Key Points
The reduced thrombosis in Klkb1−/− mice is not by defective contact activation.
Overexpressed renal Mas with elevated plasma prostacyclin increases aortic Sirt1 and KLF4 and reduces aortic TF protecting Klkb1−/− mice.
Abstract
The precise mechanism for reduced thrombosis in prekallikrein null mice (Klkb1−/−) is unknown. Klkb1−/− mice have delayed carotid artery occlusion times on the rose bengal and ferric chloride thrombosis models. Klkb1−/− plasmas have long-activated partial thromboplastin times and defective contact activation–induced thrombin generation that partially corrects upon prolonged incubation. However, in contact activation–induced pulmonary thromboembolism by collagen/epinephrine or long-chain polyphosphate, Klkb1−/− mice, unlike F12−/− mice, do not have survival advantage. Klkb1−/− mice have reduced plasma BK levels and renal B2R mRNA. They also have increased expression of the renal receptor Mas and plasma prostacyclin. Increased prostacyclin is associated with elevated aortic vasculoprotective transcription factors Sirt1 and KLF4. Treatment of Klkb1−/− mice with the Mas antagonist A-779, COX-2 inhibitor nimesulide, or Sirt1 inhibitor splitomicin lowers plasma prostacyclin and normalizes arterial thrombosis times. Treatment of normal mice with the Mas agonist AVE0991 reduces thrombosis. Klkb1−/− mice have reduced aortic tissue factor (TF) mRNA, antigen, and activity. In sum, Klkb1−/− mice have a novel mechanism for thrombosis protection in addition to reduced contact activation. This pathway arises when bradykinin delivery to vasculature is compromised and mediated by increased receptor Mas, prostacyclin, Sirt1, and KLF4, leading to reduced vascular TF.
Introduction
Prekallikrein (PK), the precursor for plasma kallikrein (KK), circulates in complex with plasma high-molecular-weight kininogen (HK).1 PK is converted to KK by activated factor XII (αXIIa) on biological or artificial surfaces by the process of contact activation, in plasma or solution by soluble Hageman factor fragment (βXIIa) or by the endothelial cell–bound serine protease prolylcarboxypeptidase (PRCP).2-4 In the contact activation system (CAS), formed KK, a serine protease, activates zymogen factor XII (XII) to αXIIa in a reciprocal manner, amplifying XII autoactivation to initiate the intrinsic pathway of coagulation, leading to thrombin generation and fibrin formation.5 KK also promotes inflammation through the kallikrein/kinin system (KKS) by cleaving HK in solution and bound to endothelium to liberate the vasoactive peptide bradykinin (BK).6,7 BK binds to constitutively expressed bradykinin B2 receptor (B2R), regulating kininogen binding sites, inducing vasodilation and vascular permeability, and reducing thrombus formation.8-12 Intravascular PK activation and BK release are both physiologic and pathophysiologic processes, because BK formation regulates vascular tone, and the deficiency of the major KK and αXIIa inhibitor, C1 inhibitor, causes constitutive BK-mediated angioedema.13
PK deficiency in humans (Fletcher trait) has a prolonged activated partial thromboplastin time (aPTT) that corrects on longer incubation of plasma in glass tubes.14 Although PK activation promotes blood coagulation through the CAS, PK-deficient patients have no hemostatic defect.14 Selective reduction of murine PK by antisense oligonucleotides yields reduced thrombus size without bleeding.15 PK-deficient mice (Klkb1−/−) have delayed ferric chloride–induced carotid artery thrombosis.16 These combined studies indicate that PK deficiency reduces thrombosis without change in hemostasis.
The precise mechanism(s) leading to delayed thrombosis in PK-deficient mice is not known. Recent investigations indicate that Bdkrb2−/− mice, B2R-deficient animals, have delayed thrombosis by a novel mechanism whereby 2 receptors from the renin-angiotensin system (RAS), the angiotensin receptor 2 (AT2R) and Mas, become overexpressed to bind angiotensin II (AngII) and angiotensin-(1-7) [Ang-(1-7)], respectively, to increase nitric oxide (NO) and prostacyclin (PGI2).17,18 We asked whether the thrombosis delay in PK-deficient mice is caused by reduced contact activation or less BK delivery to tissues, or both. This investigation presents a novel mechanism for thrombosis reduction in Klkb1−/− mice. Klkb1−/− mice have reduced thrombosis risk by a mechanism related to the Bdkrb2−/− mice whereby overexpression of the Mas receptor is associated with elevated plasma PGI2. The enhanced Mas-prostacyclin axis produces increased aortic mRNA and protein of the vasculoprotective transcription factors sirtuin-1 (Sirt1) and Kruppel-like factor 4 (KLF4) with decreased vascular tissue factor (TF). This pathway for thrombosis reduction highlights the interaction between the KKS and RAS independent of the CAS and the importance of the Mas-prostacyclin axis in the modulation of arterial thrombosis risk in vivo.
Materials and methods
Materials
Sirt1 inhibitor splitomicin was purchased from Tocris and Cayman Chemicals. Mas antagonist A-779 was obtained from Bachem. Mas agonist AVE0991 sodium salt was custom synthesized by MedChem Express. Carbaprostacyclin (cPGI2) was purchased from Cayman Chemicals. Insoluble high-molecular-weight bacterial sodium polyphosphate (>75 U per polymer) (LC polyp) was generously provided by Dr James Morrissey (University of Illinois) (see supplemental Methods, available on the Blood Web site). Collagen-related peptide (CRP) was a gift from Dr Debra Newman (BloodCenter of Wisconsin). Monoclonal antibody to murine fibrin (59D8) was generously provided by Dr Harmut. Weiler (BloodCenter of Wisconsin). rHA-Infestin-4 was generously provided by Dr Marc Nolte (CSL Behring, Marburg, Germany).
Animals
Prekallikrein-deficient mice (Klkb1−/−) were generated at Texas Genomic Institute and generously provided by Dr Edward Feener of Harvard University (see supplemental Methods). The knockout (KO) mice were produced in a B6/129 background and backcrossed 4 generations into a C57BL/6 background. These animals were mated with wild-type (WT) mice (C57BL/6, Jackson Laboratories) to make heterozygous animals and were re-derived into Klkb1−/−. Klkb1−/− mice and littermate WT colonies were maintained by brother/sister mating. Every 10 generations, the Klkb1−/− mice are mated with C57BL/6J to re-derive KOs from the heterozygous mice. The genotyping of Klkb1−/− mice was determined with 2 sets of primers: Forward 5′-CTTCCAGGTAGCTGCTTTCTACC-3′ and Reverse 5′-TCACCCACAACCTTCACAGAAAGG-3′ for WT (245-bp band), and Forward 5′-CGCTGCTTAGGATGGTAGGAG-3′ and Reverse 5′-GCTAGACTAGTCTAGCTAG-AGCGG-3′ for KO (391-bp band) (see the supplemental Methods). F12−/− mice (deficient in coagulation factor XII) in a C57BL/6J background were generously provided by Dr Frank Castellino of the University of Notre Dame (see supplemental Methods). Animal care and procedures were reviewed and approved by the Institutional Animal Care and Use Committees at Case Western Reserve University (CWRU) and performed in accordance with the guidelines of the American Association for Accreditation of Laboratory Animal Care and the National Institutes of Health.
All other assays and methods are described in the supplemental Methods.
Results
Characterization of Klkb1−/− mice
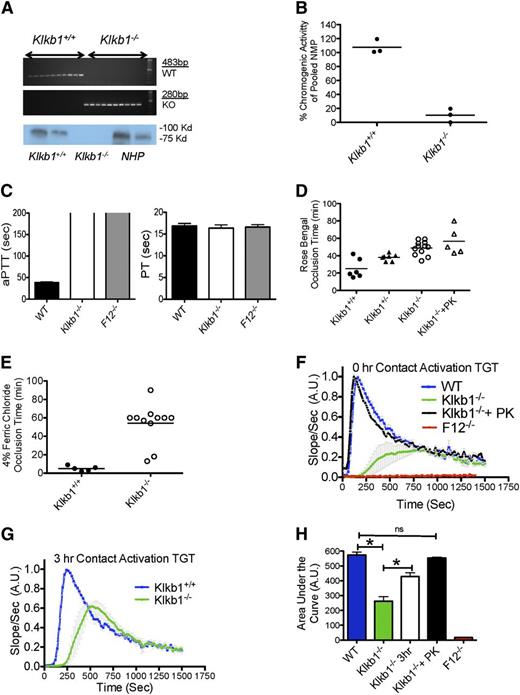
Klkb1−/− mice were prepared by exon 1 and 2 deletion of the Klkb1 gene with replacement by a LacZ/neomycin cassette.19 On polymerase chain reaction of genomic DNA, WT and Klkb1−/− produced 245-bp and 391-bp bands, respectively (Figure 1A, upper 2 panels). Immunoblot studies showed no detectable PK antigen in Klkb1−/− plasma (Figure 1A, bottom panel), unlike normal mouse and human plasmas. On a chromogenic assay for plasma PK, Klkb1−/− plasma had <10% plasma kallikrein activity compared with normal plasma (Figure 1B). Like the Fletcher trait in humans, Klkb1−/− plasma had a significantly prolonged aPTT. The mean clotting time of WT plasma was 38.6 ± 1.8 seconds, whereas the Klkb1−/− plasma was >200 seconds, like XII-deficient plasma (Figure 1C). The degree of prolongation of the aPTT of murine Klkb1−/− plasma was substantially longer than that typically observed in humans with Fletcher trait.14 Because the aPTT was prolonged in Klkb1−/− plasma, we determined the levels of the other contact system factors. In Klkb1−/− plasmas, XII was 90 ± 5.7%, factor XI (XI) was 80 ± 4.2%, and HK was 120 ± 9.3%, which were not significantly different from WT (n = 5 for each genotype) when compared with pooled normal mouse plasma. The prothrombin time was not significantly different between the 3 genotypes (16.9 ± 0.6 s in WT, 16.4 ± 0.8 s in Klkb1−/−, and 16.6 ± 0.6 s in F12−/−) (P > .05, n = 4 in each group) (Figure 1C). These combined data confirmed the absence of PK antigen and activity in Klkb1−/− mice. On complete blood counts, Klkb1−/− mice had white blood cell counts, red blood cell counts, hemoglobin values, mean corpuscular volumes, and platelet counts that were not significantly different from those of WT mice (supplemental Table 2).
Characterization of Klkb1−/− mice. (A, top) An agarose gel from a polymerase chain reaction showing genotype of WT control (Klkb1+/+) and Klkb1−/−; (bottom) immunoblots for prekallikrein antigen in murine Klkb1+/+ and Klkb1−/− plasmas and normal human plasma (NHP). Both murine and human plasmas were added at 2 different concentrations (see Materials and methods). (B) Plasma kallikrein activity from Klkb1+/+ and Klkb1−/− mice after activation of PK as determined by cleavage of chromogenic substrate HD-Pro-Phe-Arg-pNA. The data are presented as percentage of pooled normal murine plasma KK activity. Three individual mice plasmas were examined in each genotype. (C) aPTT (left 3 columns) and PT (right 3 columns) were determined in WT, Klkb1−/−, and F12−/− plasmas (n = 4 in each group). The absence of the line on the top of 2 aPTT bar graphs indicates that they were >200 seconds. (D) Carotid artery occlusion times on the rose bengal thrombosis model. Klkb1+/+, Klkb1+/−, Klkb1−/−, and Klkb1−/− mice reconstituted with purified human plasma prekallikrein to make the blood 450 nM were examined in this assay. Five to 12 animals were examined in each group. (E) Carotid artery occlusion times on the ferric chloride thrombosis model. (F-G) Contact activation–induced thrombin generation times (TGT) in WT (Klkb1+/+), Klkb1−/−, Klkb1−/− + 450 nM human prekallikrein, or F12−/− plasma without incubation (F) or after incubation for 3 hours at room temperature (G) (n = 4 in each group). The slopes of the TGT are relative values normalized to WT. (H) The AUCs were calculated and quantified from the TGT curves in (F). Data were normalized to the WT value for each set of experiments.
Characterization of Klkb1−/− mice. (A, top) An agarose gel from a polymerase chain reaction showing genotype of WT control (Klkb1+/+) and Klkb1−/−; (bottom) immunoblots for prekallikrein antigen in murine Klkb1+/+ and Klkb1−/− plasmas and normal human plasma (NHP). Both murine and human plasmas were added at 2 different concentrations (see Materials and methods). (B) Plasma kallikrein activity from Klkb1+/+ and Klkb1−/− mice after activation of PK as determined by cleavage of chromogenic substrate HD-Pro-Phe-Arg-pNA. The data are presented as percentage of pooled normal murine plasma KK activity. Three individual mice plasmas were examined in each genotype. (C) aPTT (left 3 columns) and PT (right 3 columns) were determined in WT, Klkb1−/−, and F12−/− plasmas (n = 4 in each group). The absence of the line on the top of 2 aPTT bar graphs indicates that they were >200 seconds. (D) Carotid artery occlusion times on the rose bengal thrombosis model. Klkb1+/+, Klkb1+/−, Klkb1−/−, and Klkb1−/− mice reconstituted with purified human plasma prekallikrein to make the blood 450 nM were examined in this assay. Five to 12 animals were examined in each group. (E) Carotid artery occlusion times on the ferric chloride thrombosis model. (F-G) Contact activation–induced thrombin generation times (TGT) in WT (Klkb1+/+), Klkb1−/−, Klkb1−/− + 450 nM human prekallikrein, or F12−/− plasma without incubation (F) or after incubation for 3 hours at room temperature (G) (n = 4 in each group). The slopes of the TGT are relative values normalized to WT. (H) The AUCs were calculated and quantified from the TGT curves in (F). Data were normalized to the WT value for each set of experiments.
Recent studies showed delayed thrombosis in Klkb1−/− mice.16 On the rose bengal assay, Klkb1−/− mice (49 ± 2.3 min [n = 12]) had longer occlusion times compared with WT (25.0 ± 4.6 min, n = 6) (P < .005) (Figure 1D). Klkb1+/− mice had an occlusion time in between WT and KO, 38 ± 1.4 min (n = 7). When Klkb1−/− mice were reconstituted with 1 μM purified human PK such that their aPTTs were corrected to normal (supplemental Figure 1), the time (57 ± 7 min [n = 5]) to arterial occlusion did not correct (Figure 1D). These data suggested that the mechanism(s) for delayed thrombosis in Klkb1−/− mice was not entirely caused by PK deficiency. Using 4% ferric chloride, we also observed thrombosis delay in Klkb1−/− mice. All WT animals had complete occlusion in the carotid artery within 10 minutes (5.0 ± 1.2 min, n = 5), whereas Klkb1−/− mice occluded at 54 ± 6.4 minutes (n = 11) (P < .001) (Figure 1E). These combined data showed that PK deficiency likewise was associated with reduced thrombosis in our models.
Klkb1−/− mice have reduced contact activation
KK participates in contact activation by cleaving XII into αXIIa, leading to thrombin generation through XI activation of the intrinsic pathway. We determined the plasma thrombin generation times (TGT) in Klkb1−/− mice.
Consistent with the prolonged aPTT, Klkb1−/− plasma had defective contact activation–induced TGT (Figure 1F). When the Klkb1−/− plasma was reconstituted with purified human PK to make it 450 nM, the contact activation TGT corrected to normal (Figure 1F). There was no contact activation on the TGT with F12−/− plasma (Figure 1F). The mean peak height of TGT in Klkb1−/− plasma was 37 ± 7% (n = 4) compared with WT plasma (n = 8) (P < .001) (supplemental Figure 2). Upon prolonged incubation of Klkb1−/− plasma for 3 hours at room temperature in a glass cuvette, the TGT and mean peak height partially corrected (73 ± 5% compared with WT, n = 4) but was still significantly lower than WT plasma (n = 8) (Figure 1G and supplemental Figure 2) (P < .001). Similarly, the mean total area under the curve (AUC) for contact activation–induced TGT in Klkb1−/− plasma was 46 ± 8% (n = 4) compared with WT plasma (n = 8) (P < .001), which upon 3-hour incubation partially corrected to 76 ± 4% (n = 4) (Figure 1H) (P < .001). F12−/− plasma had no AUC (Figure 1H). These combined data indicated that Klkb1−/− plasma had defective contact activation.
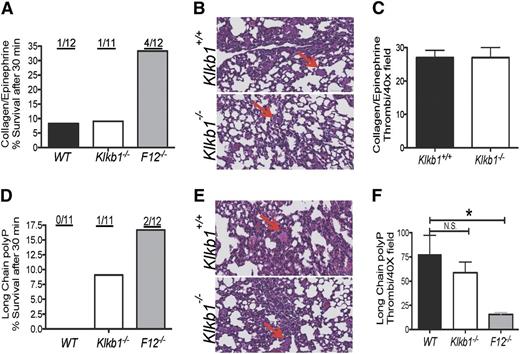
We next asked whether defective contact activation contributed to the reduced thrombosis risk as observed in F12−/− mice.20-22 Klkb1−/− mice were examined on both the collagen/epinephrine (Col/Epi) and bacterial long-chain polyphosphate (LC polyP) models of lethal pulmonary embolism.21,22 After Col/Epi injection into the jugular vein, 11 of 12 WT and 10 11 Klkb1−/− mice died within 30 minutes of challenge (survival rate 8% and 9% for WT and Klkb1−/−, respectively). However, only 8 of 12 F12−/− mice died (33% survival rate), which is consistent with a previous report showing a survival advantage of F12−/− mice in this model21 (Figure 2A). Histopathology studies with H&E staining of lung tissues suggested that Klkb1+/+ and Klkb1−/− mice died of massive pulmonary emboli by the large number of occluded vessels observed. The vessel occlusions were quantified and there was no significant difference between WT and Klkb1−/− mice in the Col/Epi model (Figure 2B-C).
Role of contact activation in thrombosis protection in Klkb1−/−. (A,D) Survival rate in WT, Klkb1−/−, and F12−/− mice after challenge with collagen/epinephrine (Col/Epi) (A) or long-chain bacterial polyphosphate (LC polyP) (D). The ratios on top of each column represent the number of mice surviving 30 minutes. (B,E) Hematoxylin and eosin (H&E) staining of lung sections from Col/Epi- (B) or LC polyp- (E) challenged Klkb1+/+ and Klkb1−/− mice. The red arrows point to representative occluded vessels. Images were obtained from a Leica SCN 400 Slide Scanner equipped with a Hamamatsu line-sensor color camera and a ×40/0.65 objective lens. The images were made through a ×2 tube lens at ×40 final magnification. (C) (Col/Epi) and (F) (LC polyP). Number of vessel occlusions quantified from H&E staining. Vessel occlusions per visual field were counted at ×40. Mean ± standard error of the mean (SEM) of 100 fields per group are shown. *Significant difference (P < .05) among the groups on 1-way analysis of variance (ANOVA).
Role of contact activation in thrombosis protection in Klkb1−/−. (A,D) Survival rate in WT, Klkb1−/−, and F12−/− mice after challenge with collagen/epinephrine (Col/Epi) (A) or long-chain bacterial polyphosphate (LC polyP) (D). The ratios on top of each column represent the number of mice surviving 30 minutes. (B,E) Hematoxylin and eosin (H&E) staining of lung sections from Col/Epi- (B) or LC polyp- (E) challenged Klkb1+/+ and Klkb1−/− mice. The red arrows point to representative occluded vessels. Images were obtained from a Leica SCN 400 Slide Scanner equipped with a Hamamatsu line-sensor color camera and a ×40/0.65 objective lens. The images were made through a ×2 tube lens at ×40 final magnification. (C) (Col/Epi) and (F) (LC polyP). Number of vessel occlusions quantified from H&E staining. Vessel occlusions per visual field were counted at ×40. Mean ± standard error of the mean (SEM) of 100 fields per group are shown. *Significant difference (P < .05) among the groups on 1-way analysis of variance (ANOVA).
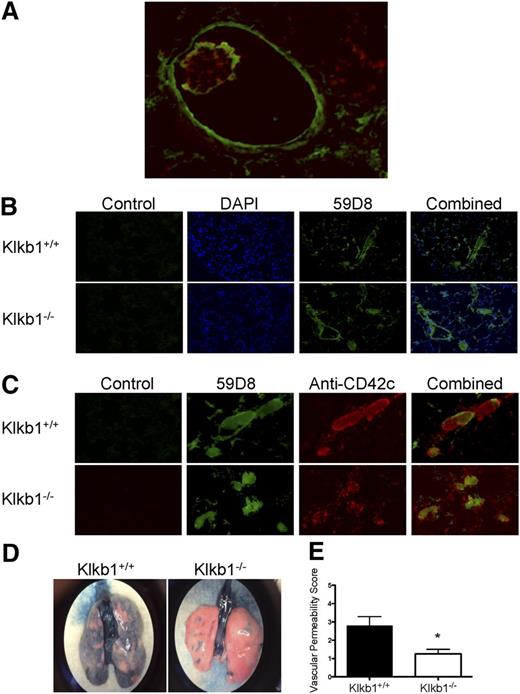
To confirm that the occluded vessels observed on H&E staining in the previous figure were caused by fibrin deposition, additional investigations were performed. Several large vessels in the Col/Epi model had an intraluminal rim of murine fibrin as detected by monoclonal antibody 59D8 (Figure 3A).21 In addition, in the lumen of these vessels, platelet thrombi as detected by anti-CD42c were observed also with a fibrin rim (Figure 3A). When the microvasculature was examined, Klkb1+/+ and Klkb1−/− mice had an equal number of fibrin-occluded vessels matching what was observed on H&E stain (Figure 3B). Moreover, these microvessels had an equal amount of platelet material within the occluded vessels and the platelet material colocalized with fibrin (Figure 3C). This assessment was based on the confluence of the green (fibrin) and red (platelet) staining to give yellow vessel occlusions (Figure 3C). Thus, both the Klkb1−/− and Klkb1+/+ mice had similar degrees of fibrin and platelet material in their vessels, the hallmark of lung injury.23 However, even though there were similar degrees of thrombosis, the degree of lung edema-leakiness was distinctly different. Using Evans blue staining, we found that Klkb1−/− mice have significantly reduced vascular permeability when compared with WT mice after the Col/Epi-induced pulmonary embolism model as indicated by reduced blue dye present in lung tissue (Figure 3D-E). These studies indicated that vascular permeability and fibrin/platelet thrombi formation were from unrelated mechanisms in the Klkb1−/− mice.
Demonstration of vessel fibrin, platelets, and permeability after contact activation injury to the lung. (A) Immunofluorescence of a large vessel with an internal fibrin rim using Mab 59D8 (green) and platelet thrombus using anti-CD42c (red) with a fibrin border after Col/Epi-induced pulmonary embolism. (B) Immunofluorescence of microvessel thrombi for fibrin after Col/Epi pulmonary embolism. (C) Immunofluorescence of colocalization of fibrin and platelets in microvessel thrombi after Col/Epi pulmonary embolism. (A-C) Images were obtained on a Nikon Eclipse TE2000-S microscope at ×10 magnification. (D) Representative whole-lung Evans blue dye permeability in Klkb1+/+ and Klkb1−/− tissue. The images were taken with an iPhone 5 through a ×2 objective of a Nikon C-W 10XB/22 dissecting microscope. (E) Vascular permeability score for Evans blue dye in Klkb1+/+ (n = 5) and Klkb1−/− (n = 4) lungs (P < .036).
Demonstration of vessel fibrin, platelets, and permeability after contact activation injury to the lung. (A) Immunofluorescence of a large vessel with an internal fibrin rim using Mab 59D8 (green) and platelet thrombus using anti-CD42c (red) with a fibrin border after Col/Epi-induced pulmonary embolism. (B) Immunofluorescence of microvessel thrombi for fibrin after Col/Epi pulmonary embolism. (C) Immunofluorescence of colocalization of fibrin and platelets in microvessel thrombi after Col/Epi pulmonary embolism. (A-C) Images were obtained on a Nikon Eclipse TE2000-S microscope at ×10 magnification. (D) Representative whole-lung Evans blue dye permeability in Klkb1+/+ and Klkb1−/− tissue. The images were taken with an iPhone 5 through a ×2 objective of a Nikon C-W 10XB/22 dissecting microscope. (E) Vascular permeability score for Evans blue dye in Klkb1+/+ (n = 5) and Klkb1−/− (n = 4) lungs (P < .036).
Similar to the aforementioned studies, when these animals were challenged with LC polyP after inferior vena cava injection, 10 of 11 Klkb1−/− and all WT (11/11) died within 30 minutes (Figure 2D). Alternatively, 2 of 12 F12−/− mice survived the LC polyP pulmonary embolism model.22 Histopathologic examination with H&E staining of lung tissues confirmed that Klkb1+/+ and Klkb1−/− mice died of massive pulmonary embolism with a similar number of vessel occlusions quantified in each animal model (Figure 2E-F). In contrast, F12−/− mice had a significantly lower number of vessel occlusions compared with WT in the LC polyP model (Figure 2F). These combined data showed that Klkb1−/− mice, unlike F12−/− mice, were not protected from Col/Epi- or LC polyP-induced pulmonary embolism, suggesting that the reduced arterial thrombosis observed in these animals may not be just defective contact activation. We sought additional mechanism(s) for the thrombosis delay in Klkb1−/− mice.
Role of Mas receptor in thrombosis protection in Klkb1−/− mice
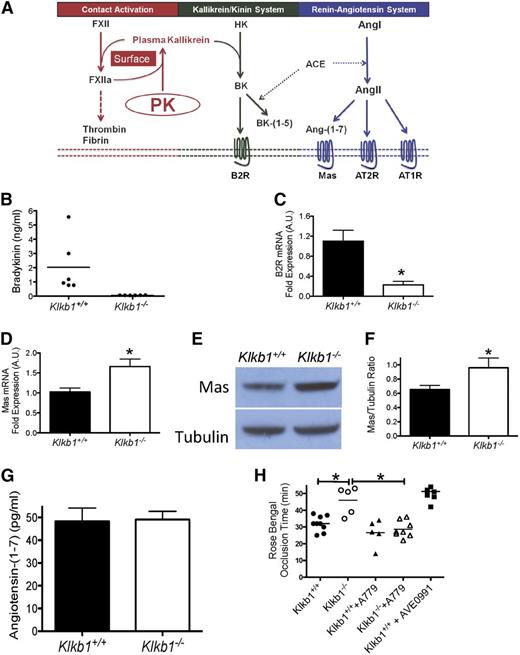
Recently, our laboratory showed that in Bdkrb2−/− mice, there is increased plasma BK, BK-(1-5), and AngII and its breakdown product Ang-(1-7).17,18 In Bdkrb2−/− mice, the elevated AngII and Ang-(1-7) interact with their additionally overexpressed receptors, AT2R and Mas, respectively, to elevate plasma NO and PGI2, leading thrombosis delay.17,18 The proteins and peptides of the KKS and RAS interact at multiple sites to influence thrombosis risk (Figure 4A).24 We examined whether there were alterations in the KKS and RAS in Klkb1−/− mice to account for their thrombosis delay. Both F12−/− and Kgn1−/− mice are BK deficient and have reduced thrombosis.20,25 Because KK cleaves HK to liberate BK, we determined plasma BK levels in PK-deficient mice.19,20 Klkb1−/− mice had plasma BK (0.07 ± 0.008 ng/mL, n = 6) levels 29-fold less than WT (2 ± 0.8 ng/mL, n = 6) (P < .0001) (Figure 4B). Further, Klkb1−/− mice have an 80% reduction in renal B2R mRNA (Figure 4C). Additional investigations revealed that renal mRNA for ACE and AT2R was reduced in Klkb1−/− mice, but mRNAs for the bradykinin B1 receptor (B1R) and AT1R were normal (supplemental Figure 3).
The influence of Mas receptor on thrombosis risk in Klkb1−/− mice. (A) The contact activation system (CAS), kallikrein/kinin system (KKS), and renin-angiotensin system (RAS) interact with each other. ACE, angiotensin-converting enzyme; Ang-(1-7), angiotensin-(1-7); AngI, angiotensin I; AngII, angiotensin II; AT1R,angiotensin receptor 1; AT2R,angiotensin receptor 2; B2R, bradykinin B2 receptor; BK, bradykinin; BK-(1-5), the ACE breakdown product of BK (peptide RPPGF); FXII, factor XII; FXIIa, activated XII; HK, high-molecular-weight kininogen; Mas, the receptor Mas; PK, prekallikrein. The CAS is initiated by XII autoactivation on a surface to convert PK to plasma kallikrein. These 2 proteases reciprocally activate each other and amplify their activation. Plasma kallikrein cleaves HK to liberate BK in the KKS. BK binds to its receptor, B2R, to activate cells or be internalized and degraded. It is also degraded to BK-(1-5) by ACE. ACE interacts with the RAS because it converts AngI to AngII to bind its receptors, AT1R or AT2R. AngII also is degraded to Ang-(1-7) to bind to its receptor Mas. (B) Plasma bradykinin (BK) levels in Klkb1+/+ and Klkb1−/− mice (n = 6 in each genotype). (C) Renal mRNA of B2R in Klkb1+/+ (n = 6) and Klkb1−/− mice (n = 5). (D) Renal mRNA of Mas in Klkb1+/+ (n = 6) and Klkb1−/− mice (n = 5). (E) Immunoblots for renal Mas showing a representative gel from multiple individual studies of kidney lysates from 8 Klkb1+/+ and 7 Klkb1−/− mice. β-tubulin served as the loading control. (F) Ratio of Mas to β-tubulin as quantified by densitometry using ImageJ software. (G) Plasma angiotensin-(1-7) levels in Klkb1+/+ and Klkb1−/− mice (n = 9 in each group). (H) The influence of Mas antagonist A-779 on thrombosis time in Klkb1−/− mice. Klkb1+/+ and Klkb1−/− were treated with the Mas antagonist A-779 (n = 5 and n = 6, respectively) or phosphate-buffered saline (n = 9 and n = 5, respectively) using osmotic pumps as previously reported.18 In additional experiments, WT mice (n = 7) were treated with the Mas agonist AVE0991 added to the drinking water at 0.1 μM. Carotid artery occlusion times were determined using the rose bengal thrombosis assay after 7 days of treatment. Data are presented as mean ± SEM for all experiments. *Significant difference (P < .05) between the 2 groups on Student t test or 1-way ANOVA test when all groups were compared.
The influence of Mas receptor on thrombosis risk in Klkb1−/− mice. (A) The contact activation system (CAS), kallikrein/kinin system (KKS), and renin-angiotensin system (RAS) interact with each other. ACE, angiotensin-converting enzyme; Ang-(1-7), angiotensin-(1-7); AngI, angiotensin I; AngII, angiotensin II; AT1R,angiotensin receptor 1; AT2R,angiotensin receptor 2; B2R, bradykinin B2 receptor; BK, bradykinin; BK-(1-5), the ACE breakdown product of BK (peptide RPPGF); FXII, factor XII; FXIIa, activated XII; HK, high-molecular-weight kininogen; Mas, the receptor Mas; PK, prekallikrein. The CAS is initiated by XII autoactivation on a surface to convert PK to plasma kallikrein. These 2 proteases reciprocally activate each other and amplify their activation. Plasma kallikrein cleaves HK to liberate BK in the KKS. BK binds to its receptor, B2R, to activate cells or be internalized and degraded. It is also degraded to BK-(1-5) by ACE. ACE interacts with the RAS because it converts AngI to AngII to bind its receptors, AT1R or AT2R. AngII also is degraded to Ang-(1-7) to bind to its receptor Mas. (B) Plasma bradykinin (BK) levels in Klkb1+/+ and Klkb1−/− mice (n = 6 in each genotype). (C) Renal mRNA of B2R in Klkb1+/+ (n = 6) and Klkb1−/− mice (n = 5). (D) Renal mRNA of Mas in Klkb1+/+ (n = 6) and Klkb1−/− mice (n = 5). (E) Immunoblots for renal Mas showing a representative gel from multiple individual studies of kidney lysates from 8 Klkb1+/+ and 7 Klkb1−/− mice. β-tubulin served as the loading control. (F) Ratio of Mas to β-tubulin as quantified by densitometry using ImageJ software. (G) Plasma angiotensin-(1-7) levels in Klkb1+/+ and Klkb1−/− mice (n = 9 in each group). (H) The influence of Mas antagonist A-779 on thrombosis time in Klkb1−/− mice. Klkb1+/+ and Klkb1−/− were treated with the Mas antagonist A-779 (n = 5 and n = 6, respectively) or phosphate-buffered saline (n = 9 and n = 5, respectively) using osmotic pumps as previously reported.18 In additional experiments, WT mice (n = 7) were treated with the Mas agonist AVE0991 added to the drinking water at 0.1 μM. Carotid artery occlusion times were determined using the rose bengal thrombosis assay after 7 days of treatment. Data are presented as mean ± SEM for all experiments. *Significant difference (P < .05) between the 2 groups on Student t test or 1-way ANOVA test when all groups were compared.
Because both Klkb1−/− and Bdkrb2−/− mice have attenuated BK signaling through B2R, we hypothesized that the reduced thrombosis in Klkb1−/− mice was mediated by the Mas receptor.18 Klkb1−/− mice had 70% increased renal Mas mRNA (P < .05) compared with WT mice (Figure 4D). Immunoblot studies also showed increased Mas antigen in renal tissue in Klkb1−/− (Figure 4E-F). We next determined whether the Mas ligand, Ang-(1-7), is increased in Klkb1−/− mice. Ang-(1-7) plasma levels were similar between WT (48 ± 6 pg/mL, n = 9) and Klkb1−/− (49 ± 4 pg/mL, n = 9) (P > .05) (Figure 4G). Additional studies also showed that renal mRNA for the Ang-(1-7)–generating enzymes ACE2 and PRCP were not significantly different between WT and Klkb1−/− mice (supplemental Figure 3). Further, investigations showed that Klkb1−/− mice had equal Ang-(1-7)–generating activities from ACE2 and PRCP as did WT mice (supplemental Figure 4). These combined studies suggested that the component in the RAS that might be contributing to reduced thrombosis in Klkb1−/− mice was increased Mas. Because the Ang-(1-7)/Mas axis modulates thrombosis risk,18,26-28 we determined whether the overexpressed Mas receptor was responsible for the antithrombotic state observed in Klkb1−/− mice. In vivo treatment with A-779, a Mas antagonist, significantly shortened the carotid artery occlusion time of Klkb1−/− mice from 46 ± 4 minutes (n = 5) to 29 ± 2 minutes (n = 6) (P < .01) but did not change the thrombosis times in treated WT (27 ± 4 min, n = 5) vs untreated WT (32 ± 2 min, n = 9) (P > .05) (Figure 4H). Further, when WT mice were treated with AVE0991, a Mas agonist, their Mas mRNA increased 6.2-fold (supplemental Figure 5) and their time to vessel occlusion was prolonged to 51 ± 2 minutes (n = 7) (Figure 4H).
Role of prostacyclin and Sirt1 in the observed reduced thrombosis
In Bdkrb2−/− mice, we observed that the antithrombotic effect of the Mas receptor was mediated by a threefold elevation of plasma prostacyclin measured as 6-keto-PGF1α18. Klkb1−/− mice (129 ± 12 pg/mL, n = 13) had a 1.72-fold increased plasma prostacyclin as determined by 6-keto-PGF1α compared with WT (75 ± 10 pg/mL, n = 8) (P < .05) (Figure 5A). When Klkb1−/− mice were treated with nimesulide, a COX-2 inhibitor, the plasma prostacyclin level fell to 79 ± 14 pg/mL (n = 6) (P < .05). Nimesulide also influenced the occlusion time of Klkb1−/− mice. After treatment, the occlusion time in Klkb1−/− mice shortened from 51 ± 3 minutes (n = 8) to 35 ± 5.5 minutes (n = 8) (P < .05), suggesting that thrombosis protection in Klkb1−/− was mediated by elevated prostacyclin (Figure 5B).
Thromboprotective mechanisms of Klkb1−/− mice are mediated by prostacyclin through Sirt1 and KLF4. (A) Plasma prostacyclin levels in Klkb1+/+ (n = 8), Klkb1−/− (n = 13), and Klkb1−/− mice + A779 (n = 6) as determined by 6-keto-PGF1α. (B) Carotid artery occlusion times in Klkb1+/+ and Klkb1−/− mice treated or untreated with nimesulide (Nimes) (n = 4-8 animals per group). (C) mRNA expression of Sirt1 and KLF4 in aortic tissues from Klkb1+/+ and Klkb1−/− mice in the absence or presence of nimesulide (n = 12 aortas per group). (D) The presence of Sirt1 and KLF4 antigen in aortic lysates. The gels on the left represent immunoblots of the murine aorta for Sirt1 or KLF4 antigen in Klkb1+/+ and Klkb1−/− mice. The graph on the right is a ratio of Sirt1 or KLF4 antigen compared with β-actin (n = 3-5 blots per condition). (E) The presence of the carotid artery Sirt1 antigen on immunofluorescence is shown on the left. The images were taken with a Nikon Eclipse TE2000-S microscope at 4× magnification. The graph on the right is the relative degree of immunofluorescence in the carotid arteries (n = 4-5 vessels per genotype). (F) The presence of carotid artery KLF4 antigen on immunofluorescence is shown on the left. The images were taken as before at 40×. The graph on the right is the relative degree of immunofluorescence in the carotid arteries (n = 10-11 vessels per genotype). (G) mRNA levels of Sirt1 and KLF4 in cultured MCECs after carbaprostacyclin (cPGI2) treatment. MCECs were treated with carbaprostacyclin (5 µg/mL) (n = 12) or vehicle (0.1% dimethyl sulfoxide [DMSO]) (n = 12) for 4 hours. (H-I) Immunoblot (H) and quantification (I) for protein expression of Sirt1 and KLF4 in untreated (UT) or cPGI2-treated (T) MCECs as in (G). (J) Carotid occlusion times in Klkb1+/+ and Klkb1−/− mice treated with splitomicin (Splito) or DMSO alone (n = 7-10 animals per group). Data are presented as mean ± SEM for all experiments. *Significant differences (P < .05) between the 2 groups on Student t test or 1-way ANOVA test when more than 2 groups of data are compared.
Thromboprotective mechanisms of Klkb1−/− mice are mediated by prostacyclin through Sirt1 and KLF4. (A) Plasma prostacyclin levels in Klkb1+/+ (n = 8), Klkb1−/− (n = 13), and Klkb1−/− mice + A779 (n = 6) as determined by 6-keto-PGF1α. (B) Carotid artery occlusion times in Klkb1+/+ and Klkb1−/− mice treated or untreated with nimesulide (Nimes) (n = 4-8 animals per group). (C) mRNA expression of Sirt1 and KLF4 in aortic tissues from Klkb1+/+ and Klkb1−/− mice in the absence or presence of nimesulide (n = 12 aortas per group). (D) The presence of Sirt1 and KLF4 antigen in aortic lysates. The gels on the left represent immunoblots of the murine aorta for Sirt1 or KLF4 antigen in Klkb1+/+ and Klkb1−/− mice. The graph on the right is a ratio of Sirt1 or KLF4 antigen compared with β-actin (n = 3-5 blots per condition). (E) The presence of the carotid artery Sirt1 antigen on immunofluorescence is shown on the left. The images were taken with a Nikon Eclipse TE2000-S microscope at 4× magnification. The graph on the right is the relative degree of immunofluorescence in the carotid arteries (n = 4-5 vessels per genotype). (F) The presence of carotid artery KLF4 antigen on immunofluorescence is shown on the left. The images were taken as before at 40×. The graph on the right is the relative degree of immunofluorescence in the carotid arteries (n = 10-11 vessels per genotype). (G) mRNA levels of Sirt1 and KLF4 in cultured MCECs after carbaprostacyclin (cPGI2) treatment. MCECs were treated with carbaprostacyclin (5 µg/mL) (n = 12) or vehicle (0.1% dimethyl sulfoxide [DMSO]) (n = 12) for 4 hours. (H-I) Immunoblot (H) and quantification (I) for protein expression of Sirt1 and KLF4 in untreated (UT) or cPGI2-treated (T) MCECs as in (G). (J) Carotid occlusion times in Klkb1+/+ and Klkb1−/− mice treated with splitomicin (Splito) or DMSO alone (n = 7-10 animals per group). Data are presented as mean ± SEM for all experiments. *Significant differences (P < .05) between the 2 groups on Student t test or 1-way ANOVA test when more than 2 groups of data are compared.
However, unlike Bdkrb2−/− mice, Klkb1−/− mice (455 ± 70 seconds, n = 5) did not have a prolonged tail bleeding time over WT mice (387 ± 88 seconds, n = 6) (P > .05) (supplemental Figure 6). Because there was no difference in platelet counts (supplemental Table 2), we examined Klkb1−/− mice for platelet function defects. Unlike Bdkrb2−/− mice, Klkb1−/− mice did not have defective α-thrombin– or CRP-induced platelet activation. As shown in supplemental Figure 7, WT and Klkb1−/− platelets had equal responses to α-thrombin (0.3-1 nM) or CRP (1-3 µg/mL) on flow cytometry with integrin α2bβ3 activation as detected by JON/A antibody and P-selectin expression as detected by Wug.E9 antibody. Likewise, ADP-induced (0.1-3 µM) fibrinogen binding was similar between the 2 genotypes (supplemental Figure 8). In addition, phalloidin staining showed that Klkb1−/− platelets spread on collagen similar to WT as determined from pixel areas analyzed by ImageJ software (supplemental Figure 9). These combined data showed that Klkb1−/− platelets functioned normally.
Because there was only a 1.72-fold increase in plasma prostacyclin in Klkb1−/− mice vs the threefold increase previously seen in Bdkrb2−/− mice,18 the influence of prostacyclin alone on vascular function was examined. Recently COX-2–derived prostacyclin has been recognized to suppress TF expression through the NAD+-dependent class III histone deacetylase sirtuin-1 (Sirt1).29-31 The mRNA for both Sirt1 and KLF4 were elevated in Klkb1−/− aorta (Figure 5C). We also found elevated aortic mRNA for Sirt1 and KLF4 in Bdkrb2−/− mice17,18 (supplemental Figure 10). In addition, we determined that in aortic lysates, Sirt1 and KLF4 antigen on immunoblot were both increased about 1.7-fold in Klkb1−/− tissue (Figure 5D). On immunofluorescence staining of the carotid artery, vessel wall Sirt1 antigen was 2.5-fold higher in Klkb1−/− mice (Figure 5E). Alternatively, the 1.9-fold increased KLF4 antigen in Klkb1−/− carotid artery was confined to the endothelium (Figure 5F). Finally, when Klkb1−/− mice were treated with nimesulide, there was a significant decrease in Sirt1 and KLF4 mRNA in aorta (Figure 5C).
We next determined whether in vitro treatment of mouse cardiac endothelial cells (MCECs) with carbaprostacyclin (cPGI2), a stable analog of PGl2, recapitulated our observations with Klkb1−/− and Bdkrb2−/− aorta. cPGI2-treated MCECs had increased Sirt1 and KLF4 mRNA compared with untreated controls (P < .05) (Figure 5G). In addition, cPGI2-treated MCECs had increased KLF4 antigen on immunoblot (Figure 5H-I). Finally, to determine the role of Sirt1 expression in the reduced thrombosis observed in Klkb1−/− mice, the animals were treated with splitomicin, a Sirt1 inhibitor,30 to determine whether it would alter their time to thrombosis. Splitomicin treatment significantly shortened occlusion times in Klkb1−/− mice (49 ± 5 min for untreated, n = 9, vs 30 ± 4 min for treated, n = 8) (P < .05) without significant change in WT thrombosis times (Figure 5J). These data suggested that the modest elevation of PGI2 in Klkb1−/− mice mostly influenced vascular function.
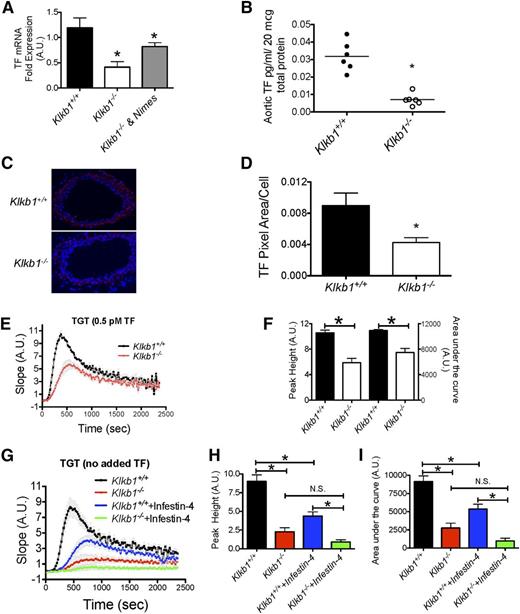
Investigations next focused on how aortic Sirt1 and KLF4 elevation in Klkb1−/− mice influenced thrombosis risk. Klkb1−/− aortas were observed to have decreased TF mRNA (P = .012) (Figure 6A). Nimesulide treatment of Klkb1−/− mice raised the aortic TF mRNA toward normal (P = .03) (Figure 6A). Investigations next determined whether there was reduced TF activity in the vessel wall. TF activity was 4.5-fold less in Klkb1−/− aorta (Figure 6B). Likewise, TF immunofluorescence antigen was 2.1-fold decreased in Klkb1−/− carotid arteries.
Tissue factor expression in Klkb1−/− mice. (A) Aortic tissue factor (mTF) mRNA determined in Klkb1+/+ (n = 19), Klkb1−/− (n = 9), and Klkb1−/− + nimesulide (Nimes) (n = 5). (B) Measurement of TF activity in aortic lysates of Klkb1+/+ and Klkb1−/− mice by chromogenic assay (n = 6 aortas per group). (C) Representative immunofluorescence of carotid artery TF antigen. The images were taken with a Nikon Eclipse TE2000-S microscope at 10× magnification. (D) The relative TF antigen presence in carotid arteries in Klkb1+/+ and Klkb1−/− tissue (n = 4 arteries per genotype). (E) Low-dose (0.5 pM) exogenous tissue factor–induced thrombin generation times (TGT) in Klkb1+/+ and Klkb1−/− plasmas (n = 3 per group). (F) Peak height and AUC were calculated and quantified from the TGT curves in (E). (G) TGT in Klkb1+/+and Klkb1−/− plasma without added TF in the absence or presence of 2 nM rHA-Infestin-4 (n = 6 per group). (H-I) Peak height and AUC, respectively, were calculated and quantified from the TGT curves in (G). In (E) and (G), the slopes of the TGT are actual values in arbitrary units. In the absence or presence of rHA-Infestin-4 (2 nM), Klkb1−/− plasma had reduced endogenous thrombin generation when compared with Klkb1+/+ plasma. Data are presented as mean ± SEM for all experiments. *Significant difference (P < .05) between the 2 groups on Student t test or 1-way ANOVA test when more than 2 groups of data are compared.
Tissue factor expression in Klkb1−/− mice. (A) Aortic tissue factor (mTF) mRNA determined in Klkb1+/+ (n = 19), Klkb1−/− (n = 9), and Klkb1−/− + nimesulide (Nimes) (n = 5). (B) Measurement of TF activity in aortic lysates of Klkb1+/+ and Klkb1−/− mice by chromogenic assay (n = 6 aortas per group). (C) Representative immunofluorescence of carotid artery TF antigen. The images were taken with a Nikon Eclipse TE2000-S microscope at 10× magnification. (D) The relative TF antigen presence in carotid arteries in Klkb1+/+ and Klkb1−/− tissue (n = 4 arteries per genotype). (E) Low-dose (0.5 pM) exogenous tissue factor–induced thrombin generation times (TGT) in Klkb1+/+ and Klkb1−/− plasmas (n = 3 per group). (F) Peak height and AUC were calculated and quantified from the TGT curves in (E). (G) TGT in Klkb1+/+and Klkb1−/− plasma without added TF in the absence or presence of 2 nM rHA-Infestin-4 (n = 6 per group). (H-I) Peak height and AUC, respectively, were calculated and quantified from the TGT curves in (G). In (E) and (G), the slopes of the TGT are actual values in arbitrary units. In the absence or presence of rHA-Infestin-4 (2 nM), Klkb1−/− plasma had reduced endogenous thrombin generation when compared with Klkb1+/+ plasma. Data are presented as mean ± SEM for all experiments. *Significant difference (P < .05) between the 2 groups on Student t test or 1-way ANOVA test when more than 2 groups of data are compared.
These results indicated that Klkb1−/− mice have significantly reduced levels of TF expression in their aortas compared with WT mice (Figure 6C-D).
We next examined TF-induced TGT with Klkb1−/− plasma. When using a standard concentration of TF (∼3 pM), Klkb1−/− plasma had slightly decreased TGT (supplemental Figure 11A). The mean peak height in Klkb1−/− plasma was 85 ± 6% (n = 6) compared with WT (n = 6) (P < .05). The mean total AUC for TF-induced TGT in Klkb1−/− plasma was 89 ± 3% (n = 6) as in WT (n = 6) (P < .005) (supplemental Figure 11B-C). When a sixfold lower dose of TF (∼0.5 pM) was used, Klkb1−/− plasma had markedly reduced thrombin generation when compared with normal plasma (Figure 6E). The peak height and AUC were both significantly lower in Klkb1−/− plasma compared with WT (Figure 6F). We next examined endogenous TF–induced thrombin generation (ie, the TGT was performed without adding exogenous TF). In this experiment, mouse plasma was collected into 0.032 g/mL sodium citrate in the absence or presence of rHA-Infestin-4, a XIIa inhibitor to block contact activation that might occur during assay incubation.32 In the presence or absence of rHA-Infestin-4, Klkb1−/− plasma had little thrombin generation compared with Klkb1+/+ plasma (Figure 6G and supplemental Figure 12). The addition of rHA-Infestin-4 significantly reduced the endogenous thrombin generation in Klkb1+/+ plasma but not in Klkb1−/− plasma (Figure 6G). In the presence of rHA-Infestin-4, Klkb1−/− plasma still had equally reduced thrombin generation compared with WT. The mean peak height in rHA-Infestin-4–treated Klkb1−/− plasma was 0.9 ± 0.3 (n = 6) vs 4.4 ± 0.6 in Infestin-4–treated WT (n = 6) (P < .05) (Figure 6H). The mean total AUC in rHA-Infestin-4–treated Klkb1−/− plasma was 973 ± 381 (n = 6) compared with 5359 ± 678 in rHA-Infestin-4–treated WT (n = 6) (P < .05) (Figure 6I). These combined studies indicated that Klkb1−/− mice were protected from thrombosis by elevated plasma prostacyclin and suppressed TF expression in the vessel wall.
Discussion
This investigation on Klkb1−/− mice indicates that the Mas-prostacyclin axis is an important modulator of arterial thrombosis risk.18,26-28 When there is reduced BK delivery to tissues, the Mas receptor becomes overexpressed and there is elevated plasma prostacyclin (Figures 5 and 7). In Bdkrb2−/− mice, the threefold elevation of prostacyclin prolongs the bleeding time and induces a platelet-spreading defect on integrins and a GPVI activation defect to reduce murine thrombosis.18 In Klkb1−/− mice, there is only a 1.72-fold increase in prostacyclin that produces a vasculoprotective effect only by elevating Sirt1 and KLF4 and suppressing TF without effect on platelet function. Our studies in Klkb1−/− and Bdkrb2−/− mice demonstrate that the antithrombotic effect of the Mas/prostacyclin axis involves degrees of vascular protection and platelet inhibition. Stimulation of this pathway may modify thrombosis risk by reducing arterial tissue factor. Inhibitor suppression of plasma kallikrein in the treatment of hereditary angioedema could confer reduced thrombosis by a similar mechanism.33,34
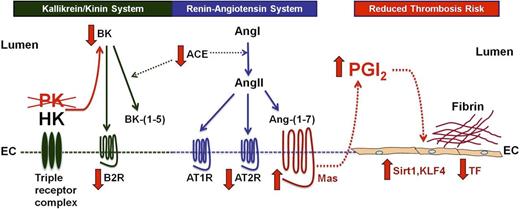
Mechanisms of thrombosis delay in Klkb1−/− mice. In the absence of prekallikrein (PK), there is reduced plasma bradykinin (BK) generation from HK bound to its triple-receptor complex. Expression of the bradykinin B2 receptor (B2R) is also reduced. Similar to our recent observation in Bdkrb2−/− (B2R KO) mice,18 the Mas receptor in the renin-angiotensin system is overexpressed to compensate for reduced BK B2R expression in Klkb1−/− mice. In Klkb1−/− mice, normal levels of Ang-(1-7) interacting with an overexpressed Mas receptor results in increased plasma prostacyclin (PGI2). The elevation of PGI2 is sufficient to increase 2 vasculoprotective transcription factors, Sirt1 and KLF4. Elevation of Sirt1 and/or KLF4 reduces the risk of thrombosis by inhibiting TF expression. In Klkb1−/− mice, the Mas/prostacyclin axis counterbalances the reduction in BK and B2R to reduce thrombosis risk independent of less contact activation.
Mechanisms of thrombosis delay in Klkb1−/− mice. In the absence of prekallikrein (PK), there is reduced plasma bradykinin (BK) generation from HK bound to its triple-receptor complex. Expression of the bradykinin B2 receptor (B2R) is also reduced. Similar to our recent observation in Bdkrb2−/− (B2R KO) mice,18 the Mas receptor in the renin-angiotensin system is overexpressed to compensate for reduced BK B2R expression in Klkb1−/− mice. In Klkb1−/− mice, normal levels of Ang-(1-7) interacting with an overexpressed Mas receptor results in increased plasma prostacyclin (PGI2). The elevation of PGI2 is sufficient to increase 2 vasculoprotective transcription factors, Sirt1 and KLF4. Elevation of Sirt1 and/or KLF4 reduces the risk of thrombosis by inhibiting TF expression. In Klkb1−/− mice, the Mas/prostacyclin axis counterbalances the reduction in BK and B2R to reduce thrombosis risk independent of less contact activation.
The murine pulmonary embolism models with collagen/epinephrine and long-chain polyphosphate are in vivo contact activation–induced thrombosis. F12−/− mice are partially protected from thrombosis and death in the Col/Epi model.21 Klkb1−/− mice were not protected from Col/Epi or LC polyP-induced lethal pulmonary embolism, suggesting that the reduced thrombosis in them is not like F12−/− mice. Klkb1−/− mice have reduced contact activation as indicated by the prolonged aPTT, reduced contact activation–induced TGT (Figure 1), and reduced lung vascular permeability (Figure 3). Unlike the human Fletcher trait, the aPTT is very prolonged in the Klkb1−/− plasma.14 Klkb1−/− plasma levels of XII, HK, and XI are mostly normal. The slightly elevated HK that is not significantly different from WT may be a compensatory response to no-plasma PK.1 These mice may have high levels of C1 inhibitor and α2-macroglobulin that retard contact activation to prolong the aPTT.35 Finally, the ellagic acid in the aPTT reagent is a contact activator that is weaker than other negatively-charged surface additives.36 Our studies with the Klkb1−/− mice suggest that other mechanisms for reduced thrombosis, in addition to delayed contact activation, need to be considered.
Plasma kallikrein is recognized as a major enzyme producing BK by cleaving plasma HK and low-molecular-weight kininogen.6,37 We confirmed that Klkb1−/− plasma has markedly reduced BK (Figure 4B), similar to what has been observed in Kgn1−/− mice.25 F12−/− mice also have reduced BK levels, but they are only half normal.20 The constitutive receptor for BK, B2R (Bdkrb2), is also significantly decreased in the Klkb1−/− kidney (Figure 4C). Both Bdkrb2−/− and Klkb1−/− mice have defective BK delivery to tissues caused by either reduced BK formation and/or reduced B2R.17,18 Like Bdkrb2−/− mice, Klkb1−/− mice have elevated Mas mRNA and antigen (Figure 4D-F). We did not observe increased plasma Ang-(1-7), the natural ligand for Mas, or increased activity for ACE2 or PRCP, the 2 main Ang-(1-7)–forming enzymes, in Klkb1−/− mice.38 Our studies show that Mas elevation alone is sufficient to modify thrombosis risk. Confirmation that Mas is a thrombosis modifier is shown by the facts that (1) the Mas antagonist A-779 shortens thrombosis times in Klkb1−/− mice and (2) the Mas agonist AVE0991 induces thrombosis delay in normal mice. How reduced BK delivery to tissues and lowered B2R expression increases Mas expression is not known. The B2R makes heterodimers with the AT1R and AT2R39 ; it is possible that it too may occur with Mas. In addition, BK binds to its receptors to (1) stimulate a signaling pathway and (2) be internalized for degradation, accounting for 40% of its metabolism.40 The absence of either mechanism also could contribute to Mas overexpression.
Our studies on Klkb1−/− and Bdkrb2−/− indicate that prostacyclin is the major mediator of the antithrombotic effect conferred by Mas receptor overexpression. In both animal models, treatment with nimesulide, a COX2 antagonist, corrects the delayed thrombosis times (Figure 5B).17 In our investigations, a less than twofold elevation of prostacyclin is sufficient to influence arterial thrombosis risk. Clinical observations tell us that administration of COX2 inhibitors to patient populations at risk for thrombosis increases thrombotic events.41 Barberi et al reported that COX-2–deficient mice have decreased Sirt1, enhanced vascular TF expression, and accelerated thrombosis.31 We observed the opposite in Klkb1−/− and Bdkrb2−/− mice. Elevated plasma PGI2 in our mice is associated with increased aortic Sirt1 and KLF4 mRNA and protein that are reduced by nimesulide (Figure 5C-F). Because the histone deacetylase inhibitor splitomicin corrects delayed thrombosis in Klkb1−/− mice, Sirt1 is a contributor to thrombosis risk (Figure 5J).30 Currently, it is not known whether the expression of increased KLF4 is interrelated to increased Sirt1 or is independently the result of elevated PGI2 itself.
Sirt1 and KLF4 are recognized vasculoprotective transcription factors.31,42 Sirt1, a deacetylase, may indirectly regulate TF expression by inactivating a coactivator for NF-κB.43 KLF4 may influence TF expression through its ability to influence endothelial nitric oxide and thrombomodulin.44,45 Decreased endothelial cell KLF4 is associated with accelerated thrombosis, and increased endothelial KLF4 is associated with reduced fibrin deposition.42 Klkb1−/− aortas have reduced TF mRNA, activity, and antigen (Figure 6A-D). The presence of reduced vessel wall TF alone is sufficient to account for the reduced thrombosis observed in Klkb1−/− mice.46 Moreover TF-induced (0, 0.5, or 3 pM) thrombin generation times are reduced in Klkb1−/− plasma, suggesting less endogenous TF is present (Figure 6E-I). Our investigations indicate how small elevations of prostacyclin profoundly affect thrombosis propensity by reducing vessel wall TF. Because the vascular transcription factors Sirt1 and KLF4 participate in these processes, there probably are additional mechanisms contributing to the reduced thrombosis.42,44
In conclusion, we have characterized the mechanism for reduced thrombosis in Klkb1−/− mice (Figure 7). In Klkb1−/− mice, there is reduced BK with reduced B2R expression, but a compensatory Mas receptor overexpression. Higher Mas expression alone increases prostacyclin production. The higher PGI2 upregulates vascular Sirt1 and KLF4 and reduces vascular TF, leading to less thrombosis (Figure 7). This reduced thrombosis mechanism is independent of and occurs before PGI2-induced platelet inhibition. Our investigations indicate that altered balance between the KKS and RAS influences arterial thrombosis risk. Our studies in Klkb1−/− and Bdkrb2−/− mice show that the Mas-prostacyclin axis modulates arterial thrombosis risk. Increasing the expression of Mas alone may be sufficient to reduce thrombosis risk, as seen in myocardial infarction and stroke, without influence on hemostasis.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Edward Feener of Harvard University and Frank Castelino of the University of Notre Dame for generously providing Klkb1−/− and F12−/− mice, respectively; Michael Sramkoski at the CWRU Cancer Center Flow Cytometry core facility; Maryanne Pendergast at the CWRU Neuroscience Imaging Center; Patty Conrad at the CWRU Genetics Imaging Core; and Denise A. Hatala and Diane M. Mahovlic at the Cleveland Clinic Lerner Research Institute Imaging Core for expert histologic section preparation.
This work was supported by the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (HL052779-18, HL112666-2) (A.H.S.), a Hemostasis and Thrombosis Research Society 2012 Mentored Research Award sponsored by Baxter Healthcare Corporation (E.X.S.), a NIH Individual Postdoctoral Fellowship (F32DK093226) (N.G.) and a NIH-National Center for Research Resources Shared Instrumentation grant (1S10RR031845).
Authorship
Contribution: E.X.S., C.F., A.M., O.A., S.A., and N.G. performed experiments; E.X.S., C.F., and A.H.S. conceptualized and planned experiments; E.X.S., C.F., and A.H.S. prepared the figures; E.X.S., C.F., N.M., and A.H.S. wrote the manuscript; and all authors reviewed the manuscript before submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alvin H. Schmaier, Case Western Reserve University, Wolstein Research Building 2-130, 10900 Euclid Ave, Cleveland, OH 44106-7284; e-mail: schmaier@case.edu.
References
Author notes
E.X.S. and C.F. contributed equally to this study.

![Figure 5. Thromboprotective mechanisms of Klkb1−/− mice are mediated by prostacyclin through Sirt1 and KLF4. (A) Plasma prostacyclin levels in Klkb1+/+ (n = 8), Klkb1−/− (n = 13), and Klkb1−/− mice + A779 (n = 6) as determined by 6-keto-PGF1α. (B) Carotid artery occlusion times in Klkb1+/+ and Klkb1−/− mice treated or untreated with nimesulide (Nimes) (n = 4-8 animals per group). (C) mRNA expression of Sirt1 and KLF4 in aortic tissues from Klkb1+/+ and Klkb1−/− mice in the absence or presence of nimesulide (n = 12 aortas per group). (D) The presence of Sirt1 and KLF4 antigen in aortic lysates. The gels on the left represent immunoblots of the murine aorta for Sirt1 or KLF4 antigen in Klkb1+/+ and Klkb1−/− mice. The graph on the right is a ratio of Sirt1 or KLF4 antigen compared with β-actin (n = 3-5 blots per condition). (E) The presence of the carotid artery Sirt1 antigen on immunofluorescence is shown on the left. The images were taken with a Nikon Eclipse TE2000-S microscope at 4× magnification. The graph on the right is the relative degree of immunofluorescence in the carotid arteries (n = 4-5 vessels per genotype). (F) The presence of carotid artery KLF4 antigen on immunofluorescence is shown on the left. The images were taken as before at 40×. The graph on the right is the relative degree of immunofluorescence in the carotid arteries (n = 10-11 vessels per genotype). (G) mRNA levels of Sirt1 and KLF4 in cultured MCECs after carbaprostacyclin (cPGI2) treatment. MCECs were treated with carbaprostacyclin (5 µg/mL) (n = 12) or vehicle (0.1% dimethyl sulfoxide [DMSO]) (n = 12) for 4 hours. (H-I) Immunoblot (H) and quantification (I) for protein expression of Sirt1 and KLF4 in untreated (UT) or cPGI2-treated (T) MCECs as in (G). (J) Carotid occlusion times in Klkb1+/+ and Klkb1−/− mice treated with splitomicin (Splito) or DMSO alone (n = 7-10 animals per group). Data are presented as mean ± SEM for all experiments. *Significant differences (P < .05) between the 2 groups on Student t test or 1-way ANOVA test when more than 2 groups of data are compared.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/4/10.1182_blood-2014-01-550285/4/m_710f5.jpeg?Expires=1769156141&Signature=m0LJ7IQHFqLtVmMVKGYuW8g3DPMp657LmpOLYZWrV8L46cCssuvpPJQ1AzpqRCVAtDPNYG3bBUvN-PGN7pqLb5Z~M8gMqiR0ACWrnZBhJw~HGprqpY8WtaIHl2lOPl5FT-DhWmQlSaPJRlux9GXBsodQT8lzjE2Gpu3hCiroPrmNI9I4qB1XBAbYrXYhAXSqKvlofysqowJYITDWmTU9OWWBwvWW7uxljQajR-3O9gnaUjeFV8kEUXEzHSxtZFM4XapVelIeL1QGbDAKRyDOKXcgTkOq1mk2OVfjx3TwvS3gEaagyktjfxd8Y2pigy9P6B0HKXuRffsTYbbV4vO-lw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal