Key Points
TLR4–MyD88–NF-κB is required for HSPC emergence in zebrafish and mouse embryos.
Notch functions downstream of inflammatory signaling to regulate HSPC emergence.
Abstract
Inflammatory signaling has been shown to be essential for stress hematopoiesis in adult bone marrow, either through increasing proliferation or by directing differentiation of hematopoietic stem and progenitor cells (HSPCs) toward myeloid or lymphoid lineages. However, its role in embryonic normal hematopoiesis has been unknown. Here, we demonstrate that in both zebrafish and mouse embryos, inflammatory signaling is necessary and sufficient for HSPC emergence, in the absence of infection or pathological inflammation. Mechanistically, inflammatory signaling regulates hemogenic endothelium-derived HSPC development through a conserved Toll-like receptor 4 (TLR4)–nuclear factor κ-light-chain enhancer of activated B core (NF-κB) signaling, which then promotes Notch activity, a well-known signal required for HSPC specification in vertebrates. Our findings establish a previously unrecognized link between inflammatory signaling and HSPC emergence, and provide new insights into regenerative medicine and novel therapies to treat innate immune-related diseases.
Introduction
Hematopoietic stem cells (HSCs) have the abilities of self-renewal and multilineage differentiation to maintain the supply of all mature blood cells for the lifetime. In vertebrate embryos, the earliest definitive hematopoietic stem and progenitor cells (HSPCs) are originated from a group of specialized endothelial cells, hemogenic endothelium (HE),1-4 through the endothelial-to-hematopoietic transition (EHT) process.5-7 In vitro generation and/or expansion of functional and transplantable bona fide HSCs with self-renewal and multilineage potential hold great promise in regenerative medicine. However, this has not been achieved yet, at least partially because a complex interplay between cell-extrinsic cues and cell-intrinsic regulatory signaling governing HE formation and HSPC specification remains incompletely understood.
Inflammatory signaling is generally thought to be active in the presence of infection and inflammation. In response to infection stimuli, innate immune cell–secreted inflammatory cytokines directly or indirectly act on myeloid precursors to differentiate and expand to meet the acute demand of effector myeloid cells. However, recent reports also showed that inflammatory cytokine can also directly act on HSPCs to instruct their fate, either increased proliferation or directed differentiation toward myeloid8 or lymphoid9 lineages, at the expense of HSC self-renewal and/or maintenance in the bone marrow.10,11 Upon Toll-like receptor (TLR) stimulation, nuclear factor κ-light-chain enhancer of activated B (NF-κB), a central player in innate immune response, integrates multiple inflammatory signaling pathways including tumor necrosis factor α (TNFα) and granulocyte colony-stimulating factor (G-CSF) pathways. NF-κB has been shown to regulate both innate and adaptive immune systems including myeloid cells and B and T cells in homeostasis and differentiation. Interestingly, the role of inflammatory signals during HSPC emergence in both zebrafish and mouse embryos has been described very recently.12-14
Inflammatory signaling pathways are well conserved in vertebrates. Previous studies in mice showed that cytokines including interleukin-1 (IL-1) and IL-3 are involved in the embryonic development of HSPCs in the aorta-gonad-mesonephros (AGM) region.15,16 We previously reported that a hematopoietic microRNA, miR-142a-3p, regulates the formation and differentiation of HSPCs by regulating the inflammatory signaling cascade irf7-gcsfr,17 indicating a possible role of inflammatory signaling in HSPC emergence. In this study, we demonstrated that in both zebrafish and mouse embryos, inflammatory signaling is required for HSPC specification. Ablation of the TLR4–myeloid differentiation primary response 88 (MyD88) pathway and inflammatory cytokine signaling pathways including TNFα and G-CSF, decreased the population of the earliest HSPCs through downregulation of their core effector NF-κB, which in turn reduced the Notch activity in endothelial cells. Attenuation of Notch activity in endothelial cells then compromised the HE and the following HSPC emergence via the EHT process. Therefore, we discovered, for the first time, an unexpected essential role of proinflammatory signaling in HSPC emergence through a conserved NF-κB–Notch pathway.
Methods
Fish strains and embryos
Zebrafish strains including Tubingen, cmyb:green fluorescent protein (GFP),18 kdrl:mCherry,5 fli1a:enhanced GFP (EGFP),19 heat shock protein (HSP)-il1b-EGFP,20 CD41:GFP,21 mpo:GFP,22 lyz:DsRed,23 myd88 mutants,24 tp1:DsRed,25 and runx1:en-GFP (P.Z. and F.L., manuscript in preparation) were raised and maintained in system water at 28.5°C. The HSP-il1b-EGFP embryos were heat-shocked from 20 hours post fertilization (hpf) for 30 minutes at 42°C. The embryos were obtained by natural spawning. This study was approved by the Ethical Review Committee in the Institute of Zoology, Chinese Academy of Sciences, China.
Morpholinos and microinjection
Antisense Morpholinos (MOs) used in this study (including runx1 MO,7 tlr4bb MO,26 myd88 MO,27 tnfr2 MO,28 gcsfr MO,29 ikbaa MO, pu.1 MO,30 irf8 MO,31 cebp1 MO,32 mismatch MOs (mis MOs), and standard control MO) were purchased from GeneTools and dissolved with distilled H2O into 1 mM as stock solutions. MOs (4 ng for runx1 MO; 1 ng for tlr4bb MO and tlr4bb mis MO; 2 ng for ikbaa MO and ikbaa mis MO; 4 ng for myd88 MO and myd88 mis MO; 3 ng for tnfr2 MO and tnfr2 mis MO; 8 ng for gcsfr MO; 6 ng for pu.1 MO; 6.4 ng for irf8 MO; 4 ng for cebp1 MO and 8 ng for control MO) were injected into 1-cell-stage zebrafish embryos at the yolk/blastomere boundary. The MO sequences are listed in supplemental Table 1 (available on the Blood Web site). For overexpression of dominant-negative ikbaa (dnikbaa) or NICD specifically in the blood vessel region, the zebrafish ikbaa lacking the first 117 N-terminal nucleotides or full-length coding sequences of zebrafish NICD were cloned into pDONR221 vector by attB and attP recombination reaction (Gateway System) to generate the entry clones, and then subcloned into a pDestTol2pA2 vector with P5E-fli1ep (fli1 enhancer/promoter)33 and p3E-V2AEGFP-pA by attL and attR recombination reaction (MultiSite Gateway Technology; Invitrogen). The pEGFP-N1-ikbaa fusion construct containing the ikbaa MO recognition site was coinjected with both ikbaa MO and ikbaa mis MO.
Whole-mount in situ hybridization
Whole-mount in situ hybridization (WISH) for embryos was performed using the ZF-A4 in situ hybridization machine (Zfand) with probes including runx1, cmyb, rag1, foxn1, ephrinB2, gata1, scl, and kdrl.34-36
RNA-seq and GO analysis
The trunk region of Tg(runx1:en-GFP) embryos at 26 to 28 hpf was dissected to sort the GFP+ cells for RNA-Seq. The runx1 GFP+ cells were sorted by AriaI (BD Biosciences). Total RNA from the sorted cells was purified and subjected to RNA-Sequencing (RNA-Seq). Beijing Genomics Institute provided the deep-sequencing service and subsequent gene ontology (GO) analysis.
Chemical treatment
Embryos were treated with dimethylsulfoxide (AMRESCO) or JSH-23 (300 μM; Selleck) from 10-somite stage to 24 hpf or 36 hpf.
Quantitative RT-PCR
Total RNA from trunk region of zebrafish embryos and the AGM regions of tlr4+/− and tlr4−/− mouse embryos at embryonic day 10.5 (E10.5) were extracted by TRNzol Reagent (Tiangen) and were reversely transcribed using reverse transcriptase (RT). The complementary DNA was diluted 5 times as the templates. Quantitative polymerase chain reaction (qPCR) was performed with GoTaq qPCR Master Mix (Promega) using the Bio-Rad CFX96 Real-Time PCR system. The PCR primers used are listed in supplemental Table 2.
Western blot
The trunk regions of zebrafish embryos were dissected for the protein extraction. The nuclear protein was extracted using the Nucl-Cyto-Mem Preparation kit (Applygen). The whole-cell protein extraction and western blot were performed as previously reported34 using anti-p65 (1:800; Cell Signaling Technology), anti-pIκBα (1:800; Cell Signaling Technology), anti-LaminB1 (1:4000; Abcam), and anti-Tubulin β (1:1000; Proteintech) antibodies.
TUNEL assay and BrdU labeling
TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling) and BrdU (5-bromo-2′-deoxyuridine) assays were performed as described previously.37
Tail amputation assay
The tail fin of Tg(mpo:GFP) embryos was cut at 24 hpf as described previously.20
Confocal microscopy
Confocal images were acquired as previously reported38 with a Nikon confocal A1 laser microscope, and 3-dimensional projections were generated using Nikon confocal software. The supplemental Videos from 33 to 54 hpf were generated with PerkinElmer spinning disk confocal microscopy38 and edited by ImageJ.
Zebrafish embryo dissociation and FACS
Embryo dissociation and fluorescence-activated cell sorter (FACS) were performed as described previously.39 The cmyb−kdrl+ and cmyb+kdrl+ cells were separately collected from cmyb:GFP/kdrl:mCherry transgenic embryos and lyz+ cells from lyz:DsRed transgenic embryos using AriaI (BD Biosciences).
Inhibition of transcription using dead-Cas9-KRAB
The plasmid with the full length of a human codon-optimized, catalytically inactive version of Cas9 (dCas9), fused with Krüppel-associated box (KRAB) repressor domain, was kindly provided by Liu Dong (Peking University). Capped dCas9-KRAB messenger RNA was synthesized using the mMessage mMachine T7 kit (Ambion) and purified with an RNA purification kit (Tiangen). The guide RNAs (gRNAs) for zebrafish genes were designed according to the Web site (http://zifit.partners.org/ZiFiT/), synthesized by T7 RNA polymerase, and purified by the mirVanaTM miRNA Isolation kit (Ambion) in vitro. Five different gRNAs targeting the downstream transcription start site at the antisense strand were designed for each gene to inhibit the transcription elongation. Cas9 messenger RNA (200 ng/μL) and 5 gRNAs (100 ng/μL per gRNA) were coinjected into 1-cell stage wild-type embryos. Injected embryos were incubated at 28.5°C and collected for quantitative RT-PCR (qRT-PCR), confocal imaging, and WISH at different stages.
Immunofluorescence
An immunofluorescence assay for mouse embryos was performed as previously reported.34 tlr4+/− and tlr4−/− embryos at E10.5 were fixed with 4% paraformaldehyde in phosphate-buffered saline for 6 to 8 hours at 4°C. The slides were blocked with 5% bovine serum albumin for 1 hour at room temperature and incubated with Runx1 (Abcam) antibody diluted in 1% bovine serum albumin overnight at 4°C. After being washed 3 times in phosphate-buffered saline with Tween 20, the slides were incubated with anti-rabbit-immunoglobulin-fluorescein for 1 hour at room temperature. The sections were counterstained with 4,6 diamidino-2-phenylindole and images were acquired by Nikon confocal A1.
Mouse embryo dissociation and FACS
E10.5 AGMs (36-40 somite pairs) were dissociated by collagenase. FACS was performed as previously reported34 using MoFlo XDP. The CD31+CD41−CD45−TER119− cells of the indicated numbers from tlr4+/− or tlr4−/− were cultured on mouse OP9 stromal cells and supplemented with hematopoietic cytokines (50 ng/mL stem cell factor, 50 ng/mL IL3, 20 ng/mL FLT3 ligand). After being cultured for 4 days, semiadherent cells were carefully harvested for flow cytometry.
CFC assay
A colony-forming cell (CFC) assay was performed as previously reported.34 The experiment was repeated in triplicate.
Flow cytometric analysis
Semiadherent cells were carefully harvested. Then, antibody staining was performed as described previously,34 using antibodies specific to C-Kit–phycoerythrin [PE]–CY7, CD45-PE, CD45.2-PE-CY7, Gr1-PE, CD3–fluorescein isothiocyanate, B220, CD4–fluorescein isothiocyanate, and CD8a-PE (eBioscience).
HSC transplantation assay
Eight-week-old male CD45.1 mice were exposed to a split dose of 9 Gy x-ray irradiation. The 2 embryo equivalent cells from tlr4+/− (CD45.2) or tlr4−/− (CD45.2) AGM regions were injected into irradiated adult recipients via tail vein, together with 20 000 CD45.1 bone marrow cells. Bone marrow, spleen, and thymus of recipients were collected at the indicated time points. Greater than 10% CD45.2-positive cells as determined by flow cytometry was considered successful reconstitution.
Statistical analysis
All experiments were performed at least 3 times. Data were given as the mean ± standard error of the mean (SEM). The Student t test was used for statistical comparisons.
Results
Expression of inflammatory signaling in HE and HSPCs
Previous studies in zebrafish and mouse embryos15-17 implicate the potential involvement of inflammatory signaling in HSPC emergence. To further test this possibility, we examined the expression profiling of the earliest HSPC population in zebrafish embryos. A mouse runx1 intronic enhancer was used to drive GFP in zebrafish embryos which can mimic zebrafish runx1 endogenous expression.40 The newly generated runx1:en-GFP line can faithfully mark the earliest HSPCs in zebrafish embryos (supplemental Figure 1). We first performed RNA-Seq with hemogenic endothelial cells by isolating runx1+ GFP cells (P.Z. and F.L., manuscript in preparation) using FACS at 26 to 28 hpf, at which stage the hemogenic endothelial cells were just specified and underwent the EHT in the ventral wall of the dorsal aorta.5,7,41 Interestingly, GO analysis showed that inflammatory signaling was highly enriched among the upregulated genes in runx1+ cells (Figure 1A-B). The qRT-PCR result confirmed the RNA-Seq data (Figure 1C). Notably, the sorted hemogenic endothelial cells expressed a high level of inflammatory signaling components including cytokine receptors and NF-κB3 (p65); the myeloid cells were used as the positive control (Figure 1D). Together, these data strongly suggest that inflammatory signaling transduced by HE and HSPCs might be involved in definitive hematopoiesis in zebrafish.
Inflammatory signaling is enriched in hemogenic endothelium. (A) runx1 GFP+ cells from Tg(runx1:en-GFP) transgenic embryos at 26 to 28 hpf were sorted for deep sequencing. The GO analysis of the sequencing data identified that inflammatory signaling was enriched in runx1+ cells. (B) Heat map analysis showed comparison of gene expression between runx1 GFP+ and runx1 GFP− cells at 26 to 28 hpf. Multiple inflammatory signaling genes were found enriched in GFP+ cells. (C) cmyb−kdrl+ and cmyb+kdrl+ cells from Tg(cmyb:GFP/kdrl:mCherry) embryos were sorted at 26 to 28 hpf, and lyz+ cells from Tg(lyz:DsRed) embryos were sorted at 48 hpf. (D) qRT-PCR results showed that a panel of inflammatory signaling genes was enriched in cmyb+kdrl+ hemogenic endothelial cells. Each bar represents the mean ± SEM of 3 independent samples. *P < .05, **P < .01.
Inflammatory signaling is enriched in hemogenic endothelium. (A) runx1 GFP+ cells from Tg(runx1:en-GFP) transgenic embryos at 26 to 28 hpf were sorted for deep sequencing. The GO analysis of the sequencing data identified that inflammatory signaling was enriched in runx1+ cells. (B) Heat map analysis showed comparison of gene expression between runx1 GFP+ and runx1 GFP− cells at 26 to 28 hpf. Multiple inflammatory signaling genes were found enriched in GFP+ cells. (C) cmyb−kdrl+ and cmyb+kdrl+ cells from Tg(cmyb:GFP/kdrl:mCherry) embryos were sorted at 26 to 28 hpf, and lyz+ cells from Tg(lyz:DsRed) embryos were sorted at 48 hpf. (D) qRT-PCR results showed that a panel of inflammatory signaling genes was enriched in cmyb+kdrl+ hemogenic endothelial cells. Each bar represents the mean ± SEM of 3 independent samples. *P < .05, **P < .01.
Loss of function of TLR4–MyD88–NF-κB leads to HSPC defects
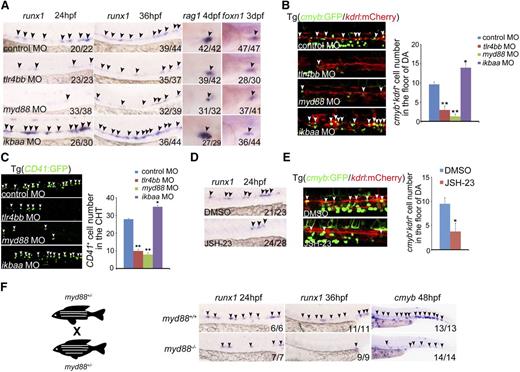
To investigate whether proinflammatory signaling is required for HSPC specification, we first performed loss-of-function experiments for the core of inflammatory signaling players, the TLR4–MyD88–NF-κB axis.42 The AGM expression of runx1, which is a well-known HSPC marker in zebrafish and mammals, was drastically reduced in tlr4bb or myd88 morphants (Figure 2A, supplemental Figure 2A-B). Additionally, the number of cmyb:GFP/kdrl:mCherry double-positive HSPCs5 in the AGM region was significantly reduced in tlr4bb- or myd88-deficient embryos, compared with control embryos (Figure 2B, supplemental Figure 2C). The number of CD41+ cells, that is, the hematopoietic progenitor cells, was also significantly reduced in deficient embryos at 48 hpf (Figure 2C). A well-known readout for HSPC function in zebrafish is its capability to differentiate into T lymphoid. We thus examined expression of T-cell marker rag1. WISH showed that rag1 expression was remarkably reduced in tlr4bb or myd88 morphants, whereas the expression of thymic epithelial cell marker foxn1 was normal (Figure 2A), further supporting that T-cell defects in these deficient embryos resulted from early HSPC defects. We further used the newly developed clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 technology to manipulate expression of these signaling components. Guide RNAs targeting gene-specific promoters, together with a dead form of Cas9 fused with transcriptional repressor KRAB43 (supplemental Figure 3A), were coinjected into zebrafish embryos at the 1-cell stage to achieve conditional repression of genes of interest. Transcriptional repression of TLR4-MyD88 (supplemental Figure 3B) remarkably reduced runx1 expression and the number of cmyb+kdrl+ cells in the AGM region and CD41+ cells in the caudal hematopoietic tissue (CHT) region (supplemental Figure 3C-E).
TLR4–MYD88–NF-κB signaling is essential for HSPC emergence. (A) runx1 and rag1 expression was decreased in tlr4bb and myd88 morphants, but increased in ikbaa morphants; thymic epithelial cell marker foxn1 expression was normal in all of these morphants. Black arrowheads mark expression of runx1 in the AGM region at 24 hpf and 36 hpf, rag1 in the thymus at 4 days post fertilization (dpf), and foxn1 in the thymus at 3 dpf. (B) The number of cmyb+kdrl+ cells in Tg(cmyb:GFP/kdrl:mCherry) embryos was decreased in tlr4bb and myd88 morphants and increased in ikbaa morphants. White arrowheads mark cmyb+kdrl+ cells in the AGM region at 36 hpf. Right panel, The quantification. Each bar represents the mean ± SEM of 3 independent samples. Each sample was composed of at least 5 embryos. *P < .05, **P < .01. (C) The hematopoietic cells in Tg(CD41:GFP) embryos were reduced in tlr4bb or myd88 morphants and increased in ikbaa morphants. White arrowheads mark the CD41 GFP+ cells in the CHT region at 48 hpf. Right panel, The quantification of CD41+ cells. Each bar represents the mean ± SEM of 3 independent samples. Each sample was composed of at least 5 embryos. *P < .05, **P < .01. (D) runx1 expression was decreased in JSH-23–treated embryos at 36 hpf. Black arrowheads mark expression of runx1 in the AGM region. (E) The population of cmyb+kdrl+ cells in Tg(cmyb:GFP/kdrl:mCherry) embryos was decreased in JSH-23–treated embryos. White arrowheads mark cmyb+kdrl+ cells in the AGM region at 36 hpf. Right panel, Quantification of cmyb+kdrl+ cells. Data are presented as mean ± SEM of 3 independent samples. Each sample was composed of at least 5 embryos. *P < .05, **P < .01. (F) The cartoon showed the incross between myd88+/− embryos. Right panels, The decreased expression of runx1 and cmyb expression in myd88−/− embryos. Black arrowheads mark expression of runx1 in the AGM region at 24 hpf and 36 hpf, and cmyb expression in the CHT region at 48 hpf.
TLR4–MYD88–NF-κB signaling is essential for HSPC emergence. (A) runx1 and rag1 expression was decreased in tlr4bb and myd88 morphants, but increased in ikbaa morphants; thymic epithelial cell marker foxn1 expression was normal in all of these morphants. Black arrowheads mark expression of runx1 in the AGM region at 24 hpf and 36 hpf, rag1 in the thymus at 4 days post fertilization (dpf), and foxn1 in the thymus at 3 dpf. (B) The number of cmyb+kdrl+ cells in Tg(cmyb:GFP/kdrl:mCherry) embryos was decreased in tlr4bb and myd88 morphants and increased in ikbaa morphants. White arrowheads mark cmyb+kdrl+ cells in the AGM region at 36 hpf. Right panel, The quantification. Each bar represents the mean ± SEM of 3 independent samples. Each sample was composed of at least 5 embryos. *P < .05, **P < .01. (C) The hematopoietic cells in Tg(CD41:GFP) embryos were reduced in tlr4bb or myd88 morphants and increased in ikbaa morphants. White arrowheads mark the CD41 GFP+ cells in the CHT region at 48 hpf. Right panel, The quantification of CD41+ cells. Each bar represents the mean ± SEM of 3 independent samples. Each sample was composed of at least 5 embryos. *P < .05, **P < .01. (D) runx1 expression was decreased in JSH-23–treated embryos at 36 hpf. Black arrowheads mark expression of runx1 in the AGM region. (E) The population of cmyb+kdrl+ cells in Tg(cmyb:GFP/kdrl:mCherry) embryos was decreased in JSH-23–treated embryos. White arrowheads mark cmyb+kdrl+ cells in the AGM region at 36 hpf. Right panel, Quantification of cmyb+kdrl+ cells. Data are presented as mean ± SEM of 3 independent samples. Each sample was composed of at least 5 embryos. *P < .05, **P < .01. (F) The cartoon showed the incross between myd88+/− embryos. Right panels, The decreased expression of runx1 and cmyb expression in myd88−/− embryos. Black arrowheads mark expression of runx1 in the AGM region at 24 hpf and 36 hpf, and cmyb expression in the CHT region at 48 hpf.
To further support that inflammatory signaling is required for HSPC development, we treated embryos with JSH-23, a commonly used inhibitor for the nuclear translocation of NF-κB.44 First, the efficiency of JSH-23 treatment was validated in zebrafish embryos. The nuclear p65 level was decreased in JSH-23–treated embryos (supplemental Figure 4A). Furthermore, the tail amputation assay also showed that the mpo+ myeloid cell number was decreased at the wound region when treated with JSH-23 (supplemental Figure 4B), suggesting that the injury-induced inflammation response was attenuated by JSH-23 treatment. Then, similar HSPC defects were observed in JSH-23–treated embryos (Figure 2D-E). Given that we demonstrated that inflammatory signaling was necessary for HSPC emergence, we wondered whether it was also sufficient to promote this process. Knockdown of IκBaa, the NF-κB inhibitory protein that normally binds to NF-κB and prevents the translocation of NF-κB to the nucleus,45 increased the number of HSPCs (Figure 2A-B). Consistently, the decreased expression of HSPC markers was also observed in the myd88 mutants (Figure 2F).
To pinpoint the stage of HSPC development at which inflammatory signaling was required, we performed time-lapse live confocal imaging with cmyb:GFP/kdrl:mCherry double transgenic lines. The number of cmyb+kdrl+ cells was reduced at the onset of HSPC emergence at 30 to 36 hpf in inflammatory signaling-deficient embryos (supplemental Videos 1-3). In addition, runx1 expression was reduced at 24 hpf, agreeing well with a defect in HE specification (Figure 2A,F). Taken together, these data demonstrated that proinflammatory signaling is required for HE-derived HSPC emergence in zebrafish embryos.
Endothelial cell–derived NF-κB mediates the TLR regulation of HSPC emergence
We went further to determine whether TLR4–MyD88–NF-κB regulation of HSPC development is mediated by NF-κB derived from endothelial cells rather than other sources. First, the western blotting result showed that the nuclear localization of NF-κB was indeed decreased in the tlr4bb or myd88 morphants, whereas increased in ikbaa morphants (supplemental Figure 4C). Then, we generated a gateway construct driven by the fli1a promoter to express dominant-negative IκBaa (dnikbaa), which lacks the first 117 N-terminal nucleotides,46 fused with GFP. The truncated IκBaa cannot be phosphorylated by the IκB kinase, and therefore prevents the nucleus translocation of NF-κB.14 The endothelial GFP signals indicated the specific expression of dnikbaa (Figure 3A), and the phosphorylated level of IκBa was decreased in these GFP+ embryos (Figure 3A). This endothelial-specific attenuation of NF-κB signaling mimicked the HSPC defects observed in tlr4bb or myd88 morphants (Figure 3B-C). Importantly, coinjection of ikbaa MOs efficiently restored the decreased population of HSPCs and il1b expression in tlr4bb or myd88 morphants (Figure 3D-F, supplemental Figure 4D). Furthermore, overexpression of il1b by heat shock treatment of a transgenic line with heat-shock–inducible il1b embryos [Tg(HSP-il1b-EGFP),20 from 20 hpf, when the artery-vein specification has been accomplished] can also rescue the HSPC defects in the absence of tlr4bb or myd88 (Figure 3G). Together, these data demonstrated that endothelial TLR4–MyD88–NF-κB signaling is both necessary and sufficient for HSPCs in zebrafish.
NF-κB derived from endothelial cells mediates the TLR regulation of HSPC emergence. (A) Top panel, Endothelial dnIκBaa-GFP+ signals. Red arrowheads mark GFP signal in the endothelial cells. Bottom panels, The decreased expression of p-IKBa in dnikbaa− and dnikbaa+ embryos. (B-C) Overexpression of dnikbaa in endothelial cells led to decreased expression of runx1. (D) Decreased expression of runx1 at 36 hpf in tlr4bb or myd88 morphants was rescued by coinjection of ikbaa MO. (E) The decreased population of cmyb+kdrl+ cells in Tg(cmyb:GFP/kdrl:mCherry) embryos injected with tlr4bb or myd88 MO, was rescued by coinjection with ikbaa MO. White arrowheads mark cmyb+kdrl+ cells in the AGM region at 36 hpf. (F) Quantification of cmyb+kdrl+ cells. Data are presented as mean ± SEM of 3 independent samples. Each sample was composed of at least 5 embryos. *P < .05, **P < .01. (G) Decreased expression of runx1 at 36 hpf in tlr4bb or myd88 morphants was rescued by the overexpression of il1b from 20 hpf.
NF-κB derived from endothelial cells mediates the TLR regulation of HSPC emergence. (A) Top panel, Endothelial dnIκBaa-GFP+ signals. Red arrowheads mark GFP signal in the endothelial cells. Bottom panels, The decreased expression of p-IKBa in dnikbaa− and dnikbaa+ embryos. (B-C) Overexpression of dnikbaa in endothelial cells led to decreased expression of runx1. (D) Decreased expression of runx1 at 36 hpf in tlr4bb or myd88 morphants was rescued by coinjection of ikbaa MO. (E) The decreased population of cmyb+kdrl+ cells in Tg(cmyb:GFP/kdrl:mCherry) embryos injected with tlr4bb or myd88 MO, was rescued by coinjection with ikbaa MO. White arrowheads mark cmyb+kdrl+ cells in the AGM region at 36 hpf. (F) Quantification of cmyb+kdrl+ cells. Data are presented as mean ± SEM of 3 independent samples. Each sample was composed of at least 5 embryos. *P < .05, **P < .01. (G) Decreased expression of runx1 at 36 hpf in tlr4bb or myd88 morphants was rescued by the overexpression of il1b from 20 hpf.
Because the earliest HSPCs are derived from the hemogenic endothelial cells in the dorsal aorta,47,48 the observed HSPC defects in inflammatory signaling-deficient embryos were likely attributed to the defective vessel or artery specification. To explore this possibility, we examined expression of endothelial marker kdrl and arterial marker ephrinB2. Expression of these markers was not altered (supplemental Figure 5A-B), indicating a proper vessel development and artery-vein establishment. Similarly, we examined primitive hematopoiesis in these inflammatory signaling-deficient embryos. We found that expression of hemangioblast marker scl, erythroid marker gata1, and myeloid marker l-plastin was relatively normal, suggesting that primitive hematopoiesis was not affected (supplemental Figure 5C). In addition, TUNEL and BrdU staining showed no significant changes in the AGM region in the morphants, compared with control embryos (supplemental Figure 5D-E), implying that the observed HSPC defects were not caused by abnormal cell proliferation or excessive apoptosis.
Notch signaling functions downstream of inflammatory signaling to regulate HSPC emergence
TLR signaling has been reported to cooperate with Notch signaling to promote production of inflammatory cytokines, and Notch signaling acts upstream of Runx1 to specify HSPCs.49-51 To explore the possible link between Notch signaling and inflammatory signaling in HSPC emergence, we examined expression of a panel of Notch target genes by qRT-PCR in the AGM region of inflammatory signaling-deficient zebrafish embryos. A significant reduction in expression of Notch target genes was observed specifically in tlr4bb or myd88 morphants (Figure 4A, supplemental Figure 6A). Furthermore, the expression of hey2 was also confirmed in the myd88 mutants (Figure 4B). Consistently, using a Notch reporter transgenic line tp1:DsRed/fli1a:EGFP,25 tp1+fli1a+ positive cells in the AGM region were greatly reduced (Figure 4C), suggesting that Notch activity was attenuated in these morphants. Repression of these inflammatory genes using dead Cas9 technology further confirmed this result (supplemental Figure 6B). Next, we investigated whether overexpression of Notch can rescue the HSPC defects in the morphants. Endothelial-derived Notch overexpression with fli1a promoter-driven Notch intracellular domain (NICD) efficiently rescued the number of runx1+ cells in the AGM region in inflammatory signaling-defective embryos (Figure 4D). Together, endothelial Notch signaling is downstream of TLR4–MyD88–NF-κB–mediated inflammatory signaling to control the emergence of HSPCs in zebrafish embryos.
Notch signaling functions downstream of inflammatory signaling to regulate HSPC emergence. (A) qRT-PCR results from the dissected trunk region showed that expression of Notch target genes hey1, hey2, and her15.1 was decreased in tlr4bb and myd88 morphants and increased in ikbaa morphants at 28 hpf. Each bar represents the mean ± SEM of 3 independent samples. *P < .05, **P < .01. (B) hey2 expression was decreased in myd88−/− embryos. Black arrowheads mark expression of hey2 in the dorsal aorta at 24 hpf. (C) tp1+fli1a+ cells in Tg(tp1:DsRed/fli1a:EGFP) transgenic embryos were decreased in tlr4bb or myd88 morphants and increased in ikbaa morphants. Left panels, Confocal images of the tp1+fli1a+ cells in the AGM region at 36 hpf, which were labeled by the white arrowheads. Right panel, Quantification of the tp1+fli1a+ cells. Each bar represents the mean ± SEM of 3 independent samples. Each sample was composed of at least 6 embryos. *P < .05, **P < .01. (D) Overexpression of NICD in endothelial cells partially rescued the HSPC defect in tlr4bb or myd88 morphants. Top panel, The NICD-GFP+ signals. Bottom panels, The expression of runx1 at 36 hpf in the embryos coinjected with tlr4bb or myd88 MOs and fli1a-ep-NICD-GFP construct. Red arrowheads mark GFP signal in the endothelial cells. Black arrowheads mark the expression of runx1 in the AGM region at 36 hpf.
Notch signaling functions downstream of inflammatory signaling to regulate HSPC emergence. (A) qRT-PCR results from the dissected trunk region showed that expression of Notch target genes hey1, hey2, and her15.1 was decreased in tlr4bb and myd88 morphants and increased in ikbaa morphants at 28 hpf. Each bar represents the mean ± SEM of 3 independent samples. *P < .05, **P < .01. (B) hey2 expression was decreased in myd88−/− embryos. Black arrowheads mark expression of hey2 in the dorsal aorta at 24 hpf. (C) tp1+fli1a+ cells in Tg(tp1:DsRed/fli1a:EGFP) transgenic embryos were decreased in tlr4bb or myd88 morphants and increased in ikbaa morphants. Left panels, Confocal images of the tp1+fli1a+ cells in the AGM region at 36 hpf, which were labeled by the white arrowheads. Right panel, Quantification of the tp1+fli1a+ cells. Each bar represents the mean ± SEM of 3 independent samples. Each sample was composed of at least 6 embryos. *P < .05, **P < .01. (D) Overexpression of NICD in endothelial cells partially rescued the HSPC defect in tlr4bb or myd88 morphants. Top panel, The NICD-GFP+ signals. Bottom panels, The expression of runx1 at 36 hpf in the embryos coinjected with tlr4bb or myd88 MOs and fli1a-ep-NICD-GFP construct. Red arrowheads mark GFP signal in the endothelial cells. Black arrowheads mark the expression of runx1 in the AGM region at 36 hpf.
Deficiency of inflammatory receptors leads to HSPC defects
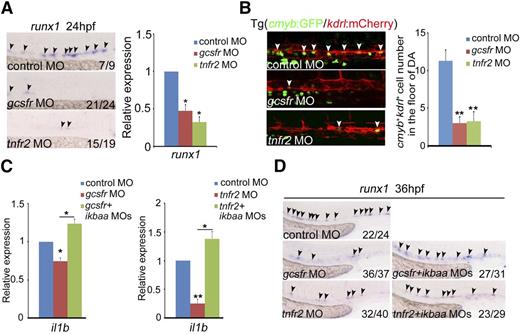
NF-κB can respond to multiple inflammatory cytokines in innate immune responses. We thus examined whether attenuation of this cytokine-mediated signaling, including TNFα and G-CSF,52,53 would cause similar HSPC defects as observed in TLR–NF-κB–deficient embryos. runx1 expression was remarkably decreased in tnfr2- and gcsfr-deficient embryos by MO knockdown, compared with control embryos (Figure 5A). The number of cmyb+kdrl+ HSPCs in the AGM region was significantly reduced (Figure 5B). The HSPC phenotype in gcsfr morphants was consistent with a recent report by another group.54 To demonstrate whether the cytokine signaling is mediated by NF-κB, we examined expression of il1b and found that il1b expression was decreased to the similar level as that in NF-κB–deficient embryos (Figure 5C), suggesting that the HSPC defects observed in tnfr2- and gcsfr-deficient embryos were likely attributed to dysregulated NF-κB signaling. Importantly, coinjection of ikbaa MO efficiently restored the decreased population of HSPCs in these morphants, indicating that NF-κB acts downstream of inflammatory cytokine signaling (Figure 5D). Taken together, these data support that inflammatory cytokines activate NF-κB signaling to control HE and HSPC specification.
The deficiency of inflammatory receptors leads to HSPC defects. (A) Expression of HSPC marker runx1 was decreased in mcsfr, gcsfr, and tnfr2 morphants at 24 hpf. Black arrowheads labeled expression of runx1 in the AGM region. (B) Fluorescence-labeled hemogenic endothelial cells were decreased in Tg(cmyb:GFP/kdrl:mCherry) embryos injected with gcsfr or tnfr2 MO at 36 hpf. White arrowheads mark cmyb+kdrl+ cells in the AGM region. Each bar represents the mean ± SEM of 3 independent samples. Each sample was composed of at least 5 embryos. **P < .01. (C) qRT-PCR analysis of the NF-κB target gene il1b showed that the decreased expression of il1b in gcsfr and tnfr2 morphants at 36 hpf were rescued by coinjection of ikbaa MO. Each bar represents the mean ± SEM of 3 independent samples. *P < .05, **P < .01. (D) The decreased expression of HSPC marker runx1 in gcsfr and tnfr2 morphants were rescued by coinjection of ikbaa MO. Black arrowheads mark runx1 expression in the AGM region at 36 hpf.
The deficiency of inflammatory receptors leads to HSPC defects. (A) Expression of HSPC marker runx1 was decreased in mcsfr, gcsfr, and tnfr2 morphants at 24 hpf. Black arrowheads labeled expression of runx1 in the AGM region. (B) Fluorescence-labeled hemogenic endothelial cells were decreased in Tg(cmyb:GFP/kdrl:mCherry) embryos injected with gcsfr or tnfr2 MO at 36 hpf. White arrowheads mark cmyb+kdrl+ cells in the AGM region. Each bar represents the mean ± SEM of 3 independent samples. Each sample was composed of at least 5 embryos. **P < .01. (C) qRT-PCR analysis of the NF-κB target gene il1b showed that the decreased expression of il1b in gcsfr and tnfr2 morphants at 36 hpf were rescued by coinjection of ikbaa MO. Each bar represents the mean ± SEM of 3 independent samples. *P < .05, **P < .01. (D) The decreased expression of HSPC marker runx1 in gcsfr and tnfr2 morphants were rescued by coinjection of ikbaa MO. Black arrowheads mark runx1 expression in the AGM region at 36 hpf.
Consistently, both the expression of Notch targets and the number of tp1+fli1a+ cells in the AGM region were greatly reduced in these inflammatory receptor-defective embryos (supplemental Figure 7A-C). Furthermore, the endothelial-derived NICD overexpression efficiently rescued the population of runx1+ cells in the AGM region in inflammatory signaling-defective embryos (supplemental Figure 7D). Together, endothelial Notch signaling also functions downstream of inflammatory signaling to control the emergence of HSPCs in zebrafish embryos.
To determine which subset of the myeloid lineages is responsible for the production of inflammatory cytokines, we used pu.1 MO which can eliminate all myeloid lineages in zebrafish, irf8 MO which attenuates macrophages, and cebp1 MO which decreases neutrophils. The runx1 expression was not much affected in irf8 knockdown embryos, whereas decreased in pu.1 and cebp1 knockdown embryos (supplemental Figure 8A-B), suggesting that neutrophils are the main source for inflammatory cytokines responsible for HSPC emergence, consistent with the findings published recently.13,14
Inflammatory signaling plays an evolutionarily conserved role in mouse definitive hematopoiesis
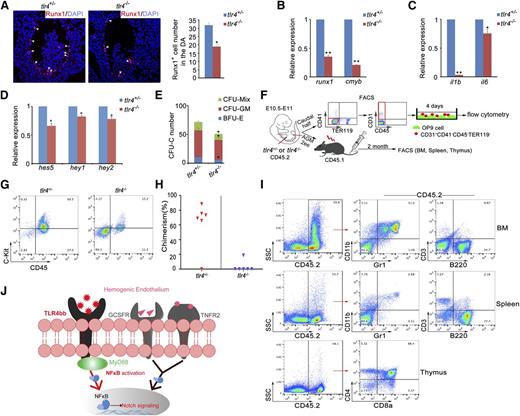
To determine whether inflammatory signaling is evolutionarily conserved in HSPC emergence in mammals, we examined the AGM region of tlr4−/− knockout mice.55 Immunofluorescence analysis showed that Runx1 expression was severely attenuated in the AGM (Figure 6A). qRT-PCR confirmed decreased levels of runx1 and cmyb, as well as downregulated expression of il1b and il6, in NF-κB signaling (Figure 6B-C). As observed in zebrafish, expression of Notch target genes was also decreased (Figure 6D). The colony-forming unit-cell (CFU-C) assay showed compromised colony formation abilities of AGM tissues in tlr4−/− mice (Figure 6E). To test whether inflammatory signaling acts on endothelial cells cell-autonomously in mice, endothelial cells (CD31+CD41−CD45−TER119−)56 from the AGM tissues in tlr4+/− or tlr4−/− were cultured for 4 days, then flow cytometry was performed (Figure 6F). The C-Kit+CD45+ hematopoietic cells were significantly decreased in tlr4−/− embryos (Figure 6G). The HSC transplantation is widely accepted as the most reliable assay for HSC activity. Five of 6 recipients were reconstituted after 2 months when transplanted with cells from tlr4+/− embryos, whereas only 1 of 6 reconstituted when transplanted with cells from tlr4−/− embryos (Figure 6H-I). Together, these data suggest that the role of inflammatory signaling in HSPC emergence is evolutionarily conserved in mammals.
Inflammatory signaling plays an evolutionarily conserved role in mouse definitive hematopoiesis. (A) Immunofluorescence of Runx1 in the E10.5 AGM region of tlr4+/− and tlr4−/− embryos. Right panel, The quantification of Runx1+ cells in AGM region on the sections (tlr4+/−, n = 3; tlr4−/−, n = 3). (B-D) qRT-PCR analysis showed attenuated expression of HSC markers (B), NF-κB signaling target genes (C), and Notch target genes (D), respectively. (E) CFC assay of AGM regions showed the numbers of CFU-Mix, CFU-GM, and BFU-E were decreased in tlr4−/− embryos. One embryo equivalent was used. (F) The scheme of cell sorting and HSC transplantation assay in mouse embryos. (G) Flow cytometry results showed an increased amount of C-Kit+CD45+ cells in tlr4−/− embryos after 4 days of OP9 coculture. (H) Donor-derived chimerism in recipients. Symbols represent the donor chimerism in bone marrow of each recipient at 2 months posttransplantation. (I) The donor-derived multilineage reconstitution was shown by the presence of CD45.2 cells in the myeloid (Gr1+/CD11b+), B lymphoid (B220+), and T lymphoid (CD3+, or CD4+/CD8+) populations of bone marrow, spleen, and thymus in a representative recipient at 2 months posttransplantation. (J) Model of inflammatory signaling during HSPC emergence. The inflammatory signals were required to activate NF-κB–Notch signaling in the hemogenic endothelial cells, and then regulate HSPC development. (B-E) Each bar represents the mean ± SEM of 3 independent samples. *P < .05, **P < .01.
Inflammatory signaling plays an evolutionarily conserved role in mouse definitive hematopoiesis. (A) Immunofluorescence of Runx1 in the E10.5 AGM region of tlr4+/− and tlr4−/− embryos. Right panel, The quantification of Runx1+ cells in AGM region on the sections (tlr4+/−, n = 3; tlr4−/−, n = 3). (B-D) qRT-PCR analysis showed attenuated expression of HSC markers (B), NF-κB signaling target genes (C), and Notch target genes (D), respectively. (E) CFC assay of AGM regions showed the numbers of CFU-Mix, CFU-GM, and BFU-E were decreased in tlr4−/− embryos. One embryo equivalent was used. (F) The scheme of cell sorting and HSC transplantation assay in mouse embryos. (G) Flow cytometry results showed an increased amount of C-Kit+CD45+ cells in tlr4−/− embryos after 4 days of OP9 coculture. (H) Donor-derived chimerism in recipients. Symbols represent the donor chimerism in bone marrow of each recipient at 2 months posttransplantation. (I) The donor-derived multilineage reconstitution was shown by the presence of CD45.2 cells in the myeloid (Gr1+/CD11b+), B lymphoid (B220+), and T lymphoid (CD3+, or CD4+/CD8+) populations of bone marrow, spleen, and thymus in a representative recipient at 2 months posttransplantation. (J) Model of inflammatory signaling during HSPC emergence. The inflammatory signals were required to activate NF-κB–Notch signaling in the hemogenic endothelial cells, and then regulate HSPC development. (B-E) Each bar represents the mean ± SEM of 3 independent samples. *P < .05, **P < .01.
Discussion
Many if not all developmental signaling pathways during developmental hematopoiesis and homeostasis have been identified. Inflammatory signaling has been well studied in infection and inflammation, but its physiological and developmental roles are poorly understood. In the present study, we have demonstrated that inflammatory signaling plays a highly conserved role in HSPC emergence in zebrafish and mice by investigating the expression pattern, requirement for HSPC specification, and regulatory mechanisms. Inflammatory signaling functions upstream of Notch signaling pathway via NF-κB. Our findings strongly support that proinflammatory signaling is a common master regulator of HSPC emergence and is shared by normal hematopoiesis and stress hematopoiesis in vertebrates.
The relationship between NF-κB and Notch is controversial and has been studied in different systems. The Notch signaling pathway is essential to maintain HSPC homeostasis by regulating the inflammatory signaling level in the bone marrow niche because inhibition of Notch signaling activates NF-κB via mir-155 and leads to myeloproliferation.57 On the one hand, Notch may prevent the NF-κB activation by directly interacting with NF-κB in the nucleus.58 On the other hand, Notch signaling has been reported to activate NF-κB59,60 as well. In hematopoietic progenitor cells, Notch1 has been shown to regulate NF-κB activity through transcriptional regulation of NF-κB subunits.61 In the T-cell leukemia, the Notch/Hes1 pathway sustains the NF-κB activation by negatively regulating IκB kinase.62 Furthermore, the cooperation of Notch3 and canonical NF-κB signaling pathways can regulate Foxp3 transcription to maintain the regulatory T-cell homeostasis and function.63 In this work, we identified the NF-κB regulation of the Notch signaling pathway by upregulating Notch signaling at the onset of definitive hematopoiesis, which is consistent with the NF-κB–dependent activation of Notch in endothelial cells64 and peripheral lymphoid tissues.65 Further studies are warranted to fully characterize the interaction between these 2 pathways in HE and HSPC specification.
Baseline inflammatory signaling might be crucial for the emergence of HSPCs under the physiological condition in that a low level of inflammatory signaling promotes HSPC specification or “priming.” Once in demand, that is, in response to infection, inflammatory signaling will rapidly induce the programming for cytokine production within the earliest HSPCs, and efficiently instruct HSPC differentiation into effector immune cells, such as myeloid and lymphoid cells.8,66 However, the sources of endogenous TLR ligands and inflammatory cytokines during normal hematopoiesis remain unclear. It is possible that the reported heat shock proteins and signals from damaged or stressed cells67 firstly induce the TLR4–NF-κB pathway during normal embryonic hematopoiesis, and then activate the Notch pathway to regulate the HSPC emergence.
In conclusion, our results support that proinflammatory signaling is a novel and potent regulator during HSPC emergence and is shared by normal and stress hematopoiesis in vertebrates. This may prove to be a useful theoretical basis in treating patients with bone marrow failure or following transplantation with clinically applicable cytokines and point to a new strategy for developing a new protocol for in vitro generation and expansion of inducible HSPCs by modulation of inflammatory signaling.
Note added in proof. During the preparation of the revision for this work, 3 recent publications reported that proinflammatory signaling mediated by TNFα or interferons plays an essential role in HSPC development in zebrafish and mouse embryos,12-14 consistent with our findings here.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dangsheng Li and Zilong Wen for helpful discussions and/or critical reading of the paper. The authors also thank Zilong Wen and Jing-Wei Xiong for providing the reagents used in this work.
This work was supported by grants from the National Basic Research Program of China (2010CB945300, 2011CB943900), the National Natural Science Foundation of China (31271570, 31425016), and the Strategic Priority Research Program of the Chinese Academy of Sciences (CAS) (XDA01010110).
Authorship
Contribution: Q.H., C.Z., L.W., P.Z., D.M., and J.L. performed the experiments; F.L. conceived the project, analyzed the data, and wrote the paper; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Feng Liu, State Key Laboratory of Biomembrane and Membrane Biotechnology, Institute of Zoology, Chinese Academy of Sciences, Beijing 100101, China; e-mail: liuf@ioz.ac.cn.
References
Author notes
Q.H., C.Z., and L.W. contributed equally to this work.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal