Key Points
Generation and function of specific human Tregs.
Specific regulation of FVIII responses by engineered human Tregs.
Abstract
Expansion of human regulatory T cells (Tregs) for clinical applications offers great promise for the treatment of undesirable immune responses in autoimmunity, transplantation, allergy, and antidrug antibody responses, including inhibitor responses in hemophilia A patients. However, polyclonal Tregs are nonspecific and therefore could potentially cause global immunosuppression. To avoid this undesirable outcome, the generation of antigen-specific Tregs would be advantageous. Herein, we report the production and properties of engineered antigen-specific Tregs, created by transduction of a recombinant T-cell receptor obtained from a hemophilia A subject’s T-cell clone, into expanded human FoxP3+ Tregs. Such engineered factor VIII (FVIII)-specific Tregs efficiently suppressed the proliferation and cytokine production of FVIII-specific T-effector cells. Moreover, studies with an HLA-transgenic, FVIII-deficient mouse model demonstrated that antibody production from FVIII-primed spleen cells in vitro were profoundly inhibited in the presence of these FVIII-specific Tregs, suggesting potential utility to treat anti-FVIII inhibitory antibody formation in hemophilia A patients.
Introduction
The immunogenicity of therapeutic proteins can lead to undesirable immune responses and render treatments ineffective. For example, a complication of factor VIII (FVIII) replacement therapy for hemophilia A patients is that ∼25% to 30% will generate a T cell-mediated neutralizing antibody response (termed inhibitor formation).1-3 Like other monogenic diseases, hemophilia A subjects lack all or part of FVIII and thus may not have immunologic tolerance to some FVIII epitopes. The ability to induce tolerance to prevent and/or reverse inhibitor responses would be highly desirable.4 One approach is the expansion of regulatory T cells (Tregs)5-7 capable of downregulating immune responses. Indeed, clinical applications of Tregs are considered a next-generation cellular therapy for many autoimmune and inflammatory immune disorders.5,8 However, polyclonal Tregs have critical potential drawbacks: they reflect a broad repertoire and are less robust than activated antigen-specific Tregs. To overcome these limitations, design and production of antigen-specific Tregs would be preferable.9-11
The success of specific T-cell receptor (TCR) gene therapy in cancer treatment suggests that antigen-specific Treg therapy with chimeric antigen receptors or engineered TCRs could be developed to treat immune disorders.12-15 In contrast to polyclonal Tregs, antigen-specific Tregs can recognize the disease-associated antigen and exert their suppressive action at sites of inflammation, eg, islets of Langerhans or the central nervous system.16,17 Recently, the generation of antigen-specific human Tregs via viral transduction of a tumor-associated antigen-specific TCR was reported.9,11,18 These results indicated that transduction of specific TCR could render Tregs antigen specific (monoclonal) and able to suppress immune responses to specific antigens. In these earlier studies, however, functional stability of the Tregs was not clearly addressed. Maintaining Treg functional stability after expansion in vitro is a key requirement for translation of TCR-engineered human Tregs and thus is a significant challenge. Although previous studies demonstrated antigen-specific suppression of T-effector responses,11,12,16,17 no studies have been reported on suppression of adverse humoral immunity, eg, inhibitor formation.
To generate functional FVIII-specific human Tregs, polyclonal human Tregs were transduced to express TCRs derived from a well-characterized FVIII-specific T-effector clone expanded from the blood of a hemophilia A inhibitor subject.19,20 We hypothesized that such TCR-transduced Tregs would recognize the same HLA-DRB1*01:01-restricted epitope as the T-effector clone, thus rendering them antigen specific. The present study describes the generation of antigen-specific FoxP3+ human Tregs and their functional suppression of FVIII-specific T- and B-cell responses.
Methods
General
Recombinant human interleukin (IL)-2 was provided by the National Cancer Institute Biological Resources Branch (Frederick, MD). Phosphorothioate-backboned oligodeoxynucleotides (ODN; 25 bp) were synthesized with “machine mixed bases” by Integrated DNA Technologies (Coralville, IA). Viability fluorescence dye, Cell proliferation Dye eFluor 450, and anti-human CD28 antibody (clone CD28.2) were purchased from eBioscience (San Diego, CA), and anti-human CD3ε antibody (clone 64.1) was purified in-house. For sorting, anti-human CD4-fluorescein isothiocyanate, anti-human CD25-PECy7, anti-human CD127-PE, and anti-human CD45RA-Ag-presenting cell (APC) were purchased from BioLegend (San Diego, CA). Treg surface markers, anti-LRRC32 (GARP)-PE, anti-latent transforming growth factor β-associated protein (LAP)-PE, and anti-glucocorticoid-induced tumor necrosis factor receptor-related protein (GITR) were purchased from eBioscience and BioLegend. Recombinant human FVIII (rFVIII) was kindly provided by Dr Birgit Reipert (Baxter, Vienna, Austria).
Identification and generation of a recombinant TCR recognizing peptide FVIII-2191-2220
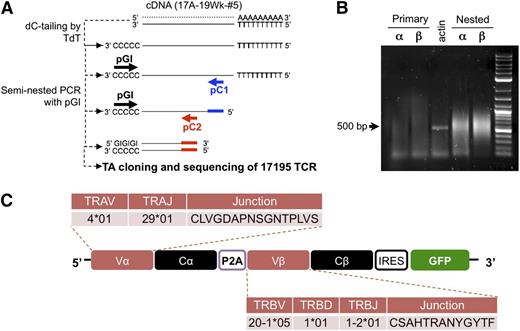
A T-cell clone from a hemophilia A subject19,20 was used to isolate DNA encoding its FVIII-specific TCR. This clone, designated 17195, proliferates and produces IL-4 in response to a C2 domain peptide, FVIII-2191-2220. (The clone designation reflects the time point following initial inhibitor detection: 17A-19wk-clone #5.) The cloning strategy is shown in Figure 1A.21 Briefly, cDNA was tagged with a poly-C tail using a terminal transferase reaction. The V regions were amplified using a common poly-GI forward primer (pGI: 5′-CACCGGGIIGGGIIGGGII-3′) and 2 different sets of human constant region-specific reverse primers (pC1 and pC2: pCa1, 5′-AGTCAGATTTGTTGCTCCAGGCC-3′; pCb1, 5′-TTCACCCACCAGCTCAGCTCC-3′; pCa2, 5′-ATACGCGTTCTCTCAGCTGGTACACGG-3′; pCb2, 5′-ATACGCGTAGATCTCTGCTTCTGATGGC-3′). After a second round of polymerase chain reaction (PCR), amplified V regions (500-600 bp) were cloned into a TA cloning vector (Invitrogen) (Figure 1B). Insertion of PCR product was confirmed by restriction enzyme digestion of plasmid DNA. Extracted individual sequences were nBlast-matched with reference database sequences from the International ImMunoGeneTics and the National Center for Biotechnology Information (NCBI). Once the α and β chain V regions were identified, the TCR of this clone, abbreviated 17195TCR, was constructed using the human TCR constant region C region reference sequence. To incorporate the α and β sequences, the α and β chain regions were connected by a P2A peptide linker22 to create a single construct (Figure 1C), and cDNA for green fluorescent protein (GFP) was inserted downstream of the TCR coding region separated by an internal ribosomal entry site (IRES). The redesigned retroviral 17195TCR construct was synthesized by GenScript USA (Piscataway, NJ). Retrovirus was produced using a Phoenix-Ampho packaging system. Empty retroviral vector (pRetroX-IRES-Zsgreen1; Clontech) was used in this study as mock vector control.
Identification and retroviral expression of an FVIII-2191-2220–specific TCR in primary CD4 T cells. (A) Scheme of FVIII-2191-2220–specific TCR cloning from a hemophilia A subject’s T-cell clone. The cloning procedure is described in “Methods.” Briefly, to amplify TCR cDNA from the clone, poly C oligonucleotide was linked onto the 3′ terminus of total cDNAs by terminal deoxynucleotidyl transferase. The variable region was amplified using seminested PCR with a poly GI primer and 2 reverse primers (pC1 and pC2) corresponding to 2 different 5′ upstream coding regions of the α and β chain constant regions. (B) Amplification of V regions of the 17195TCR using seminested PCR. Primary amplification and nested amplification were carried out with pC1 and pC2 (A). PGI was commonly used as the forward primer for the primary and nested PCR step. Bold arrows indicate the predicted sizes of the amplified Vα and Vβ PCR products. (C) Retroviral expression construct of the FVIII-2191-2220–specific TCR (17195TCR). To build the FVIII-2191-2220–specific TCR, the variable regions (Vα and Vβ) from the FVIII-2191-2220–specific T-effector clone were combined with human constant regions (Cα and Cβ) extracted from the NCBI database. To produce the individual α and β TCR chains from a single transcript, a P2A cleavage peptide was inserted between the α and β chain sequences. Expression of the GFP reporter is controlled by IRES, which is located downstream of the TCR construct.
Identification and retroviral expression of an FVIII-2191-2220–specific TCR in primary CD4 T cells. (A) Scheme of FVIII-2191-2220–specific TCR cloning from a hemophilia A subject’s T-cell clone. The cloning procedure is described in “Methods.” Briefly, to amplify TCR cDNA from the clone, poly C oligonucleotide was linked onto the 3′ terminus of total cDNAs by terminal deoxynucleotidyl transferase. The variable region was amplified using seminested PCR with a poly GI primer and 2 reverse primers (pC1 and pC2) corresponding to 2 different 5′ upstream coding regions of the α and β chain constant regions. (B) Amplification of V regions of the 17195TCR using seminested PCR. Primary amplification and nested amplification were carried out with pC1 and pC2 (A). PGI was commonly used as the forward primer for the primary and nested PCR step. Bold arrows indicate the predicted sizes of the amplified Vα and Vβ PCR products. (C) Retroviral expression construct of the FVIII-2191-2220–specific TCR (17195TCR). To build the FVIII-2191-2220–specific TCR, the variable regions (Vα and Vβ) from the FVIII-2191-2220–specific T-effector clone were combined with human constant regions (Cα and Cβ) extracted from the NCBI database. To produce the individual α and β TCR chains from a single transcript, a P2A cleavage peptide was inserted between the α and β chain sequences. Expression of the GFP reporter is controlled by IRES, which is located downstream of the TCR construct.
Isolation, transduction, and expansion of TCR-transduced T effectors and Tregs
Buffy-coat fractions from healthy 20- to 70-year-old men were provided by the Department of Transfusion Medicine at the National Institutes of Health or purchased from the American Red Cross. All procedures were approved by the Uniformed Services University of the Health Sciences Institutional Review Board, and all blood donors provided written informed consent in accordance with the Declaration of Helsinki. To obtain CD4 T-naïve and Treg cells, peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats by ficoll separation (Ficoll-Paque Plus; GE Healthcare). CD4 T cells were enriched by positive selection with magnetic-activated cell sorting (MiltenyiBiotech, Auburn, CA). Naïve CD4 T cells (CD4+CD25−CD127+CD45RA+) and Tregs (CD4+CD25hiCD127lo) were then sorted on a FACSAria (BD Biosciences).
For transduction of mock vector or 17195TCR, T-naïve cells or Tregs (2 × 105/mL) were stimulated with plate-coated anti-CD3ε (5 µg/mL) and anti-CD28 antibodies (2 μg/mL) for 48 to 72 hours plus recombinant human IL-2 (200 U/mL). Stimulated cells were transferred into 17195TCR viral particle-coated, retronectin (10 µg/mL)-pretreated plates and incubated for 24 hours. After transduction, the cells were expanded for 3 to 5 weeks with 6000r γ-irradiated HLA-DRB1*01:01 (abbreviated DR1) PBMCs and peptide FVIII-2191-2220 (0.2-0.5 μg/mL) plus IL-2 (200 U/mL). Irradiated PBMCs (PBMCs:T cells = 2:1) and FVIII-2191-2220 (0.5 μg/mL) were added to the culture every 2 weeks. Expanded 17195TCR-effectors and 17195TCR-Tregs were rested in the media (RPMI1640 supplemented with 10% fetal bovine serum [FBS], 1% human AB serum, 1× Glutamax, and antibiotics) for 48 to 72 hours.
Intracellular staining for Foxp3, Helios, and cytokines
Rested cells were restimulated with phorbol myristate acetate (50 ng/mL) and ionomycin (1 μg/mL) for 4 hours in the presence of Golgi Stop (0.75 μL/mL). Cells were then fixed with 4% paraformaldehyde solution and permeabilized in bovine serum albumin-containing 0.1% Triton X-100/phosphate-buffered saline (PBS). Permeabilized cells were stained for Foxp3-APC, Helios-PE, IL-2-PE, and interferon (IFN)γ-PECy7 (BD Bioscience).
Cell proliferation and suppression assays
For standard suppression assays, freshly isolated or expanded T effectors were washed twice in PBS and labeled with 10 μM eFluor-450 for 20 minutes. Labeled cells were washed in FBS-supplemented medium and 2 to 5×104 cells cocultured with γ-irradiated DR1-PBMCs as APCs in the presence of FVIII-2191-2220 or an irrelevant peptide (OVA) for 4 to 5 days. Proliferation was measured by a flow cytometric dye dilution assay.
An in vitro Treg suppression assay was also performed as previously described.23,24 Briefly, 3- to 4-week expanded 17195TCR-effectors (4 × 104) and γ-irradiated DR1-PBMCs were mixed at a ratio of 1:2 in the absence of IL-2. 17195TCR-Tregs were then added at various ratios and stimulated with soluble anti-CD3 antibody (0.5 μg/mL), FVIII-2191-2220 (0.5 μg/mL), or rFVIII (0.2 μg/mL) for 4 to 6 days, and cell proliferation was assayed by dye dilution or by thymidine incorporation following incubation with [3H]-thymidine (1 μCi/well) during the final 16 to 18 hours of culture. To measure suppression of cytokine production by Tregs, T effectors were incubated with Tregs (or 17195TCR) for 24 hours in the presence of FVIII-2191-2220 with irradiated DR1-PBMCs and without IL-2. The supernatants were harvested and assayed for cytokine expression using a Th1, Th2, and Th17 human CBA kit (BD Bioscience).
DNA methylation analysis of human Treg-specific de-methylation regions
Three-week expanded 17195TCR-Tregs were harvested and washed, and genomic DNA was extracted using a Wizard Genomic DNA Purification Kit (Promega). Epitect Bisulfite kits (Qiagen) were used to convert unmethylated cytosines in the genomic DNAs to uracils. A Promark Q24 instrument was used to detect and quantify methylated Treg-specific de-methylation region (TSDR) CpGs in the TSDR, using human TSDR-specific primers. Nine CpGs were determined to be methylation-sensitive TSDR CpGs within the Foxp3 genome (−2376 to −2263 from the translation start site).
ELISPOT assay and in vitro suppression of FVIII-specific antibody production by splenocytes from immunized hemophilia A mice
To quantify in vitro anti-FVIII suppression, “humanized” hemophilia A mice were created by crossing E16-FVIII knockout (KO) mice25 with DR1-transgenic mice (Dr Chella David, Mayo Clinic). These mice, which recognize p2191-2220,26,27 were immunized subcutaneously with 2 µg rFVIII in incomplete Freund’s adjuvant and boosted twice with 2 µg rFVIII in PBS intraperitoneally 3 and 5 weeks later. Pooled splenocytes from 2 immunized mice having established titers against FVIII were used as responders. HLA-DR1-FVIII-KO splenocytes (107) were cocultured with 17195TCR-Tregs or mock-transduced Tregs at various ratios in T25 flasks for 6 days in RPMI 1640 medium supplemented with 10% FBS, 2 mM l-glutamine, 50 µM 2-mercaptoethanol, and 1 µg/mL rFVIII28,29 without CD138+ cell depletion. The effect of these human Tregs on FVIII-specific antibody secreting cell (ASC) formation was measured using an enzyme-linked immunospot (ELISPOT) assay with rFVIII-coated plates.28,29 Cells were washed, added to ELISPOT wells in triplicate, and cultured overnight. The captured anti-FVIII antibodies were detected by horseradish peroxidase-conjugated anti-mouse IgG (H+L) (Invitrogen), and anti-FVIII ASCs revealed with AEC substrate (BD Biosciences). Plates were read on a CTL ELISPOT plate reader (Cellular Technology Limited, Shaker Heights, OH).
Results
Generation of an engineered TCR recognizing an epitope within FVIII-2191-2220
Because direct isolation and expansion of antigen-specific Tregs from patients remains a challenge, we elected to transduce polyclonal Foxp3+ T cells with an engineered TCR specific for a defined epitope in FVIII. The variable regions of TCR α and β chains were cloned from a FVIII-reactive T-effector clone derived from a hemophilia A inhibitor subject as described in “Methods” (Figure 1A-B).19,20 Cloned TCR-V regions, separated by a P2A peptide, were then combined with human TCR-C regions (Figure 1C).30-32
Retroviral expression of an engineered FVIII-specific TCR and FVIII-mediated proliferation of polyclonal CD4 T effectors
To prepare T effectors for retroviral transduction of 17195TCR, PBMCs from normal healthy donors were sorted to obtain a naïve CD4+ T population (CD4+CD25−CD127+CD45RA+); natural T-regulatory cells (CD4+CD25hiCD127low) were also sorted and transduced as described below, stimulated with plate-coated anti-CD3ε antibody and anti-CD28 antibody, and transduced with retroviral particles encoding the 17195TCR. Transduced cells were maintained and rested in the presence of IL-2 for 8 days without additional stimuli. Twenty-four hours after transduction, initial transfection efficiency was determined by measuring their GFP expression. GFP+ cells comprised 3% to 17% of the virus-treated CD4 cells, and this proportion did not change during the maintenance period (2-4 days) (supplemental Figure 1A available on the Blood Web site; data not shown). Transduction yields were comparable for mock-transduced cells and 17195TCR-transduced T effectors. The transduced GFP+ CD4 cells were next stained with an anti-Vβ2 antibody. GFP+ cells were predominantly Vβ2+ (67% of the GFP+ cells), whereas 10% of the nontransduced (GFP−) cells were Vβ2+, indicating endogenous TCR expression levels (supplemental Figure 1B). Expression of 17195TCR appeared to increase with increasing intensity of GFP in the transduced cells.
To validate the antigen specificity of the 17195TCR, the transduced T-effector cells were stained with DR1 tetramers loaded with FVIII-2191-2220. Despite the strong expression of GFP in transduced cells, only a small percentage of expanded naïve T cells (<5% of the transduced and 0.2% of the total CD4 population) showed a tetramer+ phenotype (dot plot in the bottom center of supplemental Figure 1A), presumably due to pairing of the α and β chains with endogenous TCR chains. Despite the low percentage of tetramer+ 17195TCR-transduced cells, their tetramer+ staining intensity was >10-fold higher and distinguishable from the nonspecific tetramer staining (0.41% of the transduced and 0.01% of the total naïve CD4+ population in top center dot plot in supplemental Figure 1A) or of mock-transduced cells (bottom right dot plot in supplemental Figure 1A). These results indicated that a subset of the primary CD4+ cells transduced with the 17195TCR expressed a TCR that recognized FVIII-2191-2220 presented on HLA-DR1.
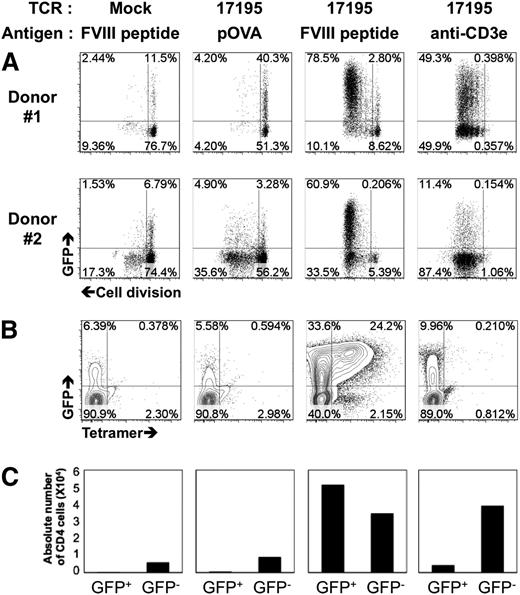
The functional activity of these 17195TCR-transduced T effectors was tested directly as follows. Ten-day rested TCR-transduced CD4 cells were labeled with a cell proliferation dye, and a proliferation assay was performed with DR1-PBMC presenting FVIII-2191-2220 or an OVA negative control peptide (pOVA). When 17195TCR-effectors were cultured with FVIII-2191-2220, ∼100% of the GFP+ cells proliferated (Figure 2A), and the proportion of GFP+ cells increased from 61% to 81%, more than the nonproliferating cells. Expanded GFP+ 17195TCR T effectors bound specifically to DR1-tetramers loaded with FVIII-2191-2220: 24% of 17195TCR T effectors were tetramer+ (Figure 2B). Moreover, the increase in absolute cell count of 17195TCR T effectors with FVIII-2191-2220 suggests that initial stimulation with FVIII-2191-2220 efficiently enriched the specifically transduced T-effector population (Figure 2C).
Activation of 17195 T effectors by FVIII-2191-2220. (A) Proliferation of 17195TCR-transduced CD4 T cells with FVIII-2191-2220. Prestimulated primary CD4 T cells were transduced with 17195TCR or with a mock vector and maintained for 10 days in culture media supplemented with IL-2. For proliferation assays, cells were labeled with cell proliferation dye (eFluor 450) and then reactivated with irradiated DR1-PBMCs plus peptide FVIII-2191-2220 (0.5 μg/mL), pOVA (0.5 μg/mL), or anti-CD3ε antibody (0.5 μg/mL) for 4 days. Cell proliferation was measured by flow cytometry using a standard dye dilution assay. Representative results are shown for CD4 cells from 2 different donors that were transduced with 17195TCR. (B) Staining of transduced, expanded GFP+ 17195 T effectors shown in A using PE-labeled DR1 tetramers loaded with FVIII-2191-2220. (C) Proliferation of GFP− and GFP+ T cell effectors stimulated with OVA peptide, FVIII-2191-2220, or anti-CD3ε antibody. Transduced T effectors (2 × 106 per well) were stimulated using irradiated DR1-APCs (Responder:Stimulator = 1:5) as in A. After culturing for 10 days, the cells were harvested, and viable GFP− and GFP+ CD4 cells were counted using flow cytometry.
Activation of 17195 T effectors by FVIII-2191-2220. (A) Proliferation of 17195TCR-transduced CD4 T cells with FVIII-2191-2220. Prestimulated primary CD4 T cells were transduced with 17195TCR or with a mock vector and maintained for 10 days in culture media supplemented with IL-2. For proliferation assays, cells were labeled with cell proliferation dye (eFluor 450) and then reactivated with irradiated DR1-PBMCs plus peptide FVIII-2191-2220 (0.5 μg/mL), pOVA (0.5 μg/mL), or anti-CD3ε antibody (0.5 μg/mL) for 4 days. Cell proliferation was measured by flow cytometry using a standard dye dilution assay. Representative results are shown for CD4 cells from 2 different donors that were transduced with 17195TCR. (B) Staining of transduced, expanded GFP+ 17195 T effectors shown in A using PE-labeled DR1 tetramers loaded with FVIII-2191-2220. (C) Proliferation of GFP− and GFP+ T cell effectors stimulated with OVA peptide, FVIII-2191-2220, or anti-CD3ε antibody. Transduced T effectors (2 × 106 per well) were stimulated using irradiated DR1-APCs (Responder:Stimulator = 1:5) as in A. After culturing for 10 days, the cells were harvested, and viable GFP− and GFP+ CD4 cells were counted using flow cytometry.
Transduction of a recombinant TCR renders polyclonal Tregs antigen specific
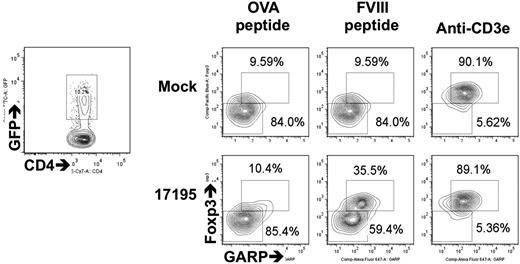
To prepare antigen-specific Tregs, sorted CD4+CD25hiCD127low T cells were transduced with 17195TCR. After 48 hours, the percentage of tetramer+ cells was equivalent or slightly higher in GFP+ Tregs than in the transduced 17195TCR T effectors (supplemental Figure 1). After resting in IL-2–containing media for 8 days, these 17195TCR-transduced Tregs were stimulated with FVIII-2191-2220 for 24 hours. Expression levels of Foxp3, GARP, LAP, and GITR were measured in transduced GFP+ cells by flow cytometry. These markers are induced within 24 to 72 hours after TCR signaling of activated Tregs in humans.9-11,33-35 Increased expression of these Treg-specific markers occurred only in 17195TCR-transduced Tregs stimulated with FVIII-2191-2220 (35.5%; bottom middle panel in Figure 3; supplemental Figure 1C), and not in mock-transduced Tregs. Helios expression was well maintained in both cell types during the activation (data not shown).
Expression of 17195TCR on human Tregs and upregulation of Foxp3 and GARP by stimulation with FVIII-2191-2220. 17195TCR and mock-transduced Tregs (prepared as in Figure 2A) were maintained in culture for 10 days and then reactivated for 48 hours with irradiated DR1-PBMCs and FVIII-2191-2220 (0.5 μg/mL) or OVA peptide (0.5 μg/mL) plus IL-2 (200 U/mL). GFP expression in transduced cells was measured by FACS (left contour plot). The right contour plots indicate subsequent Foxp3 and GARP expression in gated GFP+ cells. Induction of Foxp3 and GARP by specific antigen was calculated by comparing the Foxp3−GARP− cell counts (lower left gate and number) and the Foxp3+GARP+ cell counts (upper right gate and number) in the right contour plots.
Expression of 17195TCR on human Tregs and upregulation of Foxp3 and GARP by stimulation with FVIII-2191-2220. 17195TCR and mock-transduced Tregs (prepared as in Figure 2A) were maintained in culture for 10 days and then reactivated for 48 hours with irradiated DR1-PBMCs and FVIII-2191-2220 (0.5 μg/mL) or OVA peptide (0.5 μg/mL) plus IL-2 (200 U/mL). GFP expression in transduced cells was measured by FACS (left contour plot). The right contour plots indicate subsequent Foxp3 and GARP expression in gated GFP+ cells. Induction of Foxp3 and GARP by specific antigen was calculated by comparing the Foxp3−GARP− cell counts (lower left gate and number) and the Foxp3+GARP+ cell counts (upper right gate and number) in the right contour plots.
Thus, these data confirm that a FVIII-specific TCR can be expressed on polyclonal Tregs and that Treg-specific activation markers can be induced by FVIII-2191-2220.
Functional stability of transduced Tregs after long-term ex vivo expansion using specific FVIII-2191-2220 and ODN
The function of expanded human Tregs in vitro can be stabilized using random ODNs.36 Thus, we hypothesized that long-term expansion in the presence of FVIII-2191-2220 and ODN would optimize the enrichment of antigen-specific, transduced Tregs expressing high levels of Foxp3 and Helios. Transduced Tregs were cultured with anti-CD3ε antibody, FVIII-2191-2220, or FVIII-2191-2220 and ODN (FVIII-2191-2220/ODN), in the presence of DR1-PBMCs plus recombinant IL-2 (supplemental Figure 2). After 1 round of expansion (8 days), the rate of cellular division slowed. The cell numbers and GFP fluorescence intensity after this expansion step were comparable in all groups (data not shown). Foxp3 and Helios in the GFP+ cells were well maintained at levels similar to those of freshly isolated Tregs (70-80% of Foxp3 and Helios coexpressed this phenotype; supplemental Figure 3).
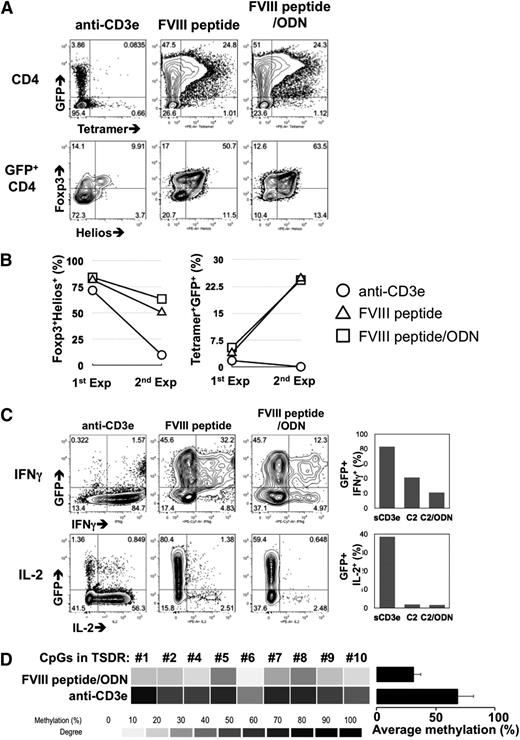
After a second round expansion, GFP+ cells were dramatically enriched only in the FVIII-2191-2220 and FVIII-2191-2220/ODN culture conditions (72% and 74%), compared with anti-CD3ε stimulation (top dot plots in Figure 4A). Moreover, the percentage of tetramer+ cells in the GFP+ cell population was increased in both FVIII-2191-2220 and FVIII-2191-2220/ODN conditions. Foxp3 and Helios expression after FVIII-2191-2220 stimulation showed a significantly different phenotype than of cells stimulated with anti-CD3ε. Specifically expanded GFP+ cells maintained Foxp3 and Helios expression, but cells cultured with anti-CD3ε did not (bottom dot plots in Figure 4A). The data in Figure 4B suggest that the FVIII-2191-2220 signal given to 17195TCR Tregs during expansion contributed to the enrichment of these FVIII-specific Tregs.
Optimized ex vivo expansion of 17195TCR-Tregs with FVIII-2191-2220 plus oligodeoxynucleotides. (A) FVIII-2191-2220–specific enrichment of transduced Foxp3+Helios+ Tregs via long-term expansion. After a second round of expansion (described in supplemental Figure 2), the CD4 T cells were stained using DR1 tetramers loaded with FVIII-2191-2220 (top contour plots). Expression of Foxp3 and Helios in the gated CD4+GFP+ population were also evaluated by intracellular staining (bottom contour plots). (B) Comparison of FVIII-2191-2220 specificity and Fox3+Helios+ phenotypes of short-term and long-term expanded 17195TCR-Tregs. First-round expanded (Exp) cells (1st Exp) were harvested 8 days after viral transduction, and second-round expanded cells (2nd Exp) were harvested 16 days after transduction. FVIII-2191-2220 specificity was determined by measuring the increase of tetramer+CD4+ cells as in A (right line graph). The Foxp3 and Helios expression levels were measured by intracellular staining, and the Foxp3+Helios+ cell count was obtained by gating of the GFP+CD4+ population (left line graph). Representative data from 1 of 2 experiments are shown. (C) Plasticity of long-term expanded 17195TCR-Tregs and Treg phenotype stability induced by antigen. Second-round expanded 17195TCR-transduced Tregs were rested for 3 days of culture without IL-2 and then restimulated for 4 hours with phorbol myristate acetate and ionomycin in the presence of Golgi-block reagent. Intracellular IFNγ (top contour plots) and IL-2 (bottom contour plots) levels were measured by FACs analysis. The bar graphs on the right indicate the relative expression levels of IFNγ (top graph) and IL-2 (bottom graph) in GFP+CD4+ cells from each treated group. (D) DNA methylation of TSDR in long-term expanded 17195TCR Tregs with FVIII-2191-2220 plus ODN. To analyze DNA methylation in the TSDR, mock-transduced and 17195TCR-transduced Tregs were expanded with anti-CD3ε antibody or FVIII-2191-2220 in the presence of ODN, as in A. TSDR is an unmethylated CpG-enriched region within the Foxp3 genome of natural Treg. Heat map analysis indicated the methylation status of 9 of the total 11 CpGs. The histogram summarizes the mean percent methylation of 9 TSDR CpGs in 17195TCR-transduced Tregs produced by FVIII 2191-2220/ODN stimulation vs Tregs produced by anti-CD3ε antibody stimulation.
Optimized ex vivo expansion of 17195TCR-Tregs with FVIII-2191-2220 plus oligodeoxynucleotides. (A) FVIII-2191-2220–specific enrichment of transduced Foxp3+Helios+ Tregs via long-term expansion. After a second round of expansion (described in supplemental Figure 2), the CD4 T cells were stained using DR1 tetramers loaded with FVIII-2191-2220 (top contour plots). Expression of Foxp3 and Helios in the gated CD4+GFP+ population were also evaluated by intracellular staining (bottom contour plots). (B) Comparison of FVIII-2191-2220 specificity and Fox3+Helios+ phenotypes of short-term and long-term expanded 17195TCR-Tregs. First-round expanded (Exp) cells (1st Exp) were harvested 8 days after viral transduction, and second-round expanded cells (2nd Exp) were harvested 16 days after transduction. FVIII-2191-2220 specificity was determined by measuring the increase of tetramer+CD4+ cells as in A (right line graph). The Foxp3 and Helios expression levels were measured by intracellular staining, and the Foxp3+Helios+ cell count was obtained by gating of the GFP+CD4+ population (left line graph). Representative data from 1 of 2 experiments are shown. (C) Plasticity of long-term expanded 17195TCR-Tregs and Treg phenotype stability induced by antigen. Second-round expanded 17195TCR-transduced Tregs were rested for 3 days of culture without IL-2 and then restimulated for 4 hours with phorbol myristate acetate and ionomycin in the presence of Golgi-block reagent. Intracellular IFNγ (top contour plots) and IL-2 (bottom contour plots) levels were measured by FACs analysis. The bar graphs on the right indicate the relative expression levels of IFNγ (top graph) and IL-2 (bottom graph) in GFP+CD4+ cells from each treated group. (D) DNA methylation of TSDR in long-term expanded 17195TCR Tregs with FVIII-2191-2220 plus ODN. To analyze DNA methylation in the TSDR, mock-transduced and 17195TCR-transduced Tregs were expanded with anti-CD3ε antibody or FVIII-2191-2220 in the presence of ODN, as in A. TSDR is an unmethylated CpG-enriched region within the Foxp3 genome of natural Treg. Heat map analysis indicated the methylation status of 9 of the total 11 CpGs. The histogram summarizes the mean percent methylation of 9 TSDR CpGs in 17195TCR-transduced Tregs produced by FVIII 2191-2220/ODN stimulation vs Tregs produced by anti-CD3ε antibody stimulation.
Analysis of the cytokine expression profile (Figure 4C) showed that cells undergoing 2 rounds of expansion with FVIII-2191-2220/ODN had the lowest percentage of IFNγ (20%) vs anti-CD3ε–conditioned cells (80%) and FVIII-2191-2220 cells (40%) (top dot plots and graph in Figure 4C). The FVIII-2191-2220–stimulated groups had few IL-2+ cells (bottom dot plots and graph in Figure 4C). These results demonstrate that the FVIII-2191-220/ODN cotreatment protocol stabilizes Foxp3 and Helios expression and prevents the conversion of Tregs to IFNγ-secreting T effectors.
The status of TSDRs in ex vivo expanded Foxp3+Helios+ human and murine natural Tregs is an important marker for stabilization of Tregs.36-38 GFP+ cells were fluorescence-activated cell sorter (FACS) sorted from expanded 17195TCR Tregs, and the methylation status of their TSDR CpGs was analyzed (Figure 4D). FVIII-2191-2220/ODN–conditioned Tregs showed well-preserved demethylation for each of the TSDR CpGs compared with anti-CD3ε–conditioned GFP+ cells (Figure 4D).
Specific engineered T-regulatory cells exhibit FVIII-specific suppression of proliferation and cytokine production
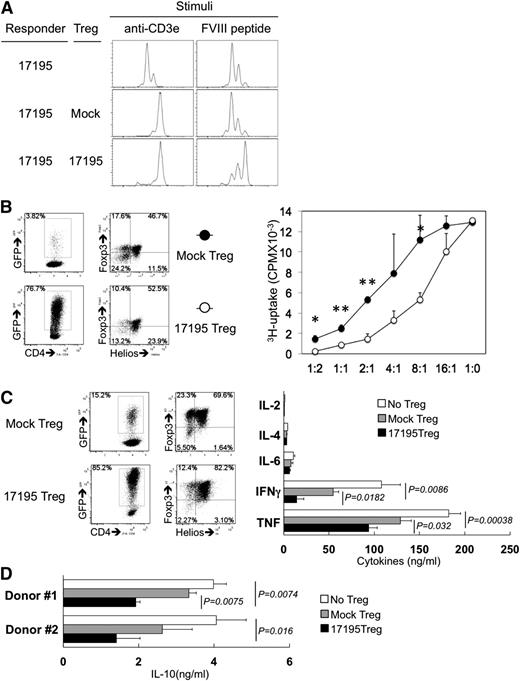
To test for antigen-specific regulatory activity of the engineered Tregs, an in vitro suppression experiment was performed by coculture of transduced T effectors and Tregs. Cells were then cocultured with FVIII-2191-2220 or anti-CD3ε antibody for 4 to 5 days without IL-2. T effectors clearly proliferated in response to FVIII-2191-2220 and anti-CD3ε stimulation in the absence of Tregs (top histograms in Figure 5A). The 17195TCR-transduced Tregs successfully blocked proliferation of T effectors stimulated by either FVIII-2191-2220 or CD3ε (bottom histograms in Figure 5A), whereas mock-transduced Tregs suppressed proliferation only following CD3ε stimulation, as expected (middle histograms in Figure 5A).
FVIII-selective immunosuppression by expanded 17195TCR-Tregs. (A) FVIII-specific immunosuppression by 17195TCR-Tregs. To measure FVIII-2191-2220–specific proliferation of T effectors as FVIII-specific responder cells, 3-week expanded 17195TCR-T effectors were labeled with cell proliferation dye (as in Figure 2A) and cocultured with 3-week expanded mock-transduced Tregs or 17195TCR-transduced Tregs in the presence of anti-CD3ε antibody or FVIII-2191-2220 for 4 days without IL-2. The ratio of responder:Treg:irradiated APCs was 1:2:1. The histogram shows dye dilution indicating proliferation of only the GFP+ cell population. Representative results are shown for 1 of 3 repeat experiments carried out with cells from different donors. (B) FVIII-selective immunosuppression by 17195TCR-Tregs. Mock-transduced and 17195TCR-transduced CD4 T cells were expanded for 3 weeks following the FVIII-2191-2220/ODN protocol as in Figure 4B. GFP+-transduced Tregs were enriched by FACS sorting. The experimental setup was similar to that shown in A but with different Treg:T effector ratios, and the cells were stimulated with rFVIII (0.5 μg/mL) instead of FVIII-2191-2220. Immunosuppression was evaluated using a 3H-thymidine incorporation assay. Dot plots indicate GFP expression in mock-transduced and 17195TCR-transduced Tregs (left) and the expression of Foxp3 and Helios in GFP+ cells (right). Results were analyzed using a 1-tailed t test (*P < .05, **P < .005). Representative data from 1 of 3 experiments are shown. (C) Suppression of FVIII-specific cytokine secretion by 17195TCR-Tregs. Four-week expanded mock-transduced Tregs or 17195TCR-Tregs were mixed with 17195TCR-T effectors (responders) at the indicated ratios with γ-irradiated DR1-PBMCs and rFVIII (0.2 μg/mL) and cultured for 36 hours without IL-2. Cytokines in culture media were measured using a human Th1, Th2, Th17 CBA kit (BD Bioscience). The quality of the mock-transduced and 17195TCR-transduced Tregs was evaluated by measuring their GFP expression (left) and the expression of Foxp3 and Helios in the GFP+ cells (right). Raw mean fluorescence intensity data from the CBA assays were converted to cytokine concentrations according to standard curves generated for each experiment. Data are presented as mean ± standard deviation. (D) IL-10 is suppressed in the presence of 17195TCR-Treg. Production of IL-10 was measured by CBA assay (results using cells from 2 different donors). The experimental protocol was identical that described for C.
FVIII-selective immunosuppression by expanded 17195TCR-Tregs. (A) FVIII-specific immunosuppression by 17195TCR-Tregs. To measure FVIII-2191-2220–specific proliferation of T effectors as FVIII-specific responder cells, 3-week expanded 17195TCR-T effectors were labeled with cell proliferation dye (as in Figure 2A) and cocultured with 3-week expanded mock-transduced Tregs or 17195TCR-transduced Tregs in the presence of anti-CD3ε antibody or FVIII-2191-2220 for 4 days without IL-2. The ratio of responder:Treg:irradiated APCs was 1:2:1. The histogram shows dye dilution indicating proliferation of only the GFP+ cell population. Representative results are shown for 1 of 3 repeat experiments carried out with cells from different donors. (B) FVIII-selective immunosuppression by 17195TCR-Tregs. Mock-transduced and 17195TCR-transduced CD4 T cells were expanded for 3 weeks following the FVIII-2191-2220/ODN protocol as in Figure 4B. GFP+-transduced Tregs were enriched by FACS sorting. The experimental setup was similar to that shown in A but with different Treg:T effector ratios, and the cells were stimulated with rFVIII (0.5 μg/mL) instead of FVIII-2191-2220. Immunosuppression was evaluated using a 3H-thymidine incorporation assay. Dot plots indicate GFP expression in mock-transduced and 17195TCR-transduced Tregs (left) and the expression of Foxp3 and Helios in GFP+ cells (right). Results were analyzed using a 1-tailed t test (*P < .05, **P < .005). Representative data from 1 of 3 experiments are shown. (C) Suppression of FVIII-specific cytokine secretion by 17195TCR-Tregs. Four-week expanded mock-transduced Tregs or 17195TCR-Tregs were mixed with 17195TCR-T effectors (responders) at the indicated ratios with γ-irradiated DR1-PBMCs and rFVIII (0.2 μg/mL) and cultured for 36 hours without IL-2. Cytokines in culture media were measured using a human Th1, Th2, Th17 CBA kit (BD Bioscience). The quality of the mock-transduced and 17195TCR-transduced Tregs was evaluated by measuring their GFP expression (left) and the expression of Foxp3 and Helios in the GFP+ cells (right). Raw mean fluorescence intensity data from the CBA assays were converted to cytokine concentrations according to standard curves generated for each experiment. Data are presented as mean ± standard deviation. (D) IL-10 is suppressed in the presence of 17195TCR-Treg. Production of IL-10 was measured by CBA assay (results using cells from 2 different donors). The experimental protocol was identical that described for C.
Importantly, when T effectors were stimulated by FVIII protein, the 17195TCR-transduced Tregs showed stronger dose-dependent immunosuppression, whereas mock-transduced Tregs were only effective at high Treg:T effector ratios (Figure 5B). FVIII-specific immunosuppression was effective with 17195TCR-transduced Tregs even when T effectors were in excess.
Because Tregs are known to inhibit the production of inflammatory cytokines,9,11,18,39,40 we next determined the levels of IL-2, IFNγ, IL-4, and IL-17 produced by T effectors in the presence of Tregs after 24 hours. At a ratio of 8:1 (T effectors:Tregs), FVIII-specific Tregs significantly suppressed the dominant Th1 cytokine (IFNγ and tumor necrosis factor) production by T effectors compared with mock-transduced Tregs (Figure 5C). Interestingly, FVIII-specific IL-10 production was also reduced in a FVIII-specific Treg-dependent manner (Figure 5D).
Effective suppression of FVIII-specific ASC formation by 17195TCR Tregs in vitro
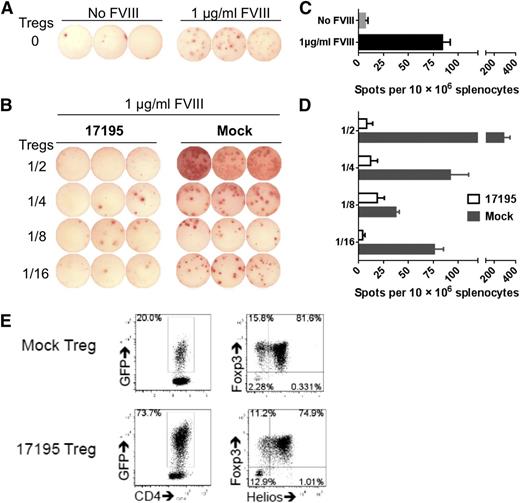
To determine whether human FVIII-specific Tregs could suppress the humoral anti-FVIII response as well, splenocytes from FVIII-primed HLA-DR1-FVIII-KO mice were cocultured with rFVIII plus 17195TCR-Tregs or mock-transduced Tregs at different ratios (Figure 6). FVIII-specific 17195TCR-Tregs dramatically inhibited driving of memory B cell to anti-FVIII antibody formation at all ratios of Tregs:total splenocytes. Mock-transduced Tregs did not suppress the anti-FVIII antibody response, but at high Treg:splenocyte ratios, more ASCs were detected, presumably because cytokines produced from xenogeneic recognition. The almost complete suppression of ASC formation by 17195TCR-Tregs suggests involvement of both FVIII-specific and nonspecific xenogeneic responses (Figure 6).
In vitro suppression of anti-FVIII antibody production by engineered FVIII-specific human Tregs. Pooled splenocytes from 2 immunized DR1/E16-FVIII-KO mice were used as B-cell responders. In a T25 flask, 1 × 107 splenocytes were cocultured with 17195TCR-transduced or mock-transduced Tregs at different ratios in the absence/presence of 1 µg/mL rFVIII. After 6 days in culture, FVIII-specific ASCs were detected using an ELISPOT assay as described in “Methods.” (A) FVIII-specific ASCs detected in splenocyte cultures that contained no TCR- or mock-transduced Tregs. (B) FVIII-specific ASCs detected in splenocytes cocultured with various ratios of 17195TCR-transduced or mock-transduced Tregs. Coculture with 17195TCR-Tregs profoundly inhibited anti-FVIII ASC formation, even with responder to suppressor ratios as low as 1:0.0625 (1/16). The histograms in C and D summarize the ELISPOT data in A and B, respectively. Data are presented as mean ± standard error of the mean. (E) The quality of the Tregs used in these experiments was evaluated by measuring their GFP expression (left) and the expression of Foxp3 and Helios in the GFP+ cells (right).
In vitro suppression of anti-FVIII antibody production by engineered FVIII-specific human Tregs. Pooled splenocytes from 2 immunized DR1/E16-FVIII-KO mice were used as B-cell responders. In a T25 flask, 1 × 107 splenocytes were cocultured with 17195TCR-transduced or mock-transduced Tregs at different ratios in the absence/presence of 1 µg/mL rFVIII. After 6 days in culture, FVIII-specific ASCs were detected using an ELISPOT assay as described in “Methods.” (A) FVIII-specific ASCs detected in splenocyte cultures that contained no TCR- or mock-transduced Tregs. (B) FVIII-specific ASCs detected in splenocytes cocultured with various ratios of 17195TCR-transduced or mock-transduced Tregs. Coculture with 17195TCR-Tregs profoundly inhibited anti-FVIII ASC formation, even with responder to suppressor ratios as low as 1:0.0625 (1/16). The histograms in C and D summarize the ELISPOT data in A and B, respectively. Data are presented as mean ± standard error of the mean. (E) The quality of the Tregs used in these experiments was evaluated by measuring their GFP expression (left) and the expression of Foxp3 and Helios in the GFP+ cells (right).
Discussion
Expansion of human FoxP3+ Tregs has been proposed for the treatment of undesirable autoimmune responses, as well as to modulate immune responses to therapeutic proteins. Expanded polyclonal FoxP3+ Tregs may recognize multiple antigens; therefore, nonspecific immunosuppression by these cells could produce undesirable side effects. In the present study, antigen-specific human Tregs were prepared by transducing expanded polyclonal Tregs with a TCR expressed from a well-characterized hemophilia A subject’s effector T-cell clone that responded strongly to FVIII-2191-2220 presented by HLA-DRB1*01:01 (DR1).19,20 The results presented herein demonstrate that retroviral transduction of T effectors with this FVIII-specific TCR provided an expandable source of human Tregs (or T effectors) for potential therapeutic translation. These transduced cells respond to FVIII-2191-2220 by proliferation and cytokine secretion (T effectors) or upregulation of Treg markers (Tregs). Importantly, the 17195TCR-Tregs suppressed proliferation and cytokine secretion of expanded 17195TCR T effectors (Figure 5) and the parent clone (Y.C.K. and D.W.C., unpublished data, 2014). The 17195TCR-Tregs also suppressed anti-FVIII antibody production in vitro, thus supporting potential translation to modulating inhibitor responses in vivo.
Interestingly, 17195TCR T effectors secreted IL-2, IL-4, IL-6, IL-10, tumor necrosis factor, and IFNγ, whereas the expanded parent T-effector clone secreted only IL-4. This is not surprising, because the expanded T cells subjected to TCR transduction were polyclonal and reflected the phenotypes of the normal donor’s total CD4 population. Hence, we speculate that the 17195TCR acted as a trigger to turn on the transcriptional programs of the transduced cells. Further experiments stimulating cells with different doses of FVIII-2191-2220 and transducing with TCR from other FVIII-specific clones (eg, Th1/Th17 clones20,41 ) are in progress to test whether the TCR dictates the cytokine repertoire.
In contrast to transduced T effectors, the 17195TCR-Tregs did not secrete IL-2 or IFNγ; they upregulated Treg markers and suppressed both T-effector and humoral responses to FVIII. Moreover, FVIII-2191-2220–specific expansion with ODN selectively enriched the TCR-transduced Tregs and stabilized their Treg-unique characteristics, preventing conversion to T effectors. Thus, cotreatment with specific antigen plus ODN is an effective means of preparing stable TCR-transduced Tregs for antigen-specific, suppressive infusion therapy.
On FVIII-2191-2220 stimulation, GFP+ 17195TCR-Tregs expanded until they accounted for 60% to 78% of the CD4 T cells cultured from 2 different donors (Figure 2). GFP− (primarily nontransduced) CD4 T cells also proliferated to a certain degree in response to pOVA or FVIII-2191-2220; this proliferation varied greatly for cells from different donors (Figure 2A). We suggest that the variable nonspecific proliferation was due to an HLA mismatch between the donor’s endogenous TCR and the DR1 of irradiated APCs, because the normal blood donors were not prescreened for HLA haplotype. In contrast, autologous APCs and T cells would be used in eventual clinical applications of this type of therapy.
It has been postulated that Tregs possess different repertoires of TCRs with respect to specificity and/or binding affinity and that these properties may be important for the maintenance of their unique phenotype and functional stability.19,20,42-44 Our data show that transduction of polyclonal CD4 Tregs with the 17195TCR from a T-effector (TH2) clone produced Tregs specific for FVIII-2191-2220 and promoted the enrichment of transduced Tregs for long-term in vitro expansion (Figures 3 and 4). Indeed, these expanded cells maintained the key Treg markers Foxp3 and Helios (Figure 4A). This was in contrast to Tregs stimulated with anti-CD3ε, which lost FoxP3 and/or Helios expression over time. Thus, transduction with an antigen-specific TCRs together with ODN treatment promoted functional stability of expanded antigen-specific Tregs. Presumably coengagement of FVIII-specific TCRs and APC-mediated CD4 signaling resulted in maintenance of Foxp3 and Helios. Interestingly, in a recent phase 2 clinical trial for rheumatoid arthritis, a humanized CD4-specific monoclonal antibody (tregalizumab; BT-061) enhanced Treg-mediated suppression of allo-specific CD8 proliferation via binding to domain 2 of the CD4 molecule.45
IL-10 is a key immunosuppressive cytokine produced by type 1 Tregs.23,24,46,47 Expression of IL-10 by thymic Tregs and human CD4+CD25+ T cells with regulatory cell phenotypes has been reported.48-50 However, there is no correlation between Foxp3 and IL-10 expression, and the role played by IL-10 in immunosuppression by human Foxp3+ cells remains controversial. We found that 17195TCR Foxp3+ Tregs expressed membrane-bound transforming growth factor β (LAP; supplemental Figure 1C) but not IL-10 (data not shown). Moreover, 17195TCR-Tregs suppressed IL-10 secretion by FVIII-stimulated 17195TCR-T effectors (Figure 5D), clearly suggesting that IL-10 is not a critical factor for immunosuppression by 17195TCR-Tregs. Similarly, IL-10–deficient Tregs were shown to suppress antibody formation against factor IX.50 The precise mechanism of suppression requires further study.
Suppression of antigen-specific T-effector proliferation has been shown recently using in vitro generated antigen-specific human Tregs.9,11,51 Whether this suppressive activity could be extended to a humoral response was unknown. Herein, we provide the first evidence demonstrating this function of human Tregs, which suppressed anti-FVIII antibody secretion in vitro. Assays that used humanized HLA-DR1-FVIII-KO mice plus human mock-transduced Tregs led to xenogeneic stimulation (Figure 6B,D). Nonetheless, complete inhibition of anti-FVIII ASC formation by 17195TCR-Tregs incubated at various ratios with responder splenocytes clearly shows that FVIII-2191-2220–specific human Tregs could produce effective suppression of humoral immune responses to FVIII. Future studies will determine whether these FVIII-specific Tregs blocked T-cell help or directly targeted FVIII-specific memory B cells. In fact, we suggest that they could be targeting T-helper cells or APCs, with secondary effects on B cells.
Importantly, the present study provides in vitro evidence that TCR-engineered antigen-specific human Tregs effectively suppressed not only a FVIII-specific T-effector response, but also anti-FVIII ASC formation from HLA-transgenic splenocyte cultures. The extension of this approach to generate FVIII-specific Tregs in vivo using HLA-transgenic humanized mice will be an important step toward future translation, in which subjects’ PBMC-Tregs could be expanded and transduced to engender clinically effective Tregs.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Arthur Thompson (Puget Sound Blood Center Hemophilia Clinic) for enrolling hemophilia patients. The authors also thank the blood donors.
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institue grant RO1-HL061883-15 (to D.W.S.), funding from Bayer and CSL Behring (to K.P.P.), and intramural support from the National Institute of Allergy and Infectious Diseases (to E.M.S.).
Authorship
Contribution: Y.C.K., A.-H.Z., E.M.S., K.P.P., and D.W.S. designed the experiments; Y.C.K., A.-H.Z., Y.S., S.A.R., R.A.E., and R.J.R. did the work; and Y.C.K., A.-H.Z., K.P.P., E.M.S., and D.W.S. wrote the paper.
Conflict-of-interest disclosure: Y.C.K. and D.W.S. have a provisional patent filing via the Henry Jackson Foundation on “Design and use of specific regulatory T cells to induce immune tolerance.” The remaining authors declare no competing financial interests.
Correspondence: David W. Scott, Department of Medicine (MED), Uniformed Services University of Health Sciences, 4301 Jones Bridge Rd, Bethesda, MD, 20814; e-mail: david.scott@usuhs.edu.