolus injections, with clinical response in 42% of patients. It appears that combination therapy with IL-21 will also be a way forward in future trials including MCL; however, with the emergence of other new drugs in this indication, the ideal combination partners of IL-21 have to be carefully considered based on theoretical biological synergies. Overall, Bhatt et al report a promising therapeutic intervention in MCL that reinforces the concept of concomitant “tumor and host” treatment. This type of dual treatment might represent a new standard for clinical trial design that should incorporate assessment of microenvironment biology and related biomarker development. Leveraging this concept, a brighter future might be ahead for patients with MCL and other hard-to-treat malignancies.
Conflict-of-interest disclosure: The author declares no competing financial interests.