In this issue of Blood, Cheng et al have identified a novel and previously unrecognized nuclear function of double-stranded RNA-activated protein kinase (PKR) in the pathogenesis of acute myeloid leukemia (AML). Increased PKR promotes genomic instability and is associated with inferior outcomes in both AML and a mouse model of myelodysplastic syndrome (MDS) and leukemia. Thus, nuclear PKR has an oncogenic function and can be a novel therapeutic target to prevent leukemia progression or relapse and improve clinical outcomes.1
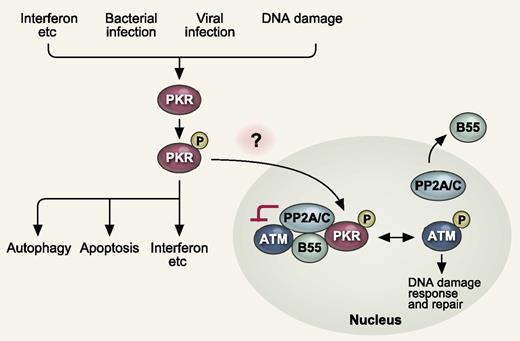
PKR in the cytoplasm is activated by multiple stimuli, such as cytokines (IFNs, etc), bacterial and viral infection, and DNA damage. Active PKR triggers production of IFNs and proinflammatory cytokines, apoptosis, and autophagy. In this study, Cheng et al showed that nuclear PKR activates PP2A by promoting nuclear localization of the regulatory B subunit (B55α). Activated PP2A in turn antagonizes autophosphorylation and activation of ATM, thereby inhibiting DNA damage response. P indicates phosphorylation. See Figure 4I in the article by Cheng et al beginning on page 1585.
PKR in the cytoplasm is activated by multiple stimuli, such as cytokines (IFNs, etc), bacterial and viral infection, and DNA damage. Active PKR triggers production of IFNs and proinflammatory cytokines, apoptosis, and autophagy. In this study, Cheng et al showed that nuclear PKR activates PP2A by promoting nuclear localization of the regulatory B subunit (B55α). Activated PP2A in turn antagonizes autophosphorylation and activation of ATM, thereby inhibiting DNA damage response. P indicates phosphorylation. See Figure 4I in the article by Cheng et al beginning on page 1585.
PKR is a ubiquitously expressed serine and threonine protein kinase that was initially characterized as an antiviral protein induced by interferon (IFN).2 PKR is now known to have multifaceted roles in the regulation of inflammatory immune responses (see figure).3 PKR in the cytoplasm is activated by multiple stimuli, such as cytokines (IFNs, etc), bacterial and viral infection, and DNA damage through a mechanism involving its dimerization and autophosphorylation. Active PKR triggers signaling of several pathways and regulates transcription to produce IFNs and proinflammatory cytokines. In addition, PKR triggers apoptosis through Fas-associated protein with death domain (FADD)–mediated activation of caspase-8, and autophagy through eukaryotic initiation factor 2α (eIF2α)–mediated activation of the microtubule-associated protein LC3. PKR is also required for inflammasome activation and promotes the release of inflammasome-dependent cytokines, such as IL-1β, IL-18, and HMGB1.4
Because of its proapoptotic functions, PKR has been considered to have tumor-suppressor activities. Indeed, the loss of PKR catalytic activity and an inactivating mutation in PKR have been detected in B-cell chronic lymphocytic leukemia and T-cell acute lymphoblastic leukemia, respectively.5,6 However, the authors have previously demonstrated that mice expressing a PKR transgene specifically in hematopoietic cells develop an MDS-like phenotype with pancytopenia and bone marrow dysplasia. Furthermore, increased PKR has been reported in patients with acute leukemias.7 This evidence suggested that PKR has a previously unrecognized role in tumorigenesis. Although PKR’s role in the cytoplasm has been well characterized as described above, PKR also resides in the nucleus but the function of nuclear PKR remained unclear. Of interest, active PKR appeared to be mainly nuclear in high-risk MDS patient samples and acute leukemia cell lines deficient in phosphatase and tensin homologue (PTEN), whereas it was mostly cytoplasmic in low-risk MDS patient samples and PTEN-positive acute leukemia cell lines, supporting that nuclear PKR may play a role in tumorigenesis.8
In this study, the authors first demonstrated that high PKR expression in CD34+ AML cells from 414 newly diagnosed AML patients correlated with worse survival and shortened remission duration. This trend was also true in large cohort studies of breast, lung, and ovarian cancer patients available from published reports and databases. These findings contradict the previously believed tumor-suppressive role of PKR based on its proapoptotic function in the cytoplasm.
Therefore, the authors next focused on the role of nuclear PKR. Phospho(threonine 451)-PKR (p-PKR), indicative of activated PKR, was observed mainly in the nucleus of CD34+ cells isolated from AML patients as previously reported, suggestive of the specific role of nuclear PKR in AML. Eventually, the authors found that PKR has a novel nuclear function to inhibit DNA damage response signaling and double-strand break repair. Mechanistically, they found that nuclear PKR activates protein phosphatase 2A (PP2A), which consists of a dimeric core enzyme composed of the structural A and catalytic C subunits and a regulatory B subunit (B55α), by promoting nuclear localization of B55α. Activated PP2A in turn antagonizes autophosphorylation and activation of ataxia-telangiectasia mutated (ATM) and its association with downstream targets. Thus, inhibition of PKR expression or activity promotes ATM activation and promotes more rapid and complete repair of double-strand breaks (see figure).
The authors then validated this new function of nuclear PKR by crossing PKR-transgenic mice or PKR knockout mice with the NUP98-HOXD13 (NHD13) MDS mouse model to produce NHD13-TgPKR and NHD13-PKRKO mice, respectively. PKR-transgenic but not PKR-null mice demonstrate a mutator phenotype that leads to more rapid MDS evolution to acute leukemia. Furthermore, the age-associated accumulation of somatic mutations that occurs in the NHD13 mouse model was significantly elevated by coexpression of a PKR transgene whereas knockout of PKR expression or treatment with a PKR inhibitor reduced the frequency of spontaneous mutations in vivo.
Taken together, these results establish that increased nuclear PKR has an oncogenic function that promotes the accumulation of deleterious mutations by inhibiting DNA damage response and double-strand break repair. Thus, PKR inhibition may represent a novel therapeutic strategy to prevent leukemia progression and potentially tumorigenesis of other cancers.
Finally, several important questions remain unsolved. How does PKR become upregulated and activated, and then shuttle into the nucleus in AML and other cancers? As mentioned above, PKR becomes activated in response to multiple stimuli, such as cytokines, bacterial and viral infection, and DNA damage. Chronic inflammatory stress or DNA damage signals could activate PKR, but there might be unknown pathways. Enhanced shuttling of activated PKR into the nucleus might also be regulated by some AML-related signals. In addition, the precise mechanism of activation of PP2A by PKR remains undetermined. Addressing these questions would promote our understanding of the complex functions of PKR and provide more therapeutic options targeting the PKR signaling pathway in AML.
Conflict-of-interest disclosure: The authors declare no competing financial interests.