Key Points
EphB2 regulates initial platelet activation in the absence of ligand binding in a contact-independent manner.
EphB2-mediated signaling regulates thrombus formation and clot retraction.
Abstract
The Eph kinases, EphA4 and EphB1, and their ligand, ephrinB1, have been previously reported to be present in platelets where they contribute to thrombus stability. Although thrombus formation allows for Eph-ephrin engagement and bidirectional signaling, the importance specifically of Eph kinase or ephrin signaling in regulating platelet function remained unidentified. In the present study, a genetic approach was used in mice to establish the contribution of signaling orchestrated by the cytoplasmic domain of EphB2 (a newly discovered Eph kinase in platelets) in platelet activation and thrombus formation. We conclude that EphB2 signaling is involved in the regulation of thrombus formation and clot retraction. Furthermore, the cytoplasmic tail of this Eph kinase regulates initial platelet activation in a contact-independent manner in the absence of Eph-ephrin ligation between platelets. Together, these data demonstrate that EphB2 signaling not only modulates platelet function within a thrombus but is also involved in the regulation of the function of isolated platelets in a contact-independent manner.
Introduction
Sustained contact-dependent signaling by integrin αIIbβ3 in platelets is believed to be important to ensure thrombus stability, and may control the size and multilayered architecture of a thrombus as it grows, contracts, and adopts a conformation to ensure cessation of blood loss.1 The repertoire of receptors that may support contact-dependent signaling within a thrombus extends beyond integrin αIIbβ3, and incorporates activators of platelet function such as Eph kinases2 and their cell-surface ligands, the ephrins, and negative regulators such as platelet endothelial cell adhesion molecule-1 (PECAM-1),3,4 junctional adhesion molecule A (JAM-A),5,6 and endothelial cell-specific adhesion molecule (ESAM).7 Sustained platelet signaling within thrombi has also been recently shown to involve intercellular signaling through gap junctions.8-10 Knowledge of the signaling mechanisms that platelets use within a thrombus may therefore offer new perspectives on strategies to prevent thrombotic disease. This led us to explore the mechanisms that allow a newly identified platelet Eph kinase, EphB2, to control platelet function.
Eph kinases comprise a family of cell-surface receptor tyrosine kinases that play roles in development of the central nervous system and vasculature,11-13 and their ligands, ephrins, are integral membrane or transmembrane proteins. Eph kinases are classified as EphA (bind ephrin A) and EphB (bind ephrin B) family members, although more promiscuous cross-family interactions have been reported.14 Ephrin A family members are glycosylphosphatidylinositol (GPI) anchored and ephrin B family members possess a transmembrane domain. Eph kinase ligation occurs in trans, that is, the receptor on one cell binds a ligand on the opposing cell thereby transmitting signals in both directions (forward signaling by Eph kinases and reverse signaling by ephrins).11 The presence of EphA4, EphB1, and ephrinB1 in human platelets has been reported,15 where forced clustering of EphA4 or ephrinB1 resulted in cytoskeletal rearrangements, fibrinogen binding, and granule secretion. EphA4 was also reported to form complexes in platelets with tyrosine kinases Fyn, Lyn, and cell adhesion molecule, L1. Blockade of Eph/ephrin interactions resulted in reduced platelet activation.15 Further studies have emphasized the role of EphA4 in the regulation of integrin αIIbβ3-mediated outside-in signaling controlling platelet spreading and clot retraction, and provide compelling evidence for the role of Eph kinases and ephrins in the activation of platelets.2,15-17 How they contribute, and whether this is consistent with a role in the initiation of sustained platelet signaling within a thrombus, however, remains unknown.
Because ephrinB1 was the only Eph ligand identified in platelets, we sought to explore the potential role of EphB2, one of its principal receptors that we report to be present in platelets. Given the complexities of bidirectional signaling, we used a strategy that would enable us to ask whether signaling elicited through the cytoplasmic domain of EphB2 controls platelet function. This was achieved using transgenic mice (EphB2LacZ) expressing EphB2 with the cytoplasmic tail replaced with β-galactosidase rendering it signaling defective while maintaining capability for ephrin binding. We demonstrate that the EphB2-mediated signaling is not only involved in thrombus formation where sustained bidirectional signaling may occur but also in initial platelet activation in the absence of cell-cell contact. Furthermore, we report that the EphB2 intracellular domain plays important roles in the regulation of phosphoinositide 3-kinase (PI3K) and integrin αIIbβ3-mediated signaling, thereby controlling platelet function.
Methods
The preparation of washed platelets, immunoblotting, immunoprecipitation and platelet functional assays such as aggregation, dense granule secretion, fibrinogen binding, P-selectin exposure, calcium mobilization, clot retraction, platelet spreading, thrombus formation, and tail bleeding were performed as described previously.8,9,18,19 Detailed materials and methods are provided in supplemental Methods (see supplemental Data available on the Blood Web site).
Generation of EphB2LacZ mice
EphB2LacZ mice were developed by replacing the intracellular region including kinase domain, sterile alpha motif (SAM) and PDZ motifs (ie, after 621 aa at the N terminus including extracellular, transmembrane and juxtamembrane domains) by a full-length β-galactosidase gene as reported previously20,21 and maintained on a C57BL6 genetic background. Littermate controls were used in all experiments. All animals were used following appropriate approval from the University of Reading Local Ethics Review Panel and a license from the British Home Office.
Results
Characterization of EphB2LacZ mouse platelets
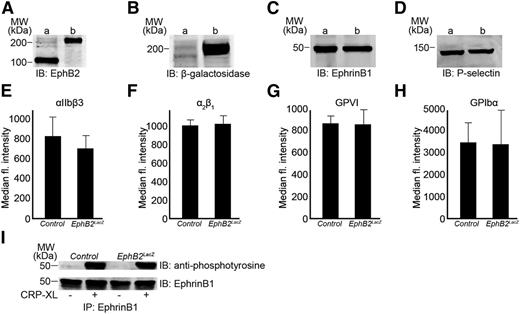
The presence of EphB2 was confirmed in control mice platelets by immunoblot analysis (Figure 1A). The fusion of β-galactosidase with EphB2 in transgenic mice resulted in a protein that was larger than native EphB2, and detected with an apparent molecular mass of ∼200 kDa (Figure 1A). The presence of β-galactosidase fused with EphB2 was confirmed by immunoblot analysis (Figure 1B). The ligand, ephrinB1, was also detected in EphB2LacZ and control platelets at similar levels (Figure 1C). Platelet numbers in EphB2LacZ were similar to those in control mice and their cellular morphology (size and granule numbers, established by transmission electron microscopy) was indistinguishable (data not shown). The level of P-selectin expression in EphB2LacZ platelets was similar to control platelets (Figure 1D) as were integrin αIIbβ3 (Figure 1E), integrin α2β1 (Figure 1F), glycoprotein VI (GPVI) (Figure 1G) and GPIbα (Figure 1H).
Characterization of EphB2LacZ platelets. The presence of EphB2 (A), β-galactosidase (B), and ephrinB1 (C) was confirmed in control (a) and EphB2LacZ (b) mouse platelets by immunoblot analysis. Similarly, the expression level of P-selectin was measured in control (a) and EphB2LacZ (b) mouse platelets by immunoblot analysis (D). The expression levels of αIIbβ3 (E), α2β1 (F), GPVI (G), and GPIbα (H) were analyzed on control and EphB2LacZ platelets by flow cytometry. Data represent mean (of median fluorescence intensity) ± SD (n = 4, control and EphB2LacZ mice). (I) ephrinB1 was immunoprecipitated from control and EphB2LacZ platelets following stimulation with CRP-XL (0.5 µg/mL) and analyzed for its phosphorylation using anti-phosphotyrosine antibody by immunoblotting. The blots are representative of 3 separate experiments. CRP-XL, cross-linked collagen-related peptide; GP, glycoprotein; SD, standard deviation.
Characterization of EphB2LacZ platelets. The presence of EphB2 (A), β-galactosidase (B), and ephrinB1 (C) was confirmed in control (a) and EphB2LacZ (b) mouse platelets by immunoblot analysis. Similarly, the expression level of P-selectin was measured in control (a) and EphB2LacZ (b) mouse platelets by immunoblot analysis (D). The expression levels of αIIbβ3 (E), α2β1 (F), GPVI (G), and GPIbα (H) were analyzed on control and EphB2LacZ platelets by flow cytometry. Data represent mean (of median fluorescence intensity) ± SD (n = 4, control and EphB2LacZ mice). (I) ephrinB1 was immunoprecipitated from control and EphB2LacZ platelets following stimulation with CRP-XL (0.5 µg/mL) and analyzed for its phosphorylation using anti-phosphotyrosine antibody by immunoblotting. The blots are representative of 3 separate experiments. CRP-XL, cross-linked collagen-related peptide; GP, glycoprotein; SD, standard deviation.
Removal of EphB2 intracellular region does not affect ephrinB1 activation
Binding of EphB2 to its ligand, ephrinB1, triggers clustering and initiates signaling and phosphorylation of specific intracellular amino acid residues on each.11 Washed platelets from EphB2LacZ and control mice were activated and aggregated using cross-linked collagen-related peptide (CRP-XL) (0.5 µg/mL) and lysed prior to the immunoprecipitation of ephrinB1 followed by immunoblotting with an anti-phosphotyrosine antibody. Platelet activation was associated with the phosphorylation of ephrinB1 that was maintained in EphB2LacZ platelets (Figure 1I). These data indicate that replacement of the EphB2 cytoplasmic tail does not diminish clustering and signaling through ephrinB1 and suggests that the receptor is present at functional levels on EphB2LacZ platelets.
Replacement of EphB2 intracellular region abrogates platelet activation
To analyze the importance of the cytoplasmic tail of EphB2 for platelet activation, aggregation assays were performed using washed platelets obtained from EphB2LacZ and control mice using optical aggregometry. Collagen (1 µg/mL)–induced aggregation was reduced by ∼85% (at 5 minutes) in EphB2LacZ platelets compared with controls (Figure 2A-B), and the inhibitory effects were overcome partially when a higher concentration (5 µg/mL) of collagen was used (Figure 2C-D). These data suggest that the reduced activation in EphB2LacZ platelets is not due to the developmental defects but reflects the importance of EphB2 in the regulation of platelet function. Because collagen binds GPVI and integrin α2β1 on platelets, a GPVI-selective agonist, CRP-XL, was used in aggregation assays to determine whether reduction in aggregation occurred through the modulation of GPVI-dependent signaling. CRP-XL (0.5 µg/mL)–induced platelet aggregation was reduced by ∼60% (Figure 2E-F), although the level of inhibition was diminished at higher concentration (Figure 2G-H) (at 5 minutes) in EphB2LacZ platelets. EphB2 functions were not restricted to the GPVI pathway, as thrombin (0.1 U/mL [Figure 2I-J])–induced platelet aggregation (which activates platelets through G-protein–coupled protease-activated receptors) was also diminished in EphB2LacZ platelets although, again, the inhibitory effects were partially overcome when a higher concentration of thrombin (0.2 U/mL) was used (Figure 2K-L).
Defective aggregation of EphB2LacZ platelets. Platelet aggregation was recorded for 5 minutes by optical aggregometry using washed platelets obtained from control and EphB2LacZ mice following stimulation with collagen (1 µg/mL [A-B] or 5 µg/mL [C-D]), CRP-XL (0.5 µg/mL [E-F] or 1 µg/mL [G-H]), and thrombin (0.1 U/mL [I-J] or 0.2 U/mL [K-L]). Data represent mean ± SD (n = 3). The level of aggregation obtained with control platelets at 5 minutes was taken as 100% (Student t test: *P ≤ .05, **P ≤ .01, and ***P ≤ .001).
Defective aggregation of EphB2LacZ platelets. Platelet aggregation was recorded for 5 minutes by optical aggregometry using washed platelets obtained from control and EphB2LacZ mice following stimulation with collagen (1 µg/mL [A-B] or 5 µg/mL [C-D]), CRP-XL (0.5 µg/mL [E-F] or 1 µg/mL [G-H]), and thrombin (0.1 U/mL [I-J] or 0.2 U/mL [K-L]). Data represent mean ± SD (n = 3). The level of aggregation obtained with control platelets at 5 minutes was taken as 100% (Student t test: *P ≤ .05, **P ≤ .01, and ***P ≤ .001).
Platelet aggregation requires the conformational modulation of integrin αIIbβ3 to enable fibrinogen binding.22 Thus, fibrinogen binding was measured in EphB2LacZ and control platelets (using whole blood) upon stimulation with CRP-XL or thrombin by flow cytometry. Reduced fibrinogen binding was observed in EphB2LacZ platelets compared with controls upon stimulation with CRP-XL (Figure 3A-B) and thrombin (Figure 3C-D). To analyze whether EphB2-mediated signaling is involved in the control of α-granule secretion in platelets, P-selectin exposure was measured in EphB2LacZ and control mouse whole blood using flow cytometry. CRP-XL (0.5 µg/mL and 1 µg/mL)–induced (Figure 3E-F) and thrombin (0.1 U/mL and 0.2 U/mL)–induced (Figure 3G-H) P-selectin exposure was reduced significantly in EphB2LacZ platelets. The use of whole blood and through gating individual platelets using flow cytometry enabled the study of EphB2 on platelets in the absence of Eph-ephrin interactions. It also prevented the potential complexities that would be introduced during the preparation of washed platelets although the results are consistent as observed for aggregation assays. Similar to α-granule secretion, dense granule secretion measured upon stimulation with CRP-XL (1 µg/mL) in EphB2LacZ platelets was reduced (Figure 3I-J). These results support the notion that EphB2 is involved in the regulation of agonist-induced platelet activation through modulation of integrin αIIbβ3-mediated inside-out signaling and granule secretion.
EphB2 signaling regulates initial platelet activation. CRP-XL (0.5 µg/mL [A] or 1 µg/mL [B]) and thrombin-induced fibrinogen binding (0.1 U/mL [C] or 0.2 U/mL [D]) were measured in control and EphB2LacZ mouse whole blood by flow cytometry. Similarly, CRP-XL (0.5 µg/mL [E] or 1 µg/mL [F]) and thrombin (0.1 U/ml [G] or 0.2 U/ml [H])-induced P-selectin exposure were measured in control and EphB2LacZ mouse whole blood by flow cytometry. ATP release was measured as a marker for dense granule secretion in washed platelets upon stimulation with 1 µg/mL CRP-XL (I-J). The level of fibrinogen binding or P-selectin exposure or ATP release (at 5 minutes) obtained in control was taken as 100% to calculate the percentage of reduction. Data represent mean ± SD (n = 4) (Student t test: *P ≤ .05 and **P ≤ .01). (K) Rap1b-GTP was precipitated from control and EphB2LacZ resting and stimulated platelets with CRP-XL (0.5 µg/mL) and analyzed by immunoblotting using a Rap1b antibody. The blot is representative of 3 separate experiments. ATP, adenosine triphosphate; GTP, guanosine triphosphate.
EphB2 signaling regulates initial platelet activation. CRP-XL (0.5 µg/mL [A] or 1 µg/mL [B]) and thrombin-induced fibrinogen binding (0.1 U/mL [C] or 0.2 U/mL [D]) were measured in control and EphB2LacZ mouse whole blood by flow cytometry. Similarly, CRP-XL (0.5 µg/mL [E] or 1 µg/mL [F]) and thrombin (0.1 U/ml [G] or 0.2 U/ml [H])-induced P-selectin exposure were measured in control and EphB2LacZ mouse whole blood by flow cytometry. ATP release was measured as a marker for dense granule secretion in washed platelets upon stimulation with 1 µg/mL CRP-XL (I-J). The level of fibrinogen binding or P-selectin exposure or ATP release (at 5 minutes) obtained in control was taken as 100% to calculate the percentage of reduction. Data represent mean ± SD (n = 4) (Student t test: *P ≤ .05 and **P ≤ .01). (K) Rap1b-GTP was precipitated from control and EphB2LacZ resting and stimulated platelets with CRP-XL (0.5 µg/mL) and analyzed by immunoblotting using a Rap1b antibody. The blot is representative of 3 separate experiments. ATP, adenosine triphosphate; GTP, guanosine triphosphate.
Rap1b is a member of the Ras superfamily of proteins23,24 and is involved in the regulation of integrin αIIbβ3.25,26 Because fibrinogen binding was affected in EphB2LacZ platelets, Rap1b activation was analyzed. Resting and CRP-XL (0.5 µg/mL)–stimulated platelets were lysed and guanosine triphosphate (GTP)–bound Rap1b precipitated using Ral guanine nucleotide dissociation stimulator (Ral-GDS)-agarose beads. CRP-XL–activated control platelets showed activation of Rap1b, whereas Rap1b activation was reduced in platelets obtained from EphB2LacZ mice (Figure 3K), indicating that the EphB2 intracellular domain may signal to Rap1b in the regulation of integrin αIIbβ3 function.
EphB2 regulates platelet calcium mobilization and PI3K signaling
Elevation of intracellular calcium levels in platelets upon exposure to agonists plays a major role in the regulation of platelet function including reorganization of the actin cytoskeleton, which is necessary for shape change,27 degranulation, and modulation of integrin αIIbβ3 affinity.28 Although the initial acceleration of calcium mobilization upon stimulation with CRP-XL (1 µg/mL) measured using flow cytometry was not affected by the absence of the EphB2 cytoplasmic tail, the overall level of calcium released was reduced substantially in EphB2LacZ platelets (Figure 4A).
EphB2 influences calcium mobilization and PI3K signaling. (A) Fluo4-NW–labeled control and EphB2LacZ mouse whole blood was stimulated with 1 µg/mL CRP-XL and the intracellular calcium levels measured by flow cytometry. Data represent mean ± SD (n = 4). CRP-XL (0.5 µg/mL)–induced phosphorylation of Src (B), Lyn (B), Syk (B), LAT (B), PLCγ2 (C), and AKT (E) was analyzed by immunoblot analysis using phospho-specific antibodies or following immunoprecipitation. Similarly, thrombin (0.1 U/mL)–induced phosphorylation of PLCβ3 (D) and AKT (F) was measured using immunoblot analysis. The protein 14-3-3ζ was detected as a loading control in all of the samples. Blots are representative of 3 separate experiments. The level of phosphorylation obtained with control platelets at 90 seconds was taken as 100%. Ionomycin (5 µM [G] and 10 µM [H])–induced fibrinogen binding was measured in whole blood using flow cytometry. Data represent mean (of median fluorescence intensity) ± SD (n = 3) (Student t test: *P ≤ .01, **P ≤ .01, and ***P ≤ .001). AKT, protein kinase B.
EphB2 influences calcium mobilization and PI3K signaling. (A) Fluo4-NW–labeled control and EphB2LacZ mouse whole blood was stimulated with 1 µg/mL CRP-XL and the intracellular calcium levels measured by flow cytometry. Data represent mean ± SD (n = 4). CRP-XL (0.5 µg/mL)–induced phosphorylation of Src (B), Lyn (B), Syk (B), LAT (B), PLCγ2 (C), and AKT (E) was analyzed by immunoblot analysis using phospho-specific antibodies or following immunoprecipitation. Similarly, thrombin (0.1 U/mL)–induced phosphorylation of PLCβ3 (D) and AKT (F) was measured using immunoblot analysis. The protein 14-3-3ζ was detected as a loading control in all of the samples. Blots are representative of 3 separate experiments. The level of phosphorylation obtained with control platelets at 90 seconds was taken as 100%. Ionomycin (5 µM [G] and 10 µM [H])–induced fibrinogen binding was measured in whole blood using flow cytometry. Data represent mean (of median fluorescence intensity) ± SD (n = 3) (Student t test: *P ≤ .01, **P ≤ .01, and ***P ≤ .001). AKT, protein kinase B.
EphB2 is reported to recruit and activate Src kinase through tyrosine phosphorylation at Y605 and Y611 in the juxtamembrane domain.29,30 Because these sites are present in the EphB2 in EphB2LacZ mice, to understand the impact of the defective receptor on Src activation, the phosphorylation of Src was explored upon activation of platelets with CRP-XL (0.5 µg/mL). The phosphorylation level of Src at Y418 in EphB2LacZ platelets was unaltered (Figure 4B). To explore whether the activation of molecules that mediate initial GPVI signaling was diminished in EphB2LacZ platelets, phosphorylation levels of Lyn (Y396), Syk, and linker for activation of T cells (LAT) were assessed using immunoblot analysis. CRP-XL (0.5 µg/mL)–induced phosphorylation of Lyn (Y396), Syk, and LAT was unaffected in EphB2LacZ platelets, indicating that EphB2 is not involved in GPVI proximal signaling (Figure 4B). Phospholipase Cγ2 (PLCγ2) and phospholipase Cβ3 (PLCβ3) are activated downstream of GPVI and Gq-mediated G-protein–coupled receptor (GPCR) signaling pathways, respectively, and responsible for the liberation of inositol trisphosphate (IP3) that releases calcium from intracellular stores. Because calcium mobilization was reduced in EphB2LacZ platelets, CRP-XL (0.5 µg/mL)–induced tyrosine phosphorylation of PLCγ2 and thrombin (0.1 U/mL)–induced tyrosine phosphorylation of PLCβ3 were analyzed in washed platelets by immunoblot analysis. Both PLCγ2 and PLCβ3 were found to be phosphorylated in control platelets upon stimulation with CRP-XL and thrombin, respectively. The phosphorylation level of PLCγ2 (Figure 4C) and PLCβ3 (Figure 4D) was reduced in EphB2LacZ platelets 90 seconds following stimulation. Similarly, CRP-XL (0.5 µg/mL)–stimulated (Figure 4E) and thrombin (0.1 U/mL)–stimulated (Figure 4F) phosphorylation of protein kinase B (PKB/AKT), a downstream effector of PI3K, was found to be reduced in EphB2LacZ platelets compared with controls. Together, these data point toward reduced PI3K signaling in EphB2LacZ platelets.
Because receptor proximal signaling was relatively unaffected in EphB2LacZ platelets, experiments were performed to determine whether its mode(s) of action lies further downstream through bypassing receptor proximal signaling and directly activating platelets using a calcium ionophore. Ionomycin (5 and 10 µM)–induced fibrinogen binding was reduced significantly in EphB2LacZ mice platelets (Figure 4G-H), indicating that EphB2 is involved in the regulation of downstream signaling that modulates platelet function.
EphB2 regulates platelet integrin signaling
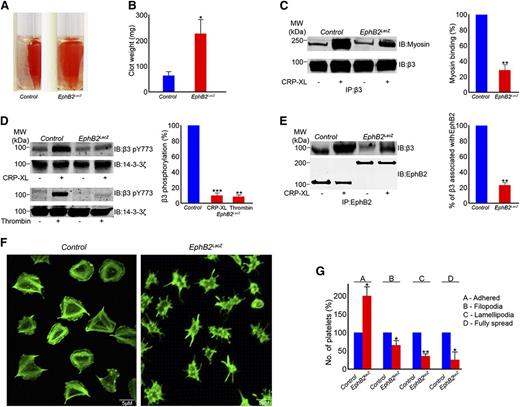
The integrin αIIbβ3 transduces signals into the cell-triggering platelet spreading and in the latter phase of thrombus formation to regulate clot retraction.31 To determine whether the EphB2 is involved in the regulation of integrin αIIbβ3-mediated outside-in signaling, clot retraction was measured. Although the initial clot formation was unchanged, clot retraction was found to be reduced in EphB2LacZ platelets at 2 hours (Figure 5A-B).
Integrin αIIbβ3-mediated outside-in signaling is reduced in EphB2LacZ platelets. (A) The effect of deletion of EphB2 intracellular domains in clot retraction was analyzed using PRP from control and EphB2LacZ mice for 2 hours. (B) Clot weight measured after 2 hours. Data represent mean ± SD (n = 3). (C) Integrin β3 was immunoprecipitated from resting and CRP-XL (0.5 µg/mL)–stimulated control and EphB2LacZ platelets and analyzed for its association with myosin by immunoblot analysis. (D) Phosphorylation of integrin β3 in resting and CRP-XL (0.5 µg/mL)– or thrombin (0.1 U/mL)–activated control and EphB2LacZ platelets was analyzed using phospho-specific integrin β3 antibody for pY773. The protein 14-3-3ζ was analyzed as a protein loading control by immunoblot analysis. (E) β3 integrin was coimmunoprecipitated with EphB2 in control and EphB2LacZ platelets, and analyzed for its association by immunoblot. Immunoblots shown are representative of 3 separate experiments. Data represent mean ± SD (n = 3) and the level of phosphorylation obtained in the control (activated) samples was taken as 100%. (F) Washed platelets obtained from control and EphB2LacZ mice were allowed to spread on fibrinogen-coated cover glass for 45 minutes prior to staining with Alexa Fluor 488 phalloidin and analyzed by confocal microscopy. Images are representative of 3 separate experiments. (G) Multiple images obtained by differential interference contrast microscopy were analyzed by counting numbers of platelets in various stages of spreading using Image J software. P values shown are as calculated by Student t test: *P ≤ .05, **P ≤ .01, and ***P ≤ .001. PRP, platelet-rich plasma.
Integrin αIIbβ3-mediated outside-in signaling is reduced in EphB2LacZ platelets. (A) The effect of deletion of EphB2 intracellular domains in clot retraction was analyzed using PRP from control and EphB2LacZ mice for 2 hours. (B) Clot weight measured after 2 hours. Data represent mean ± SD (n = 3). (C) Integrin β3 was immunoprecipitated from resting and CRP-XL (0.5 µg/mL)–stimulated control and EphB2LacZ platelets and analyzed for its association with myosin by immunoblot analysis. (D) Phosphorylation of integrin β3 in resting and CRP-XL (0.5 µg/mL)– or thrombin (0.1 U/mL)–activated control and EphB2LacZ platelets was analyzed using phospho-specific integrin β3 antibody for pY773. The protein 14-3-3ζ was analyzed as a protein loading control by immunoblot analysis. (E) β3 integrin was coimmunoprecipitated with EphB2 in control and EphB2LacZ platelets, and analyzed for its association by immunoblot. Immunoblots shown are representative of 3 separate experiments. Data represent mean ± SD (n = 3) and the level of phosphorylation obtained in the control (activated) samples was taken as 100%. (F) Washed platelets obtained from control and EphB2LacZ mice were allowed to spread on fibrinogen-coated cover glass for 45 minutes prior to staining with Alexa Fluor 488 phalloidin and analyzed by confocal microscopy. Images are representative of 3 separate experiments. (G) Multiple images obtained by differential interference contrast microscopy were analyzed by counting numbers of platelets in various stages of spreading using Image J software. P values shown are as calculated by Student t test: *P ≤ .05, **P ≤ .01, and ***P ≤ .001. PRP, platelet-rich plasma.
Association of myosin with cytoplasmic region of β3 is required for clot retraction.32,33 Hence, the association of myosin with the integrin β3 tail in EphB2LacZ and control platelets upon stimulation with 0.5 µg/mL CRP-XL was analyzed. Resting and activated platelets were lysed and myosin was coimmunoprecipitated with β3. Detection with a myosin-specific antibody demonstrated the association of myosin with β3 in activated control platelets and a reduced level of binding in EphB2LacZ platelets (Figure 5C). Myosin binding requires the phosphorylation of 2 tyrosine residues (Y773 and Y785 in humans [Y747 and Y759 in mice]) in the β3 cytoplasmic domain32 and mutation of either residue retards the retraction of blood clots in mice.34 Consistent with reduced clot retraction and retarded myosin binding, the phosphorylation of β3 at Y747 was reduced by ∼70% in EphB2LacZ platelets upon stimulation with CRP-XL compared with controls (Figure 5D). Similarly, thrombin (0.1 U/mL)–induced phosphorylation of β3 was also diminished in EphB2LacZ platelets (Figure 5D).
Because EphA4 has been reported to physically interact with integrin β3 in resting and activated platelets,16 the effects of replacement of the EphB2 intracellular region on its potential ability to associate with integrin β3 was analyzed. The immunoprecipitation of EphB2 from resting and CRP-XL (0.5 µg/mL)–activated platelets from control mice established that EphB2 physically associates with β3 in platelets, but levels were diminished in EphB2LacZ platelets (Figure 5E), indicating that the EphB2 cytoplasmic tail may be important for interaction with integrin β3 and/or subsequent signaling.
EphB2-mediated regulation of integrin β3 function does not require ephrin interaction
Platelet spreading following adhesion to fibrinogen is controlled by integrin αIIbβ3 outside-in signaling and does not require platelet-platelet contact. It is possible that defective responses of platelets to thrombin that is present in clot retraction assays may in part explain defective clot retraction exhibited by EphB2LacZ platelets. We therefore explored platelet spreading on fibrinogen where the addition of other agonists is not required and where cell density was reduced to prevent cell-cell contact.
Platelets were allowed to spread on fibrinogen-coated cover glass for 45 minutes and images were obtained following the staining with Alexa Fluor 488–conjugated phalloidin by confocal microscopy, and the results revealed that spreading was retarded in EphB2LacZ platelets (Figure 5F). To analyze different stages of spreading, multiple images were obtained by differential interference contrast microscopy and platelets at different stages of spreading counted (stage A includes platelets adhered; stage B, with filopodia; stage C, with lamellipodia; and D, fully spread). Our data suggested that in comparison with control platelets, a large proportion of EphB2LacZ platelets were retarded at stage A with only a small percentage of cells progressing to spread fully at 45 minutes (Figure 5G).
EphB2 cytoplasmic tail regulates thrombus formation and hemostasis
Because platelet aggregation, calcium mobilization, granule secretion, and clot retraction were reduced in EphB2LacZ platelets, we hypothesized that EphB2 mutation would influence thrombus formation. This was measured in vitro under arterial flow conditions (1000 S−1) using whole fluorescently labeled mouse blood perfused through collagen-coated biochips. Although similar levels of initial platelet adhesion were observed in comparison with control mouse blood, thrombus growth and volume were reduced substantially in EphB2LacZ mouse blood (Figure 6A-C).
EphB2 signaling is important for thrombus formation and hemostasis. (A) DiOC6-labeled control or EphB2LacZ mouse blood was perfused over collagen-coated Vena8 Biochips and thrombus formation monitored for 10 minutes capturing images at every 30 seconds (representative images at different time points shown). (B) The fluorescence intensity of thrombi obtained from control and EphB2LacZ mice was compared over a 10-minute period. Data represent mean (of sum intensity) ± SD (n = 4). (C) Thrombus intensity obtained in control at 10 minutes was taken as 100% and compared with EphB2LacZ at the same time point. Data represent mean (of sum intensity) ± SD (n = 4). The P values shown in figure are as calculated by 2-way ANOVA (***P ≤ .001). (D) Laser-induced arterial thrombosis was measured in mouse cremaster muscle arterioles using intravital microscopy and the fluorescence intensity of thrombi (E) was calculated using Slidebook software (version 5.5). Representative images at different time points and cumulative trace of thrombus formation are shown. Data represent mean ± SD (n = 4 of control and EphB2LacZ mice). The P values shown in figure are as calculated by 2-way ANOVA (F) Tail bleeding was assessed in control and EphB2LacZ mice by dissection of 1 mm of tail tip and monitoring the time to cessation of bleeding. Data represent mean ± SD (n = 7 of control and n = 9 EphB2LacZ mice). The P value shown was calculated using the nonparametric Mann-Whitney test. ANOVA, analysis of variance.
EphB2 signaling is important for thrombus formation and hemostasis. (A) DiOC6-labeled control or EphB2LacZ mouse blood was perfused over collagen-coated Vena8 Biochips and thrombus formation monitored for 10 minutes capturing images at every 30 seconds (representative images at different time points shown). (B) The fluorescence intensity of thrombi obtained from control and EphB2LacZ mice was compared over a 10-minute period. Data represent mean (of sum intensity) ± SD (n = 4). (C) Thrombus intensity obtained in control at 10 minutes was taken as 100% and compared with EphB2LacZ at the same time point. Data represent mean (of sum intensity) ± SD (n = 4). The P values shown in figure are as calculated by 2-way ANOVA (***P ≤ .001). (D) Laser-induced arterial thrombosis was measured in mouse cremaster muscle arterioles using intravital microscopy and the fluorescence intensity of thrombi (E) was calculated using Slidebook software (version 5.5). Representative images at different time points and cumulative trace of thrombus formation are shown. Data represent mean ± SD (n = 4 of control and EphB2LacZ mice). The P values shown in figure are as calculated by 2-way ANOVA (F) Tail bleeding was assessed in control and EphB2LacZ mice by dissection of 1 mm of tail tip and monitoring the time to cessation of bleeding. Data represent mean ± SD (n = 7 of control and n = 9 EphB2LacZ mice). The P value shown was calculated using the nonparametric Mann-Whitney test. ANOVA, analysis of variance.
To assess the contributions of EphB2 to thrombus formation in vivo, laser-induced thrombus formation was studied in cremaster muscle arterioles using intravital microscopy. Following laser injury, thrombus formation was monitored for 3 minutes, and found to be reduced substantially in EphB2LacZ mice compared with controls (Figure 6D-E).
To assess the importance of the cytoplasmic region of EphB2 for hemostasis, tail-bleeding assays were performed. The bleeding time after dissection of 1 mm of tail tip was prolonged substantially in EphB2LacZ mice compared with controls (Figure 6F). Bleeding persisted in most EphB2LacZ mice until 10 minutes at which point assays were terminated.
Discussion
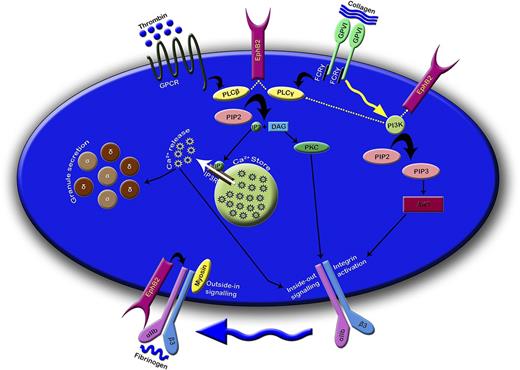
Bidirectional signaling mediated through Eph kinases and their ligands, ephrins, has been shown to play important roles in the development of the nervous system,11,35-37 skeletal patterning,38 and angiogenesis.39-42 A surprising role for Eph kinases and their ligands has also been identified on circulating platelets during thrombus formation where sustained Eph-ephrin signaling between platelets was proposed to be important for the maintenance of thrombus stability.2,15 In the present study, we analyzed the function of a newly identified platelet Eph kinase, EphB2, and focused on the contribution of its intracellular region in the stimulation of platelet signaling and activation. The results of this study suggest that EphB2-derived signaling is important for the positive regulation of platelet function through control of calcium mobilization, PI3K, and integrin αIIbβ3-mediated signaling (Figure 7).
EphB2 regulates platelet function through modulating calcium mobilization, PI3K- and integrin αIIbβ3-mediated signaling. EphB2 may directly and/or indirectly influence the activation of PLC isoforms and thereby controls PI3K signaling. The consequences of these signaling events modulate the calcium mobilization, integrin αIIbβ3 mediated inside-out signaling, and granule secretion. Because EphB2 is physically associated with integrin αIIbβ3, it may be involved in the regulation of integrin αIIbβ3 mediated outside-in signaling. α and δ in small discs represent α and dense granules, respectively. The dotted lines indicate the predictive functions. DAG, diacylglycerol; FCRγ, Fc receptor γ chain; GPCR, G-protein–coupled receptor; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C.
EphB2 regulates platelet function through modulating calcium mobilization, PI3K- and integrin αIIbβ3-mediated signaling. EphB2 may directly and/or indirectly influence the activation of PLC isoforms and thereby controls PI3K signaling. The consequences of these signaling events modulate the calcium mobilization, integrin αIIbβ3 mediated inside-out signaling, and granule secretion. Because EphB2 is physically associated with integrin αIIbβ3, it may be involved in the regulation of integrin αIIbβ3 mediated outside-in signaling. α and δ in small discs represent α and dense granules, respectively. The dotted lines indicate the predictive functions. DAG, diacylglycerol; FCRγ, Fc receptor γ chain; GPCR, G-protein–coupled receptor; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; PIP2, phosphatidylinositol 4,5-bisphosphate; PKC, protein kinase C.
The use of a genetically modified mouse in which the intracellular region of EphB2 was replaced by β-galactosidase enabled us to explore the functions of the cytoplasmic domains of EphB2 in regulating platelet function without interfering with its ligand binding. This has previously been shown to result in defects in pathfinding of specific axons in the central nervous system, suggesting the importance of the intracellular domains in EphB2 signaling.20 The previous study also confirmed the presence of EphB2LacZ fusion protein on the surface of neuronal cells and where subcellular localization was normal. In our study, the disruption of the intracellular region of EphB2 was found to reduce platelet activation, granule secretion, calcium mobilization, fibrinogen binding, and thrombus formation. These functional defects were associated with reduced signaling through PI3K and integrin αIIbβ3. The reduction in Rap1b activation in EphB2LacZ platelets suggests that EphB2 is involved in controlling agonist-induced inside-out signaling, leading to the activation of integrin αIIbβ3.
A principal focus of previous explorations of Eph-ephrin functions in platelets has been their involvement in sustained signaling between platelets within a thrombus.2 The functional and signaling defects observed on individual EphB2LacZ mouse platelets in absence of Eph-ephrin interactions, for example, while spreading on fibrinogen, fibrinogen binding, and P-selectin exposure (measured by gating on individual platelets in flow cytometry analysis), suggest that EphB2-mediated forward signaling is not entirely dependent on the binding of its ligand ephrinB1 in the regulation of platelet function. Consistent with reduced platelet activation, EphB2LacZ mouse platelets displayed diminished phosphorylation of signaling proteins such as AKT and PLCγ2 or PLCβ3 upon activation of GPVI or Gq-mediated GPCR pathways.
The tyrosine phosphorylation of PLCβ3 in platelets following stimulation with thrombin is a novel observation, although tyrosine phosphorylation sites have been previously identified in several carcinoma cell lines using mass spectrometry.43 Several tyrosine phosphorylation sites within the sequence of PLCβ3 have been predicted, including a prominent Y855 site that is highly conserved in humans, mice, and rats. The biological significance of PLCβ3 tyrosine phosphorylation, its regulation, and its impact on enzymatic activity in cell function are yet to be explored.
The reduction in phosphorylation of AKT and PLCγ2 was also apparent in EphB2LacZ platelets in the presence of agents (such as EGTA, indomethacin, and apyrase) that block the secondary activation, indicating that reduced signaling in EphB2LacZ platelets is not due to the inhibition of secretion of ADP or synthesis of thromboxane (data not shown). The phosphorylation of signaling proteins proximal to the GPVI collagen receptor such as Lyn, Syk, and LAT were unaffected, suggesting that EphB2 may not influence early signaling following engagement of GPVI. Reduced fibrinogen binding observed in EphB2LacZ platelets upon activation with ionomycin further confirms that EphB2-mediated platelet signaling regulates downstream signaling of calcium (in addition to the regulation of calcium mobilization itself) and suggests a complex signaling role.
The tyrosine phosphorylation sites 605 and 611 present in the juxtamembrane region of EphB2 were shown to bind and activate Src kinase.29,30 Because these sites are intact in the EphB2LacZ platelets, they may become phosphorylated upon ephrin ligation. Other tyrosine phosphorylation sites identified within the kinase domain such as 668, 741/751, 779, and 82144 are not present in EphB2LacZ. The phosphorylation of Y668 in the kinase domain has been suggested to bind SH2 domain-containing proteins44 and the SAM domain and PDZ- binding motif to interact with several cytoplasmic proteins. The absence of these regions in addition to the kinase domain may therefore account for reduced platelet function in the current settings. The retarded functions observed in EphB2LacZ platelets where ephrinB1 binding is potentially maintained (eg, clot retraction assays) suggest that these functions of EphB2 are not dependent on reverse signaling.
Defective spreading on fibrinogen and clot retraction assessed in EphB2LacZ mouse platelets suggest that EphB2 (particularly the intracellular region) is also involved in the regulation of integrin αIIbβ3-mediated outside-in signaling. Our data indicate that the EphB2 intracellular domain may enhance its physical association with integrin β3 and contribute to its phosphorylation that in turn regulates spreading and clot retraction. We cannot rule out that the reduced PI3K and PLC activities in EphB2LacZ platelets may also be responsible for the diminished integrin αIIbβ3-mediated signaling. The blockade of EphA4 using recombinant ligands has been reported to suppress platelet spreading and clot retraction15 and, therefore, interaction with, and regulation of, integrins in platelets may represent a shared feature of Eph kinase function in these cells. Eph kinases and ephrins have been widely reported to modulate integrin function, although specific effects appear to be cell-type and receptor-type specific. For example, activation of EphB1, EphA8, ephrinA2, and ephrinA5 have been shown to increase integrin affinity and thereby enhance cell-cell adhesion in endothelial, P19, and HEK-293 cells,45-48 whereas, EphB2, EphA2, and EphA3 regulate cell repulsion/detachment in PC-3 and HEK-293 cells.49,50 In the present study, we report a role for EphB2 in regulating integrin αIIbβ3 activation and therefore platelet-platelet adhesion. The means by which this is achieved remain to be established. In addition to a potential direct signaling role, it is possible that Eph kinases may form multimeric-protein complexes with integrin αIIbβ3 and other membrane proteins that contribute to cell adhesion and consequent integrin (outside-in) signaling. In platelets, all 3 Eph kinases identified to date appear to contribute to the upregulation of integrin activation, enhance cell-cell adhesion, and facilitate contact-dependent sustained signaling.
Although it is possible for Eph-ephrin–mediated signaling to contribute within a thrombus once platelet contact has been achieved, our studies reveal a role for EphB2 earlier in the platelet activation process and prior to cell contact. It may be speculated that the initial activation of individual platelets modulates the phosphorylation status of EphB2 and cause the recruitment of effector proteins to the cytoplasmic domains. The consequence of fibrinogen binding to integrin αIIbβ3 may also lead to enhanced clustering of EphB2 because β3 and EphB2 interact and facilitated Eph-ephrin interactions when the platelets come to close proximity. In these ways, Eph kinases may play roles during initial activation in a contact-independent manner and later, when the Eph-ephrin ligation occurs, contribute to sustained contact-dependent signaling. As shown previously,2,15 Eph-ephrin ligation and clustering may induce further activation of platelets through shape change, degranulation, fibrinogen binding, and strengthening of the growing thrombus.
The intracellular region of EphB2 incorporates several important tyrosine phosphorylation sites, a kinase domain, SAM and PDZ-binding region. Therefore, the functional and signaling defects observed in EphB2LacZ platelets may be explained through the loss of direct signaling activity (kinase domain), disruption of the interaction of the cytoplasmic tail with downstream signaling effector molecules, or a combination of these. It may be possible that steric constraints due to the introduction of a larger cytoplasmic tail may also impact platelet function. Should this prove to be the case, it would not diminish a key conclusion from this study: that the cytoplasmic tail of EphB2 is required for the regulation of platelet function. In general, Eph kinases and ephrin B family members regulate their signaling events through several cellular effector proteins in phosphorylation-dependent and phosphorylation-independent manners.2 Although the phosphorylation-dependent signaling events of Eph kinases rely on the cytoplasmic region including kinase and SAM domains, phosphorylation-independent signaling is mainly driven through the C terminus PDZ-binding motif. Many of the Eph kinase effector molecules reported in other cell types are involved in the regulation of cytoskeletal rearrangements and focal adhesions.2 Therefore, further studies are needed to identify and characterize the effector molecules that are involved in Eph signaling in platelets.
In summary, we reveal that Eph-ephrin signaling is not only restricted to sustained contact-dependent signaling during thrombus formation and clot retraction, but in the absence of Eph-ephrin ligation between platelets, EphB2 also contributes to initial platelet activation signals in a contact-independent manner. Further studies are required to dissect the selective roles of individual tyrosine phosphorylation sites and specific intracellular domains of EphB2 in the regulation of platelet function.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Prof Ralf Adams (Max Planck Institute for Molecular Biomedicine, Germany) for providing transgenic mice and Dr Mike Fry (University of Reading) for his advice and suggestions during the preparation of this manuscript.
This work was supported by the British Heart Foundation (grants PG/08/100/26125, PG/11/125/29320, and RG/09/011/28094), Medical Research Council (grant MR/J002666/1) and Biotechnology and Biological Sciences Research Council (grant BB/J014451/1).
Authorship
Contribution: S.V. designed the study, performed experiments, analyzed data, and wrote the paper; T.S., M.P.S., R.H.R., M.S.A., A.J.U., A.R.S., N.K., C.I.J., and L.A.M. performed experiments and analyzed data; and J.M.G. designed the study, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonathan Gibbins, Institute for Cardiovascular and Metabolic Research, School of Biological Sciences, University of Reading, Harborne Building, Reading, RG6 6AS, United Kingdom; e-mail: j.m.gibbins@reading.ac.uk.
References
Author notes
T.S., R.H.R., and M.P.S. have contributed equally to this study.

![Figure 2. Defective aggregation of EphB2LacZ platelets. Platelet aggregation was recorded for 5 minutes by optical aggregometry using washed platelets obtained from control and EphB2LacZ mice following stimulation with collagen (1 µg/mL [A-B] or 5 µg/mL [C-D]), CRP-XL (0.5 µg/mL [E-F] or 1 µg/mL [G-H]), and thrombin (0.1 U/mL [I-J] or 0.2 U/mL [K-L]). Data represent mean ± SD (n = 3). The level of aggregation obtained with control platelets at 5 minutes was taken as 100% (Student t test: *P ≤ .05, **P ≤ .01, and ***P ≤ .001).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/4/10.1182_blood-2014-06-585083/4/m_720f2.jpeg?Expires=1765906413&Signature=htZm8IYRO9FVqHinl0zo1GJlDl62za8nJ9DGu4fUynlQkEc5jD2c374e6SRDxezQEQCg0Gt~edEkftolUntyH83DW7-QrFd57bRsg44SPRQaPi3f6j7gdXfROf8qgJX9tX03N0gxG2D47YMM9d5m2iwwCOSSvKG1I6zXJucg-dECUfMB-jPcCKWjlvOYmjbTWTYwFZTSfee7G9VRmBUyM~k5mGhByjou-wyky44Yk-vunbzbKWDtLG8zDjeHO-gZ0uRd-4aqsF2cL32ZUW8e9Cl--WEe-DRUHLYvl7iw1Hb6ziecjYV9BMShUELmIbQA6E96sTpZonyXgVGh7lbqqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. EphB2 signaling regulates initial platelet activation. CRP-XL (0.5 µg/mL [A] or 1 µg/mL [B]) and thrombin-induced fibrinogen binding (0.1 U/mL [C] or 0.2 U/mL [D]) were measured in control and EphB2LacZ mouse whole blood by flow cytometry. Similarly, CRP-XL (0.5 µg/mL [E] or 1 µg/mL [F]) and thrombin (0.1 U/ml [G] or 0.2 U/ml [H])-induced P-selectin exposure were measured in control and EphB2LacZ mouse whole blood by flow cytometry. ATP release was measured as a marker for dense granule secretion in washed platelets upon stimulation with 1 µg/mL CRP-XL (I-J). The level of fibrinogen binding or P-selectin exposure or ATP release (at 5 minutes) obtained in control was taken as 100% to calculate the percentage of reduction. Data represent mean ± SD (n = 4) (Student t test: *P ≤ .05 and **P ≤ .01). (K) Rap1b-GTP was precipitated from control and EphB2LacZ resting and stimulated platelets with CRP-XL (0.5 µg/mL) and analyzed by immunoblotting using a Rap1b antibody. The blot is representative of 3 separate experiments. ATP, adenosine triphosphate; GTP, guanosine triphosphate.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/4/10.1182_blood-2014-06-585083/4/m_720f3.jpeg?Expires=1765906413&Signature=O~ju9ZKoPVn4sPAfLK6G6DWIrLaDTusfEH3I3wacoWDrdeSdTSU-xNkmJC50gO-q0GWQdDmj7-0CHIZTZ0eJs9TCULJNVZvyiEexc5wCIJHWrntujImHelUTyt4-pX7vz6Redwq~H9r20ltjnZgVO3QwJKggMPqTuXER5x85uW9swxTfEw-~KFj~-kB0xN8o1W031UDJ0Wfd3p5vY~8ajqaG2NrT7SEw~M9wLk3TRZu~PVy-dVXXZ19oW-rvzVvucxQvcKSKp3xNJEC0Apb~-SggdxxDLsFXk3dY1zDz1aMqh-OavM3XKB4eLegi0jz-biNhlI3fYnyMuWXMPzYezg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. EphB2 influences calcium mobilization and PI3K signaling. (A) Fluo4-NW–labeled control and EphB2LacZ mouse whole blood was stimulated with 1 µg/mL CRP-XL and the intracellular calcium levels measured by flow cytometry. Data represent mean ± SD (n = 4). CRP-XL (0.5 µg/mL)–induced phosphorylation of Src (B), Lyn (B), Syk (B), LAT (B), PLCγ2 (C), and AKT (E) was analyzed by immunoblot analysis using phospho-specific antibodies or following immunoprecipitation. Similarly, thrombin (0.1 U/mL)–induced phosphorylation of PLCβ3 (D) and AKT (F) was measured using immunoblot analysis. The protein 14-3-3ζ was detected as a loading control in all of the samples. Blots are representative of 3 separate experiments. The level of phosphorylation obtained with control platelets at 90 seconds was taken as 100%. Ionomycin (5 µM [G] and 10 µM [H])–induced fibrinogen binding was measured in whole blood using flow cytometry. Data represent mean (of median fluorescence intensity) ± SD (n = 3) (Student t test: *P ≤ .01, **P ≤ .01, and ***P ≤ .001). AKT, protein kinase B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/4/10.1182_blood-2014-06-585083/4/m_720f4.jpeg?Expires=1765906413&Signature=QInGh1VFHA1s2FRosO0s-l3NHK5BEwSy4FNlmL-iExEBaCaH4mt3ttkCIdJd1G58TSor94WUer-8-Iqoa4s4tqKMqlfmwUp1JSFYumj1oa9HlxYtJuHF4qoeO13Q1xo8PGXc74GoZqPfS8~Y-ToBARVLGZN5oKqSuEgndB1nBlYwFPe3tYSDZYolel1ejXYIUMB6MoMikz4sfP7I2aTrLMD57-mrOR1Y2558gXCeSeXTDHZ-BMKkHplzjuYAj4m~ei-Ip8oRQLsRuPHmiMTMyTnwDA2ikOtf0-dAMo6j44vPeV2OWrVSyVY9Q00VPe9vj~Ey424NWcSZBAc7GFjTow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)