In this issue of Blood, Kim et al show that engineered factor VIII (FVIII)-specific regulatory T cells inhibit immune responses to FVIII,1 suggesting the possibility of cellular therapy for FVIII inhibitors in hemophilia A.
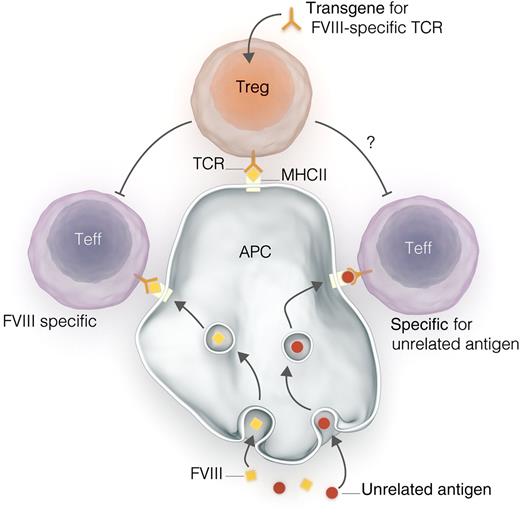
Interactions between engineered Tregs and effector T cells (Teffs). A gene construct encoding for a T-cell receptor (TCR) specific for FVIII peptide is transduced into polyclonal Tregs, producing Tregs expressing that TCR. Tregs interact with antigen-presenting cells (APCs) via human major histocompatibility complex II (MHCII), as do Teffs. Tregs exert suppressive effects on Teffs, preventing immune responses to antigen and promoting tolerance. Kim et al demonstrate in vitro that such engineered Tregs can suppress the typically polyclonal responses to whole FVIII, despite expressing TCRs specific for a single small FVIII peptide. Could this result in the off-target suppression of T-cell responses to non-FVIII antigens, such as antigens from pathogens or vaccines?
Interactions between engineered Tregs and effector T cells (Teffs). A gene construct encoding for a T-cell receptor (TCR) specific for FVIII peptide is transduced into polyclonal Tregs, producing Tregs expressing that TCR. Tregs interact with antigen-presenting cells (APCs) via human major histocompatibility complex II (MHCII), as do Teffs. Tregs exert suppressive effects on Teffs, preventing immune responses to antigen and promoting tolerance. Kim et al demonstrate in vitro that such engineered Tregs can suppress the typically polyclonal responses to whole FVIII, despite expressing TCRs specific for a single small FVIII peptide. Could this result in the off-target suppression of T-cell responses to non-FVIII antigens, such as antigens from pathogens or vaccines?
FVIII inhibitors are a potential disaster for patients with hemophilia A. Inhibitors, which affect ∼25% of patients with severe hemophilia A who are treated with FVIII concentrates,2 render subsequent replacement therapy ineffective. This leaves patients vulnerable to bleeding and dependent on bypassing agents such as recombinant activated FVII or FVIII inhibitor bypassing activity for hemostasis. The standard therapy to eliminate inhibitors, immune tolerance induction, is both expensive and inconvenient to administer because it consists of the frequent infusion of large doses of FVIII, and is ineffective in 20% to 50% of patients.3 New therapeutic options are certainly needed.
A number of medical immunomodulatory strategies have been investigated in animal models of hemophilia A,4-6 but none are close to clinical application. In patients, rituximab is sometimes used in combination with immune tolerance induction, with mixed results.7 Therefore, the search for improved therapies for inhibitor patients continues. By providing novel data regarding immunomodulation using engineered regulatory T cells (Tregs) as a strategy to eliminate anti-FVIII immune responses, Kim et al introduce a promising new avenue to this search.
After antigen-specific activation, Tregs suppress the function of effector T cells, perhaps even effector T cells with different antigen specificities8 (see figure). The generation of inhibitors—immunoglobulin G antibodies with specificity for epitopes on the FVIII molecule—is dependent on stimulation of FVIII-specific B cells by T cells; stimulated B cells then differentiate into antibody-secreting cells (ASC). It is plausible that the presence of a population of FVIII-specific Tregs that prevents this T-cell stimulation might interfere with the generation of inhibitors.
In the work of Kim et al, FVIII-specific Tregs were generated by transducing the gene for a TCR that recognizes an FVIII peptide (FVIII-2191-2220) into Tregs; the TCR gene was obtained from a hemophilia A patient and the Tregs from nonhemophiliac donors. Transduced Tregs expressed the transgene, and Treg lineage markers (eg, Foxp3) were inducible in these cells by exposure to FVIII-2191-2220. These Tregs could be expanded by exposure to FVIII-2191-2220 and oligodeoxynucleotides, and they maintained their phenotype after expansion; this suggests these FVIII-specific Tregs might be manufactured in large quantities.
Crucially, transduced Tregs were able to suppress immune responses to FVIII in vitro. When incubated with effector T cells transduced with the same TCR (and therefore with specificity for the same FVIII peptide) and irradiated APCs, engineered Tregs prevented T-cell proliferation and reduced the production of the T cell–derived cytokines interferon and tumor necrosis factor. Perhaps the most important experiment involved the incubation of engineered Tregs with FVIII and splenocytes taken from FVIII-immunized hemophilia A mice with a transgene for a MHCII allele. In this in vitro model of exposure of FVIII-specific T cells and B cells to FVIII, the generation of antibody-secreting cells, as detected by an enzyme-linked immunospot (ELISPOT) assay, was inhibited by engineered Tregs.
Further in vitro and animal studies will determine more mechanistic details about these observed effects, although the mechanisms of Treg function in general are still incompletely understood. However, the most interesting work to follow will likely be efforts to move this technology toward clinical application. In that direction, there are many important unanswered questions.
Therapeutic application would likely involve isolating a patient’s Tregs, transducing them with a FVIII-specific TCR, and then expanding these transduced cells before reinfusion back into the patient. Will it be possible to manufacture Tregs in quantities sufficient to suppress anti-FVIII immune responses in a patient? The experiments described by Kim et al require large numbers of Tregs, relative to the number of effector T cells, to efficiently suppress T-cell function.
How many different TCR clones would be required to treat a patient? In the ELISPOT experiment described, a population of Tregs with a TCR specific for a single small FVIII peptide was able to suppress ASC formation after stimulation with the entire FVIII molecule. Because anti-FVIII immune responses in hemophilia A are polyclonal,9 this suggests that these Tregs can suppress effector T cells with specificity for a variety of FVIII peptide antigens. Whether this is true in general, and in humans in particular, remains to be seen.
This potential ability of transduced Tregs to suppress responses to other peptide antigens raises concerns about more generalized immunosuppressive effects. This might be particularly concerning when antigens to which tolerance is not desired (eg, antigens from infectious pathogens or vaccines) are encountered simultaneously with FVIII (see figure). Will engineered Tregs induce tolerance to these antigens unrelated to FVIII? Further studies of this technology, both clinical and preclinical, should consider this question very carefully.
These questions notwithstanding, there is promise in the work begun by Kim et al. Imagine monitoring hemophilia A patients for FVIII-reactive T cells after beginning FVIII replacement therapy and using TCRs from those T cells to manufacture Tregs that stop the immune response against FVIII. Better still, imagine being able to do all of this before patients can develop clinically important FVIII inhibitors at all.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal