Key Points
Coexistent hyperdiploidy or t(11;14) does not abrogate the poor prognosis associated with adverse cytogenetics in myeloma patients.
Single-cell analysis reveals that hyperdiploidy may precede IGH translocation in the clonal history of a proportion of patients with both.
Abstract
The acquisition of the cytogenetic abnormalities hyperdiploidy or translocations into the immunoglobulin gene loci are considered as initiating events in the pathogenesis of myeloma and were often assumed to be mutually exclusive. These lesions have clinical significance; hyperdiploidy or the presence of the t(11;14) translocation is associated with a favorable outcome, whereas t(4;14), t(14;16), and t(14;20) are unfavorable. Poor outcomes are magnified when lesions occur in association with other high-risk features, del17p and +1q. Some patients have coexistence of both good and poor prognostic lesions, and there has been no consensus on their risk status. To address this, we have investigated their clinical impact using cases in the Myeloma IX study (ISRCTN68454111) and shown that the coexistence of hyperdiploidy or t(11;14) does not abrogate the poor prognosis associated with adverse molecular lesions, including translocations. We have also used single-cell analysis to study cases with coexistent translocations and hyperdiploidy to determine how these lesions cosegregate within the clonal substructure, and we have demonstrated that hyperdiploidy may precede IGH translocation in a proportion of patients. These findings have important clinical and biological implications, as we conclude patients with coexistence of adverse lesions and hyperdiploidy should be considered high risk and treated accordingly.
Introduction
The initiation and progression of myeloma involves a wide range of (epi)genetic lesions, including chromosomal translocations, copy-number abnormalities such as gains or losses, mutations, and epigenetic events.1 It is postulated that the acquisition of immunoglobulin gene translocations or hyperdiploidy triggers myeloma development, with the clonal progeny of the myeloma-founding cell evolving through independent acquisition of secondary lesions.1-3 Increasing evidence from our group and others supports this clonal evolution in myeloma, whereby different subclones coexist and compete for space and stromal support within the patient bone marrow microenvironment.4-7
During the past 2 decades, a number of defined cytogenetic lesions have been associated with worse patient outcomes, including the t(4;14), t(14;16), and t(14;20); the deletion of the short arm of chromosome 17 (chr17) (del17p); and the gain of the long arm of chr1 (+1q).8-16 Patients with these cytogenetic lesions either do not respond to treatment or relapse quickly following an initial response; they have a shorter overall survival (OS) and are therefore considered high-risk myeloma patients. We have previously demonstrated an additive effect of these lesions on survival with patients having worse outcomes as the number of adverse lesions increases.12 Conversely, other cytogenetic abnormalities, such as hyperdiploidy or t(11;14), are associated with more favorable outcomes.17,18
For patients with high-risk cytogenetics, it is becoming increasingly attractive to attempt to improve outcomes by using more dose-dense chemotherapy regimens as first-line therapy and/or to use consolidation or maintenance strategies to maintain responses. This raises the problem of how to treat patients who have the coexistence of both high-risk and normal-risk cytogenetic lesions. Previous studies provide no consistent answer, with one study demonstrating that the presence of an IGH translocation negatively impacts survival in hyperdiploid myeloma patients,19 whereas another group suggests that the presence of trisomies overcomes the negative impact of the adverse translocations and del17p.20
In this study, we sought to further clarify the risk status of patients with coexistence of hyperdiploidy and adverse cytogenetics in a large population of patients uniformly treated within the Medical Research Council (MRC) Myeloma IX trial. Additionally, we aimed to elucidate whether patients with hyperdiploidy and IGH translocations (HRD-Tx patients) had both genomic aberrations in all myeloma cells or whether they were segregated in different subclones with either trisomies or translocations consistently occurring first.
Patients and methods
Patient samples
The MRC Myeloma IX trial (ISRCTN68454111, 2003-2007) was a phase 3 trial that recruited 1970 newly diagnosed patients with symptomatic myeloma and compared induction treatment with a thalidomide-containing regimen to traditional chemotherapy. There was an intensive pathway for younger/fitter patients, which randomized cyclophosphamide, vincristine, doxorubicin, and dexamethasone vs cyclophosphamide, thalidomide, and dexamethasone and included an autologous stem cell transplant, and a nonintensive pathway, which randomized melphalan and prednisone vs attenuated cyclophosphamide, thalidomide, and dexamethasone. The full methods and primary results have been published previously.21-25 Median follow-up for the 1960 patients in the intention-to-treat analysis, a subset of which was used in this analysis, is 5.9 years. The Myeloma IX and XI trials with associated collection of biological materials and studies were approved by the relevant ethics committees. Research was conducted in accordance with the Declaration of Helsinki.
iFISH
Diagnostic bone marrow samples, suitable for testing, were received for 1140 patients. From these, plasma cells were selected using CD138 magnetic microbeads (Miltenyi Biotec, Bisley, United Kingdom). Interphase fluorescence in situ hybridization (iFISH) was performed on the CD138-selected cells. Adverse cytogenetics were defined as the presence of 1 or more of the following: an adverse translocation (t[4;14], t[14;16], t[14;20]), del17p, or +1q, as these were the lesions found to be associated with poor outcomes in our data set (supplemental Table 1 available on the Blood Web site). Because patients with t(14;16) and t(14;20) are rare but behave in a similar fashion to t(4;14) patients, they were grouped together to form an adverse IGH translocation group. Ploidy was classified as previously defined26 using a modification of the method of Wuilleme et al.27 An extra copy of probes for any 2 of chr5, chr9, or chr15 was defined as hyperdiploidy, with patients not meeting these criteria further examined as described by Chiecchio et al.26 Only patients with a complete, valid data set for all of the adverse cytogenetic lesions and hyperdiploidy were included (847 patients).
Survival analyses
Survival curves were plotted (Kaplan-Meier) and the statistical significance of the difference between curves tested using the log-rank test, with P < .05 as significant. Multivariate analysis was performed using a fitted backward Cox regression.
Molecular screening for the presence of translocation and hyperdiploidy
Patients from the Myeloma XI trial (ISRCTN49407852, 2009-ongoing) were also assessed for translocations and hyperdiploidy. Recurrent translocations were assessed by a translocation/cyclin classification-based predictor that involves multiplexed real-time quantitative polymerase chain reaction (RT-PCR) of the common translocation target genes in myeloma.28 Copy-number abnormalities including gains and hyperdiploidy, as well as losses, were assessed by multiplex ligation-dependent probe amplification.29,30 HRD-Tx samples were selected for single-cell genetic analysis (supplemental Figure 1).
Five patients (1 from Myeloma IX and 4 from Myeloma XI) were selected for single-cell genetic analysis (supplemental Table 3).
Translocation sequence definition
Translocation breakpoints were defined for the selected HRD-Tx patients by targeted-capture sequencing using the SureSelect (Agilent, Wokingham, Berkshire, United Kingdom) system by tiling RNA baits across the IGH/IGK/IGL loci as before.31
Single-cell sorting and multiplex qPCR for translocation and copy-number analysis
Fixed single cells from each patient (252 cells) and lymphocytes from a healthy donor (reference control for copy-number values and translocations) were sorted in a FACSAria cell sorter (BD Biosciences, Oxford, United Kingdom) using propidium iodide nuclei staining in 96-well plates as before.6 A novel approach for single-cell multiplex quantitative polymerase chain reaction (qPCR) analysis was performed (Fluidigm UK, London, United Kingdom).32 Briefly, single cells were sorted into lysis buffer followed by specific (DNA) target amplification (STA). This multiplex-STA reaction comprises the simultaneous amplification of target regions of interest using TaqMan PreAmp Master Mix and assays (Life Technologies). Three different TaqMan copy-number assays covering each chromosomal region of interest were used for copy-number analysis (supplemental Table 4). To detect each derivative chromosome from a translocation, TaqMan custom-made probes were designed to cover each breakpoint detected by targeted exome sequencing of the IGH region as before6,31 (supplemental Table 5). The STA product was diluted, and qPCR was performed using the 96.96 dynamic arrays and the BioMark HD (Fluidigm). Each multiple copy-number assay per region was used in quadruplicates to provide replicates for accurate copy-number calling.33 Translocations and copy-number aberrations were assessed at the single-cell level using Fluidigm Real-Time PCR Analysis v.3.0.2 software (Fluidigm). To estimate copy-number values, CopyCaller v.2.0 software (Life Technologies) was used. Weighted means of the calculated copy-number values for each experimental replicate were obtained following quality criteria and confidence intervals as before.6,32 A filtering strategy for wells with low-quality DNA amplification or with DNA from multiple cells and subclones without a minimum cell number was applied6,32 (supplemental Table 6).
Phylogenetic inference of clonal populations
The evolutionary history of the clonal populations defined by single-cell genetic analysis was inferred using the minimum evolution method.34 The phylogenetic tree was drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the number of differences method35 and are in the units of the binary state differences per sequence. As the likelihood of 2 independent translocations with the same IGH partner and same sequence rearrangement was considered to be negligible, this possibility was weighted against by counting translocations as 2 separate events. The minimum evolution tree was searched using the close-neighbor interchange algorithm35 at search level of 1. The neighbor-joining algorithm36 was used to generate the initial tree. Evolutionary analyses were conducted in MEGA6.37
Results
Survival data
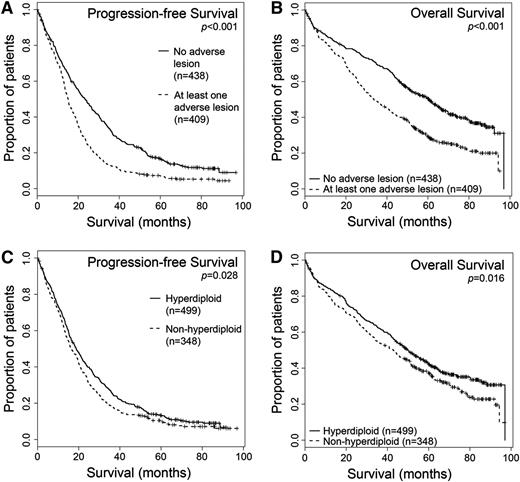
A total of 847 patients from the Myeloma IX data set had complete results for all the adverse cytogenetic lesions and hyperdiploidy, and this subset is representative of the trial as a whole. Using survival data now with a longer median follow-up than previously published,12 we reconfirmed the results demonstrating a poor prognosis associated with adverse cytogenetics (Figure 1A-B and supplemental Table 1). The median OS was 60.6 months for patients with no adverse cytogenetic lesions (438/847, 52%) vs 33.7 months for those with 1 or more of the adverse lesions t(4;14), t(14;16), t(14;20), del17p, and +1q (P < .001), whereas median progression-free survival (PFS) was 23.3 vs 15 months, respectively (P < .001). Outcomes for other individual lesions in this data set are shown in supplemental Table 1. For the first time, given the longer follow-up now available, we also demonstrate a small, but statistically significant, improvement in survival for those patients with hyperdiploidy (499/847, 59%) over the rest of the population, with a PFS 18.8 months vs 16.3 months (P = .028) and an OS of 49.7 months vs 42.8 months (P = .016) (Figure 1C-D).16
Patients with any adverse cytogenetic lesion have a shorter survival than those without, whereas patients with hyperdiploidy have a longer survival. Kaplan-Meier survival curves and log-rank P values are shown. (A-B) Survival analysis for patients with no adverse lesions (n = 438) vs at least 1 adverse lesion (n = 409). (A) Median PFS values: no adverse lesion, 23.3 months; adverse lesion, 15 months. (B) Median OS values: no adverse lesion, 60.6 months; adverse lesion, 33.7 months. (C-D) Survival analysis for patients with hyperdiploidy (n = 499) or without (n = 348). (C) Median PFS values: hyperdiploid, 18.8 months; nonhyperdiploidy, 16.3 months. (D) Median OS values: hyperdiploid, 49.7 months; nonhyperdiploidy, 42.8 months.
Patients with any adverse cytogenetic lesion have a shorter survival than those without, whereas patients with hyperdiploidy have a longer survival. Kaplan-Meier survival curves and log-rank P values are shown. (A-B) Survival analysis for patients with no adverse lesions (n = 438) vs at least 1 adverse lesion (n = 409). (A) Median PFS values: no adverse lesion, 23.3 months; adverse lesion, 15 months. (B) Median OS values: no adverse lesion, 60.6 months; adverse lesion, 33.7 months. (C-D) Survival analysis for patients with hyperdiploidy (n = 499) or without (n = 348). (C) Median PFS values: hyperdiploid, 18.8 months; nonhyperdiploidy, 16.3 months. (D) Median OS values: hyperdiploid, 49.7 months; nonhyperdiploidy, 42.8 months.
Of those patients with hyperdiploidy, 36 also had a coexistent IGH translocation: t(4;14) in 18 cases, t(6;14) in 2, t(11;14) in 12, t(14;16) in 2, and t(14;20) in 2 cases. There is, therefore, coexistence of these 2 apparently etiologic lesions in 4.3% (36/847) of myeloma patients. Of these, 22 (2.6%) patients had hyperdiploidy and coexistence of an adverse translocation t(4;14), t(14;16), and t(14;20). The other adverse lesions 17p and +1q were seen to coexist with hyperdiploidy in 31 (3.7%) and 166 (19.6%) patients, respectively. We sought to analyze the characteristics of the population characterized by hyperdiploidy and whether this good prognostic feature abrogated the effect of adverse cytogenetic lesions where they coexisted. The characteristics of the hyperdiploid population (n = 499) are shown in Table 1.
Baseline clinical characteristics for patients with hyperdiploidy (n = 499) from the Myeloma IX clinical trial and univariate analysis of the effect on OS
| Patient characteristics . | Frequency . | Median OS, mo (95% CI) . | Univariate analysis (P) . |
|---|---|---|---|
| Sex (n = 499) | |||
| Male | 326 (65%) | 52.5 (45.3, 59.7) | .029 |
| Female | 173 (35%) | 44.7 (34.1, 55.3) | |
| Age (n = 499) | |||
| <70 | 335 (75%) | 62.1 (53.7, 70.5) | <.001 |
| ≥70 | 164 (25%) | 35.1 (30.3, 39.9) | |
| Pathway (n = 499) | |||
| Intensive | 286 (57%) | 69.8 (56.3, 83.3) | <.001 |
| Nonintensive | 213 (43%) | 34.9 (30.5, 39.3) | |
| Chemotherapy (n = 499) | |||
| Thalidomide based (CTD/CTDa) | 247 (49%) | 54.4 (44.2, 64.6) | .017 |
| Traditional (CVAD/MP) | 252 (51%) | 43.7 (36.9, 50.5) | |
| ISS (n = 362)* | |||
| I | 76 (21%) | NR | <.001 |
| II/III | 286 (79%) | 44.7 (39.7, 49.7) | |
| Bone disease (n = 494)† | |||
| Present | 357 (72%) | 48.1 (41.6, 54.6) | NS |
| Absent | 137 (28%) | 54.0 (45.3, 62.7) | |
| Coexistent adverse cytogenetics (n = 499) | |||
| Present | 195 (39%) | 35.7 (27.3, 44.1) | <.001 |
| Absent | 304 (61%) | 60.9 (53.5, 68.3) |
| Patient characteristics . | Frequency . | Median OS, mo (95% CI) . | Univariate analysis (P) . |
|---|---|---|---|
| Sex (n = 499) | |||
| Male | 326 (65%) | 52.5 (45.3, 59.7) | .029 |
| Female | 173 (35%) | 44.7 (34.1, 55.3) | |
| Age (n = 499) | |||
| <70 | 335 (75%) | 62.1 (53.7, 70.5) | <.001 |
| ≥70 | 164 (25%) | 35.1 (30.3, 39.9) | |
| Pathway (n = 499) | |||
| Intensive | 286 (57%) | 69.8 (56.3, 83.3) | <.001 |
| Nonintensive | 213 (43%) | 34.9 (30.5, 39.3) | |
| Chemotherapy (n = 499) | |||
| Thalidomide based (CTD/CTDa) | 247 (49%) | 54.4 (44.2, 64.6) | .017 |
| Traditional (CVAD/MP) | 252 (51%) | 43.7 (36.9, 50.5) | |
| ISS (n = 362)* | |||
| I | 76 (21%) | NR | <.001 |
| II/III | 286 (79%) | 44.7 (39.7, 49.7) | |
| Bone disease (n = 494)† | |||
| Present | 357 (72%) | 48.1 (41.6, 54.6) | NS |
| Absent | 137 (28%) | 54.0 (45.3, 62.7) | |
| Coexistent adverse cytogenetics (n = 499) | |||
| Present | 195 (39%) | 35.7 (27.3, 44.1) | <.001 |
| Absent | 304 (61%) | 60.9 (53.5, 68.3) |
Bold values represent significant P values.
CI, confidence interval; CTD, cyclophosphamide, thalidomide, and dexamethasone; CTDa, as CTD with attenuated dosing; CVAD, cyclophosphamide, vincristine, doxorubicin, and dexamethasone; MP, melphalan and prednisolone; NR, not reached; NS, nonsignificant.
Missing data (n = 137).
Missing data (n = 5).
To examine the effect of adverse cytogenetics within the hyperdiploid population, those patients with hyperdiploidy alone (304, 61%) were compared with those with coexistent hyperdiploidy and 1 or more adverse cytogenetic lesion (195, 39%). Both PFS and OS were significantly worse for those patients with hyperdiploidy and at least 1 adverse cytogenetic lesion (median PFS 23 vs 15.4 months, P < .001; median OS 60.9 vs 35.7 months, P < .001) (Figure 2A-B). This remained true when the patients were subdivided by pathway (intensive vs nonintensive) and by treatment received (thalidomide based vs not), and results are shown in Table 2. Further univariate analysis demonstrated a significant detrimental effect on the OS of patients with hyperdiploidy of older age (≥70 years vs <70 years), International Staging System (ISS) II/III (vs I), traditional chemotherapy (vs thalidomide based), nonintensive pathway (vs intensive), female sex, and coexistent adverse cytogenetics, with no significant effect on OS for the presence of bone disease (Table 1). Multivariate analysis showed persistent significance for ISS, chemotherapy, pathway, and coexistent adverse cytogenetics (Table 3).
Coexistent adverse cytogenetic lesions shorten survival in myeloma patients with hyperdiploidy. (A-B) Patients with hyperdiploidy alone have a longer PFS and OS than patients with hyperdiploidy plus any adverse cytogenetic lesion. Kaplan-Meier survival curves demonstrate shorter survival for those patients with the presence of any adverse cytogenetic lesion. (A) PFS medians: 15.4 months for patients with the presence of any adverse lesion (n = 195) and 23 months for those without (n = 304); log-rank P < .001. (B) OS medians: 35.7 months vs 60.9 months; log-rank P < .001. (C-E) Coexistence of each adverse cytogenetic lesion with hyperdiploidy results in shorter OS. Kaplan-Meier survival analysis with log-rank P values are shown for patients with hyperdiploidy alone (median OS, 60.9 months) vs (C) patients with hyperdiploidy plus an adverse translocation (median OS, 27 months); (D) patients with hyperdiploidy plus del17p (median OS, 29.9 months); or (E) patients with hyperdiploidy plus +1q (median OS, 35.1 months). HRD, hyperdiploidy.
Coexistent adverse cytogenetic lesions shorten survival in myeloma patients with hyperdiploidy. (A-B) Patients with hyperdiploidy alone have a longer PFS and OS than patients with hyperdiploidy plus any adverse cytogenetic lesion. Kaplan-Meier survival curves demonstrate shorter survival for those patients with the presence of any adverse cytogenetic lesion. (A) PFS medians: 15.4 months for patients with the presence of any adverse lesion (n = 195) and 23 months for those without (n = 304); log-rank P < .001. (B) OS medians: 35.7 months vs 60.9 months; log-rank P < .001. (C-E) Coexistence of each adverse cytogenetic lesion with hyperdiploidy results in shorter OS. Kaplan-Meier survival analysis with log-rank P values are shown for patients with hyperdiploidy alone (median OS, 60.9 months) vs (C) patients with hyperdiploidy plus an adverse translocation (median OS, 27 months); (D) patients with hyperdiploidy plus del17p (median OS, 29.9 months); or (E) patients with hyperdiploidy plus +1q (median OS, 35.1 months). HRD, hyperdiploidy.
Effect of coexistent adverse cytogenetics on the PFS and OS of patients with hyperdiploidy when subdivided by pathway and chemotherapy
| . | Survival, median mo (95% CI) . | Log-rank test (P) . | |
|---|---|---|---|
| HRD, no adverse lesion . | HRD + any adverse lesion . | ||
| PFS | |||
| Intensive pathway (CVAD/CTD) | 34.1 (27.5, 40.7) | 20.1 (17.5, 22.6) | <.001 |
| Nonintensive pathway (MP/CTDa) | 14.5 (12.1, 16.9) | 13.1 (12.4, 14.6) | <.001 |
| Thalidomide chemotherapy (CTD/CTDa) | 25.4 (17.4, 33.4) | 17.5 (13.7, 21.3) | .002 |
| Traditional chemotherapy (CVAD/MP) | 21.6 (15.8, 27.4) | 13.9 (14.7, 19.1) | <.001 |
| OS | |||
| Intensive pathway (CVAD/CTD) | 97.0 (74, —) | 43.5 (32.0, 55.0) | <.001 |
| Nonintensive pathway (MP/CTDa) | 38.5 (31.8, 45.2) | 28.9 (22.8, 35.0) | .047 |
| Thalidomide chemotherapy (CTD/CTDa) | 73.3 (52.4, 94.2) | 44.1 (32.3, 55.9) | .014 |
| Traditional chemotherapy (CVAD/MP) | 57.6 (49.1, 66.1) | 29.9 (24.9, 34.9) | <.001 |
| . | Survival, median mo (95% CI) . | Log-rank test (P) . | |
|---|---|---|---|
| HRD, no adverse lesion . | HRD + any adverse lesion . | ||
| PFS | |||
| Intensive pathway (CVAD/CTD) | 34.1 (27.5, 40.7) | 20.1 (17.5, 22.6) | <.001 |
| Nonintensive pathway (MP/CTDa) | 14.5 (12.1, 16.9) | 13.1 (12.4, 14.6) | <.001 |
| Thalidomide chemotherapy (CTD/CTDa) | 25.4 (17.4, 33.4) | 17.5 (13.7, 21.3) | .002 |
| Traditional chemotherapy (CVAD/MP) | 21.6 (15.8, 27.4) | 13.9 (14.7, 19.1) | <.001 |
| OS | |||
| Intensive pathway (CVAD/CTD) | 97.0 (74, —) | 43.5 (32.0, 55.0) | <.001 |
| Nonintensive pathway (MP/CTDa) | 38.5 (31.8, 45.2) | 28.9 (22.8, 35.0) | .047 |
| Thalidomide chemotherapy (CTD/CTDa) | 73.3 (52.4, 94.2) | 44.1 (32.3, 55.9) | .014 |
| Traditional chemotherapy (CVAD/MP) | 57.6 (49.1, 66.1) | 29.9 (24.9, 34.9) | <.001 |
CI, confidence interval; CTD, cyclophosphamide, thalidomide, and dexamethasone; CTDa, as CTD with attenuated dosing; CVAD, cyclophosphamide, vincristine, doxorubicin, and dexamethasone; MP, melphalan and prednisolone.
Factors which retained a significant effect on overall survival on multivariate analysis within the hyperdiploid population
| Factor . | HR . | 95% CI . | P . |
|---|---|---|---|
| ISS (I vs II/III) | 1.67 | 1.15-2.43 | .007 |
| Pathway (intensive vs nonintensive) | 2.04 | 1.56-2.66 | <.001 |
| Chemotherapy (thalidomide based vs traditional) | 1.48 | 1.14-1.93 | .004 |
| Adverse cytogenetics (any of adverse IGH, del17p, or +1q present vs absent) | 1.78 | 1.36-2.33 | <.001 |
| Factor . | HR . | 95% CI . | P . |
|---|---|---|---|
| ISS (I vs II/III) | 1.67 | 1.15-2.43 | .007 |
| Pathway (intensive vs nonintensive) | 2.04 | 1.56-2.66 | <.001 |
| Chemotherapy (thalidomide based vs traditional) | 1.48 | 1.14-1.93 | .004 |
| Adverse cytogenetics (any of adverse IGH, del17p, or +1q present vs absent) | 1.78 | 1.36-2.33 | <.001 |
A backward fitted Cox regression analysis was performed and included all variables found to have a significant impact on OS in univariate analysis. A total of 137 of 499 patients were excluded, as there were incomplete ISS data.
HR, hazard ratio.
We studied the impact of each individual adverse lesion within the hyperdiploid population. Patients were divided into those who had hyperdiploidy alone (ie, without the presence of an adverse translocation, del17p or +1q) and those who had hyperdiploidy with each adverse lesion and their survival compared. The presence of each adverse cytogenetic lesion significantly shortens PFS (not shown) and OS (Figure 2C-E) compared with patients with hyperdiploidy and no adverse lesion.
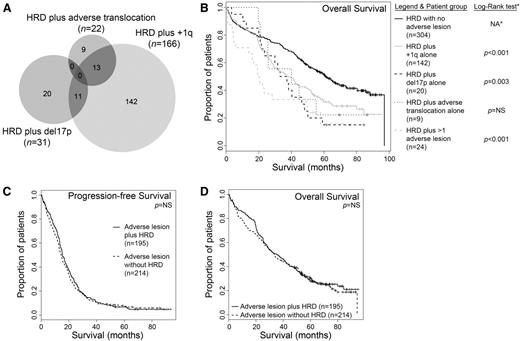
We noticed that some patients with hyperdiploidy had coexistence of several adverse lesions (Figure 3A) and that this might have affected the results for each individual lesion. We therefore divided patients into those who had coexistence of each lesion alone and those who had coexistence of >1 lesion. As shown in Figure 3B, each lesion still had a detrimental impact on patient survival when assessed alone, with the worst prognosis for patients with >1 lesion as previously demonstrated for the whole trial population.12 In this analysis, each lesion alone retains significance when compared with hyperdiploidy patients with no adverse lesions (at significance level P < .05) except adverse IGH translocation, likely due to the small number of patients.
Patient survival is shorter with increasing number of adverse lesions, regardless of the presence of hyperdiploidy. (A) Venn diagram to show that within the subset of patients with hyperdiploidy, there is cosegregation of additional adverse cytogenetic lesions. (B) Kaplan-Meier survival curves within the group of patients with hyperdiploidy predicted shorter OS with the coexistence of each adverse lesion alone independently and even shorter OS when >1 adverse lesion was present. *Pairwise comparisons were established for median OS values for HRD alone (60.9 months) vs HRD plus adverse translocation alone (40.1 months; log-rank P = .180), HRD plus del17p alone (35.2 months; log-rank P = .003), HRD plus 1q+ alone (38.1 months; log-rank P < .001), and HRD plus >1 adverse lesion (19.9 months; log-rank P < .001). NA, not applicable. (C-D) The coexistence of hyperdiploidy did not alter the PFS or OS of patients with the presence of any adverse cytogenetic lesion. Median PFS values: adverse lesion alone, 14.5 months (n = 214); adverse lesion plus hyperdiploidy, 15.4 months (n = 195). Median OS values: adverse lesion alone, 33.6 months; adverse lesion plus hyperdiploidy, 35.7 months. Log-rank P values were not significant. HRD, hyperdiploidy; NS, not significant.
Patient survival is shorter with increasing number of adverse lesions, regardless of the presence of hyperdiploidy. (A) Venn diagram to show that within the subset of patients with hyperdiploidy, there is cosegregation of additional adverse cytogenetic lesions. (B) Kaplan-Meier survival curves within the group of patients with hyperdiploidy predicted shorter OS with the coexistence of each adverse lesion alone independently and even shorter OS when >1 adverse lesion was present. *Pairwise comparisons were established for median OS values for HRD alone (60.9 months) vs HRD plus adverse translocation alone (40.1 months; log-rank P = .180), HRD plus del17p alone (35.2 months; log-rank P = .003), HRD plus 1q+ alone (38.1 months; log-rank P < .001), and HRD plus >1 adverse lesion (19.9 months; log-rank P < .001). NA, not applicable. (C-D) The coexistence of hyperdiploidy did not alter the PFS or OS of patients with the presence of any adverse cytogenetic lesion. Median PFS values: adverse lesion alone, 14.5 months (n = 214); adverse lesion plus hyperdiploidy, 15.4 months (n = 195). Median OS values: adverse lesion alone, 33.6 months; adverse lesion plus hyperdiploidy, 35.7 months. Log-rank P values were not significant. HRD, hyperdiploidy; NS, not significant.
We sought to confirm these findings by examining the impact of the presence or absence of hyperdiploidy for those patients with any adverse cytogenetic lesion. From the population of patients with a complete data set for the required lesions as described above (n = 847), we analyzed those who had 1 or more of the adverse lesions t(4;14), t(14;16), t(14;20), del17p, and +1q (n = 409). In this patient group, the coexistence of hyperdiploidy did not significantly alter the poor prognosis associated with the presence of adverse cytogenetic lesions (Figure 3C-D). This remained true for each individual cytogenetic lesion examined (data not shown).
The impact of adverse lesions with or without the coexistence of hyperdiploidy is summarized in Figure 4 (broken down by pathway) and supplemental Figure 2 (by chemotherapy), for both PFS and OS. This clearly demonstrates that across both intensive and nonintensively treated patients receiving either novel or traditional chemotherapy, there is a detrimental impact of adverse cytogenetic lesions that is not overcome by the presence of hyperdiploidy.
The presence of any adverse lesion in patients with or without hyperdiploidy is associated with worse PFS and OS, within both the intensive and nonintensive pathways. Three patient groups were compared (group 1, HRD with no adverse lesion; group 2, HRD plus any adverse lesion; group 3, non-HRD with any adverse lesion) to analyze their PFS and OS depending on treatment pathway. (A-B) Analysis of PFS for patients through the intensive pathway (A) or nonintensive pathway (B). (C-D) Analysis of OS through the intensive pathway (C) or nonintensive pathway (D). Log-rank tests are calculated in pairwise comparisons. Note that the presence of HRD in patients with adverse lesions does not significantly improve outcome (compare group 2 vs 3). HRD, hyperdiploidy.
The presence of any adverse lesion in patients with or without hyperdiploidy is associated with worse PFS and OS, within both the intensive and nonintensive pathways. Three patient groups were compared (group 1, HRD with no adverse lesion; group 2, HRD plus any adverse lesion; group 3, non-HRD with any adverse lesion) to analyze their PFS and OS depending on treatment pathway. (A-B) Analysis of PFS for patients through the intensive pathway (A) or nonintensive pathway (B). (C-D) Analysis of OS through the intensive pathway (C) or nonintensive pathway (D). Log-rank tests are calculated in pairwise comparisons. Note that the presence of HRD in patients with adverse lesions does not significantly improve outcome (compare group 2 vs 3). HRD, hyperdiploidy.
We also examined the group of patients with t(11;14), another group associated with a more favorable prognosis in other studies though not significant in our data set (supplemental Table 1), to determine the impact of adverse cytogenetic lesions (+1q, del17p). We found that the coexistence of +1q and/or del17p was associated with shorter OS in patients with t(11;14) and there was a similar, but nonsignificant, trend for PFS (supplemental Figure 3A-B). This finding did not persist across each lesion when individually analyzed, likely due to the small number of patients involved and therefore insufficient statistical power. Conversely, there was no improvement in OS or PFS for patients who had coexistence of +1q or del17p with t(11;14) vs those who had +1q or del17p alone (supplemental Figure 3C-D), though this is difficult to interpret due to the fact that there was no improvement in survival for patients with t(11;14) across the whole data set.
Hyperdiploidy may precede IGH translocation in the myeloma etiology
The coexistence of both hyperdiploidy and an IGH translocation in a small but notable population in our and other data sets raises questions about the myeloma-initiating event in these patients. We therefore performed genetic studies to define the clonal composition of HRD-Tx patients and establish which aberration is most likely to occur first in myeloma etiology.
First, we compared iFISH cell percentages for each genetic lesion in HRD-Tx patients with available information (31/36 cases) from the Myeloma IX trial (supplemental Table 2). Different cells were analyzed for each probe and the assignments to groups inferred from the data. Most cases (22/31 patients, 71%, group 1) had both lesions present in similar cellular percentages (around 90% to 100%), supporting the hypothesis either that they were acquired simultaneously or, alternatively, that no ancestral clone can be established at the analyzed time point. In contrast, a minority of cases showed slightly higher percentages of hyperdiploidy (4/31, 13%, group 2) or translocation (5/31, 16%, group 3), providing potential evidence of these lesions acting as founder events. No significant differences were seen in the group distribution between different translocation patient groups (data not shown). However, cell percentages for 1 or 2 trisomies were missing in 3 out of 5 cases from group 3 (supplemental Table 2), which could potentially alter the case group assignment if percentages were similar to the fraction of cells with translocation. Because this analysis was not conclusive, we performed simultaneous genetic analyses of both genomic aberrations on the same cells.
Due to lack of single-cell material from Myeloma IX, we screened samples from the Myeloma XI clinical trial using multiplex ligation-dependent probe amplification and RT-PCR data to assess hyperdiploidy and translocation group, respectively. We also used whole-exome sequencing data to define the breakpoint DNA sequence for each derivative chromosome of a translocation (supplemental Figure 1).28 The selected HRD-Tx patients carried t(4;14) (n = 2), t(11;14) (n = 2), and t(14;16) (n = 1); their clinical and cytogenetic data are available in supplemental Table 3.
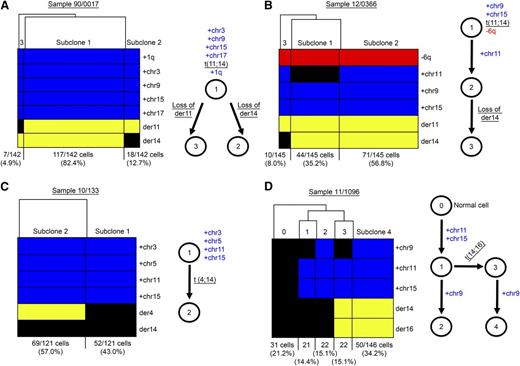
Having selected a series of HRD-Tx patients, we carried out single-cell genetic analyses using the BioMark HD (Fluidigm).6 We tested patient fixed-single cells for the simultaneous presence of trisomic chromosomes, translocation breakpoints in each derivative chromosome, and other genomic aberrations such as +1q, −6q, or −13q (Figure 5 and supplemental Figure 4). For 2 out of 5 patients, we found that the earliest ancestral clone at the analyzed time point had already accumulated most genomic aberrations: hyperdiploidy, t(11;14), and +1q or −6q (Figure 5A-B). Interestingly, both cases also showed that 1 derivative chromosome from the t(11;14) could be lost in a small cellular fraction. For instance, case 90/0017 had a major subclone that accounted for 82.4% of cells with trisomies of chr3, chr5, chr9, and chr17; a t(11;14); and +1q (subclone 1). We also described 2 minor clones (subclones 2 and 3) where der14 or der11 was lost (12.7% and 4.9% cells, respectively) (Figure 5A). Genetic analysis of sample 12/0366 also identified 3 subclones: subclone 1 (35.2%) that had trisomies for chr9 and chr15, presence of the t(11;14), and −6q; subclone 2 (56.8%) that further acquired trisomy 11; and subclone 3 (8.0%) that lost der14 (Figure 5B). Based on these data, we could not determine the HRD-Tx myeloma etiology for these cases, but the analysis of the remaining 3 HRD-Tx patients provided informative results.
Single-cell genetic analysis of 4 patients with hyperdiploidy and translocation demonstrated 2 different patterns of myeloma etiology. (A-D) Hierarchical clustering of genetic information (rows) for each single-cell (columns) established different cell groups or myeloma subclones (left). Cell numbers and subclonal proportions are shown in the bottom. Blue means gains, red means losses, and yellow means positivity for translocation breakpoint. Schematic representations of the most-plausible phylogenetic tree for the defined subclonal populations were depicted (right). (A-B) The earliest ancestral clone that can be detected at the analyzed time point already had both hyperdiploidy and IGH translocations and further evolved by acquiring additional genomic changes. (A) Sample 90/0017, with each derivative chromosomes being lost in different subclones. (B) Sample 12/0366, acquisition of an extra trisomy (HRD11) and a loss of der14. (C-D) Hyperdiploidy preceded IGH translocation as shown in the earliest ancestral clone. (C) Sample 10/133. (D) Sample 11/1096, showing HRD9 as a trisomy acquired independently in 2 subclones. HRD, hyperdiploidy. See supplemental Figure 2 for an additional case study with hyperdiploidy preceding IGH translocation. Evolutionary trees inferred by using the minimum evolution method and genetic distances are provided in supplemental Figure 3.
Single-cell genetic analysis of 4 patients with hyperdiploidy and translocation demonstrated 2 different patterns of myeloma etiology. (A-D) Hierarchical clustering of genetic information (rows) for each single-cell (columns) established different cell groups or myeloma subclones (left). Cell numbers and subclonal proportions are shown in the bottom. Blue means gains, red means losses, and yellow means positivity for translocation breakpoint. Schematic representations of the most-plausible phylogenetic tree for the defined subclonal populations were depicted (right). (A-B) The earliest ancestral clone that can be detected at the analyzed time point already had both hyperdiploidy and IGH translocations and further evolved by acquiring additional genomic changes. (A) Sample 90/0017, with each derivative chromosomes being lost in different subclones. (B) Sample 12/0366, acquisition of an extra trisomy (HRD11) and a loss of der14. (C-D) Hyperdiploidy preceded IGH translocation as shown in the earliest ancestral clone. (C) Sample 10/133. (D) Sample 11/1096, showing HRD9 as a trisomy acquired independently in 2 subclones. HRD, hyperdiploidy. See supplemental Figure 2 for an additional case study with hyperdiploidy preceding IGH translocation. Evolutionary trees inferred by using the minimum evolution method and genetic distances are provided in supplemental Figure 3.
For 3 out of 5 patients, we found that hyperdiploidy preceded t(4;14) (Figure 5C and supplemental Figure 4) or t(14;16) (Figure 5D). We described 2 subclones in case 10/133: subclone 1 (43.0%) with chr3, chr5, chr11, and chr15 gains; and subclone 2 (57.0%) with trisomies and der4 (Figure 5C). Der14, however, was not detected in any cellular fraction, suggesting that a technical assay failure occurred, as overexpression of FGFR3 and MMSET was consistently seen in the RT-PCR analysis. Sample 11/396 had a similar pattern with hyperdiploidy likely preceding t(4;14) (supplemental Figure 4A). Lastly, clonal analysis in case 11/1096 generated a complex pattern where not only hyperdiploidy occurred first but also trisomy 9 was acquired in parallel in independent lineages. Briefly, a fraction of 21.2% cells had no genomic aberrations and may represent normal CD138+ plasma cells in the bone marrow sample (subclone 0); subclone 1 had acquired trisomies in chr11 and chr15 (14.4%) and generated 2 additional clonal descendants: subclone 2 (15.1%), characterized by the additional accumulation of chr9 trisomy, and subclone 3 (15.1%) that acquired t(14;16). Remarkably, subclone 3 also acquired chr9 trisomy generating subclone 4, which represented the predominant cell fraction (34.2%) (Figure 5D). This independent acquisition of +chr9 in 2 divergent lineages represents an example of parallel evolution similar to that seen involving double hits in the RAS pathway or numerical aberrations in myeloma and other malignancies.6,38 Of note, this parallel evolutionary pattern was also seen in case 11/396 when 13q monosomy was interrogated. Both subclones 1 and 2 lost 13q generating subclones 3 and 4, respectively (supplemental Figure 4B).
Discussion
The use of cytogenetic aberrations as prognostic biomarkers can stratify high- and standard-risk myeloma patients.39 Additionally, recent genetic and single-cell studies have demonstrated that myeloma is composed of distinct subclones that all share the founding genetic aberration (IGH translocation or hyperdiploidy) but then differ from each other due to the independent accumulation of secondary genetic lesions.3-6 In this study, we reported that in 4% of patients presenting with myeloma, hyperdiploidy and IGH translocations occur concurrently. This contradicts the often-held assumption among clinicians that these founding events in myeloma pathogenesis are mutually exclusive. These results raise the mechanistic issue as to which cytogenetic abnormality occurs first as well as the more practical issue of what it means for prognosis.
We sought to answer the latter question by further analyzing patients with coexistent hyperdiploidy and adverse translocations as well as other adverse genetic lesions. We demonstrate that the coexistence of hyperdiploidy or t(11;14) does not prevent poor prognostic lesions, including adverse translocations, del17p, and +1q, from shortening survival in myeloma patients and does not even ameliorate their adverse effects (Figures 1, 2, and 4). Moreover the coexistence of increasing numbers of adverse cytogenetic lesions worsens survival further (Figure 3).
It is worth noting that the number of patients with hyperdiploidy and an adverse translocation is small (n = 22) despite the large size of the data set as a whole. In this group, the comparison of median survival to that of patients with hyperdiploidy and no adverse lesions shows significantly shorter survival (Figure 2C). The significance is lost when those patients with hyperdiploidy and adverse translocation without additional +1q or del17p are compared with patients with hyperdiploidy and no adverse lesions (Figure 3B), but the trend is the same, and loss of significance is likely due to a reduction in the number of such cases to 9.
Our data are consistent with the study of Chng et al,19 which found that adverse translocations significantly shortened survival in patients with hyperdiploidy. They also described a trend for patients with coexistent hyperdiploidy and del17p, but this did not reach significance, perhaps due to the small sample size. In contrast, the study of Kumar et al found that the presence of even 1 chromosomal trisomy was able to ameliorate the adverse effect of the t(4;14), t(14;16), and t(14;20).20 This paper took a different analysis approach to our study, where we compared patients with classically defined hyperdiploidy (≥2 trisomies). Additionally, their study was a retrospective analysis of 484 patients, whereas ours was a prospective analysis in a large clinical trial with standard induction regimens.
Our results have important implications for the clinical management of patients, as there is increasing interest in using risk-adapted therapy based on cytogenetic, ISS, and gene-expression profiling data. It is thought that by using more dose-dense chemotherapy plus consolidation and/or maintenance treatment, we might improve outcomes for those patients at high risk.40,41 We therefore need to be able to accurately identify the risk status of patients in order to avoid unnecessary excess toxicities in those genuinely at lower risk while not undertreating those at high risk. This therapeutic dilemma makes it important to understand how the clinical outcome is affected when good and poor prognostic lesions occur simultaneously. Our results clearly show that the risk status of patients with coexistence of both good and adverse lesions should be based on the adverse lesion and these patients treated as high risk.
Our study also raises several biological questions about myeloma etiology. IGH translocations and hyperdiploidy are postulated to be myeloma-founding events, but the mechanisms by which they arise and which genetic lesion occurs first in HRD-Tx patients are open questions. Although we have recently demonstrated that IGH translocations are more often the product of aberrant class-switch recombination processes at the germinal center during B-cell development, they may also occur as a consequence of an aberrant DHJH recombination, possibly at an earlier B-cell stage in up to 25% of non-t(4;14) samples.31 In contrast, the mechanism and origins of hyperdiploidy are more difficult to describe. There are 4 different biological processes proposed: first, a near-haploid cell doubles all chromosomes; second, a tetraploid cell has subsequent loss of chromosomes; third, a diploid cell has sequential gains of chromosomes during clonal evolution; or lastly, a diploid cell suffers a single mitotic catastrophe resulting in simultaneous gain of all additional chromosomes.42 According to our iFISH results, cell percentages positive for chromosomal gains for each studied chromosome are very similar, which suggests that they may be acquired simultaneously and in the same cells. However, there are cases where an additional chromosome is reported in a lower cell percentage, suggesting that sequential gains may follow initial hyperdiploidy (supplemental Table 2). We corroborated these findings when performing single-cell genetic analysis of HRD-Tx patients, as all cases had the most ancestral clones already carrying 2 or more trisomies, and 2 out of 5 patients further accumulated an extra trisomy during clonal evolution (Figure 5 and supplementary Figure 4). Based on our results, we suggest that a single mitotic catastrophe may be the most likely mechanism of acquisition of hyperdiploidy, in agreement with other studies of hyperdiploidy in childhood-acute lymphoblastic leukemia,42 but it can also be followed by secondary accumulation of extra chromosomes.
Our investigation into which genetic aberration triggers myeloma initiation in HRD-Tx patients demonstrated 2 potential evolutionary frameworks for disease progression. In the first, 2 out of 5 patients showed the earliest ancestral subclone already carrying both hyperdiploidy and the IGH translocation, supporting that either both lesions occurred simultaneously or the founding subclone with only one of these cytogenetic lesions could not be detected at the disease time point at which we performed the analysis (Figure 5A-B). Conversely, the second pattern (3 out of 5 patients) showed ancestral subclones carrying hyperdiploidy with no IGH translocation, as well as separate subclones where both lesions coexisted (Figure 5C-D and supplemental Figures 4 and 5), which supports an initiating role for hyperdiploidy and the IGH translocation as a secondary event. Alternative explanations include an unlikely simultaneous accumulation of hyperdiploidy and translocation followed by the subclonal loss of both derivative chromosomes, or the presence of an IGH oligoclonality or multiple translocations with different breakpoints not detected with the probes used.43 Additionally, all translocations in the 11 HRD-Tx patients sequenced in this study (supplemental Figure 1) originated from aberrant somatic hypermutation or class-switch recombination processes (data not shown) and did not involve VDJ rearrangements. The latter has been seen in a small fraction of myeloma patients31 and would occur in pro–B-cell stages and so could precede hyperdiploidy if trisomies were found in such myeloma plasma cells. Having said this, based on our results, we can only conclude that the initial accumulation of hyperdiploidy followed by IGH translocation in HRD-Tx patients represents the most parsimonious evolutionary framework. This model is in agreement with other temporal analyses of hyperdiploid myeloma where trisomies were shown to precede translocations and monosomies or deletions20,44 and cytogenetic studies of myeloma and monoclonal gammopathy of undetermined significance where patients had 14q32 translocations, but these were absent in the primordial aberrant myeloma clone.45,46 Therefore, hyperdiploidy may occur earlier in the B-cell development but simply confer a slight proliferative cell advantage that will be further enhanced by the acquisition of additional genetic lesions, such as an IGH translocation, loss of 13q or 17p, or RAS mutations, during the course of clonal evolution and disease progression. The presence of hyperdiploidy and its favorable prognostic value is not an exclusive event of myeloma and has been seen in other hematologic malignancies such as acute myeloid leukemias47 and childhood B-acute lymphoblastic leukemia,42,48,49 where hyperdiploidy involves different chromosomes and sometimes occurs at a prenatal stage.48 Altogether, it seems that hyperdiploidy is an important event in the initiation of the malignant process requiring the acquisition of additional genetic lesions to drive progression of the disease. The combination of clinical and biological data demonstrates that patients who have hyperdiploidy as their only cytogenetic abnormality have a good prognosis, but if these patients subsequently acquire an adverse translocation, then this drives poor prognosis.
In summary, we provide strong evidence that the presence of hyperdiploidy or t(11;14), both cytogenetic abnormalities that are good prognosis biomarkers, does not abrogate the poor prognosis associated with the presence of adverse cytogenetic lesions when found together in the same patient. The outcome of patients with hyperdiploidy and adverse lesions is worse when >1 adverse lesion is present. We establish the most likely etiologic framework of myeloma where hyperdiploidy and IGH translocations occur together is for hyperdiploidy to occur first, but studies in bigger data sets should be performed to confirm this. It may also be informative to carry out single-cell analysis of monoclonal gammopathy of undetermined significance patients with HRD-Tx, as studying this earlier time point in myeloma disease progression might yield further evidence of the time course of acquisition of these events. Nevertheless, the combined biological and clinical data from this study strongly suggest that patients with hyperdiploidy and adverse cytogenetic lesions should be treated as high-risk patients.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all of the patients, investigators, and staff who participated in the Myeloma IX and XI trials.
Financial support for the Myeloma IX trial and linked studies was obtained from the MRC, the National Institutes of Health Biomedical Research Centre at the Royal Marsden Hospital, and Institute of Cancer Research and Leukaemia & Lymphoma Research, with additional funding in the form of unrestricted educational grants from Novartis, Schering Health Care, Chugai, Pharmion, Celgene, and Ortho Biotech, mainly to support trial coordination and laboratory studies. Myeloma XI sample collection and processing was supported by a Myeloma UK program grant, a Cancer Research UK Clinical Trials Awards and Advisory Committee sample collection grant, and a Cancer Research UK Biomarkers and Imaging Discovery and Development grant as well as funds from the National Institutes of Health Biomedical Research Centre at the Royal Marsden Hospital and Institute of Cancer Research. C.P. is a Wellcome Trust Clinical Research Fellow, and F.E.D. is a Cancer Research UK Senior Cancer Fellow.
Authorship
Contribution: C.P., L.M., F.E.D., and G.J.M. conceived and designed the study; L.M., B.A.W., D.B.B., N.B.D., P.P., M.T.D., G.H.J., F.M.R., F.E.D., and G.J.M. acquired data; C.P., L.M., A.M., C.P.W., A.B., E.M.B., M.F.K., B.A.W., D.B.B., N.B.D., P.P., W.M.G., and F.M.R. analyzed data; and C.P., L.M., A.M., C.P.W., A.B., E.M.B., M.F.K., B.A.W., D.B.B., N.B.D., P.P., W.M.G., M.T.D., G.H.J., F.E.D., and G.J.M. wrote, reviewed, or revised the manuscript.
Conflict-of-interest disclosure: C.P. and A.B. have received honoraria and travel support from Celgene, M.F.K. has received honoraria and research funding from Celgene, G.H.J. and W.M.G. have received honoraria from Celgene and Janssen, and F.E.D. and G.J.M. have received honoraria and travel support from and participated in advisory boards for Celgene, Janssen, Millennium, and Onyx. The remaining authors declare no competing financial interests.
The current affiliation for B.A.W. is Centre for Molecular Pathology, Royal Marsden Hospital NHS Foundation Trust, London, United Kingdom.
The current affiliation for F.E.D. and G.J.M., in addition to the Institute of Cancer Research, London, is Myeloma Institute for Research and Therapy, University of Arkansas for Medical Sciences, Little Rock, AR.
Correspondence: Gareth J. Morgan, Myeloma Institute for Research and Therapy, University of Arkansas for Medical Sciences, 4302 West Markham, #816, Little Rock, AK; e-mail: gjmorgan@uams.edu.
References
Author notes
C.P. and L.M. contributed equally to this study.