Key Points
Monoclonal antibody blockade of the common γ chain attenuates acute and chronic GVHD.
Common γ-chain cytokines increase granzyme B levels in CD8 T cells, which are reduced upon CD132 blockade in vivo.
Abstract
The common γ chain (CD132) is a subunit of the interleukin (IL) receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. Because levels of several of these cytokines were shown to be increased in the serum of patients developing acute and chronic graft-versus-host disease (GVHD), we reasoned that inhibition of CD132 could have a profound effect on GVHD. We observed that anti-CD132 monoclonal antibody (mAb) reduced acute GVHD potently with respect to survival, production of tumor necrosis factor, interferon-γ, and IL-6, and GVHD histopathology. Anti-CD132 mAb afforded protection from GVHD partly via inhibition of granzyme B production in CD8 T cells, whereas exposure of CD8 T cells to IL-2, IL-7, IL-15, and IL-21 increased granzyme B production. Also, T cells exposed to anti-CD132 mAb displayed a more naive phenotype in microarray-based analyses and showed reduced Janus kinase 3 (JAK3) phosphorylation upon activation. Consistent with a role of JAK3 in GVHD, Jak3−/− T cells caused less severe GVHD. Additionally, anti-CD132 mAb treatment of established chronic GVHD reversed liver and lung fibrosis, and pulmonary dysfunction characteristic of bronchiolitis obliterans. We conclude that acute GVHD and chronic GVHD, caused by T cells activated by common γ-chain cytokines, each represent therapeutic targets for anti-CD132 mAb immunomodulation.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) is an important treatment option not only for different hematologic malignancies, but also for some nonmalignant hematologic disorders, such as sickle cell anemia, aplastic anemia, and thalassemia.1 In the latter group, the graft-versus-leukemia (GVL) effect mediated by donor T cells is less important, and prevention of graft-versus-host disease (GVHD), which occurs in 40% to 50% of allo-HCT patients,2 is a major priority.
Proinflammatory cytokines produced by not only different myeloid but also nonhematopoietic cells play a central role in the pathogenesis of acute GVHD3-6 and have therefore been targeted by antagonistic antibodies. Such strategies have included, for example, the anti–tumor necrosis factor (TNF) therapy infliximab in patients with acute GVHD.7 However, because of the high redundancy of different proinflammatory pathways which may have prevented the success of anti-TNF therapy,7 or high treatment-related mortality and relapse rates observed when giving, for instance, daclizumab for the treatment of acute GVHD,8 none of these approaches has become a standard initial clinical therapy for acute GVHD. In chronic GVHD, new therapies are urgently needed as there is a dearth of agents beyond steroids that have been shown to be efficacious in patients with multiorgan system disease.
The common γ chain (CD132), is a constituent of the receptor complexes for at least 6 different interleukins (ILs): IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21.9 More recently, the role of CD132 in CD8 T-cell lineage fate has also been demonstrated.10 Besides its presence in multiple cytokine receptors, CD132 is expressed on most lymphocytes, and therefore could be a potent target for the reduction of GVHD. Here, we demonstrate that a neutralizing monoclonal antibody (mAb) against CD132 did reduce acute GVHD by mitigating the perforin/granzyme B–mediated cytotoxicity of CD8 T cells. Furthermore, T cells activated in the presence of anti-CD132 had lower levels of Janus kinase 3 (JAK3), p38 mitogen-activated protein kinase (MAPK), and signal transducer and activator of transcription 5 (STAT5) phosphorylation, and expressed a gene signature characteristic for naive CD8 T cells compared with T cells activated in the absence of anti-CD132. Consistent with a role for JAK3 in GVHD, mice receiving JAK3-deficient T cells developed less severe GVHD compared with mice receiving wild-type (WT) T cells. Besides the studies in the mouse model, we observed that granzyme B and perforin levels were increased in CD8 T cells from patients developing GVHD compared with patients without GVHD or compared with healthy individuals, suggesting that these cytotoxic molecules could be a target for anti-CD132 treatment in humans. Although these analyses were performed in the setting of acute GVHD, we also found that anti-CD132 treatment ameliorated disease manifestations in a mouse model of organ-specific fibrosis with features of chronic GVHD.
Materials and methods
Human subjects
We collected all samples after approval by the ethics committee of the Albert-Ludwigs-University (Freiburg, Germany) (protocol no. 267/11) and after written informed consent in accordance with the Declaration of Helsinki. Blood samples were collected from individuals undergoing allo-HCT. Lymphocytes were isolated using the Pancoll separation buffer (PAN-Biotech). The patients’ characteristics are detailed in supplemental Table 1 (see supplemental Data available at the Blood Web site).
Mice
For acute GVHD experiments, C57BL/6 (H2b, Thy-1.2) and BALB/c (H2d, Thy-1.2) mice were purchased from the local stock of the animal facility at Freiburg University. BALB.B mice (C.B10-H2b/LilMcdJ) were purchased from The Jackson Laboratory. Perforin-deficient C57BL/6 mice (Perf−/−; H2b, Thy-1.2)11 and JAK3-deficient C57BL/6 mice (Jak3−/−; H2b)12 have been previously described. The animal protocols (G-12/34, X-10/13H) were approved by the Committee on the Use of Laboratory Animals at Albert-Ludwigs University Freiburg (Freiburg, Germany). Mice were 6 to 12 weeks of age and sex matched for each allo-HCT.
For the experiments on chronic GVHD, C57BL/6 (B6; H2b) mice (8-12 weeks of age; female recipients) were purchased from the National Cancer Institute. B10.BR (H2k) mice were purchased from The Jackson Laboratory. Mice were housed in a pathogen-free facility and used with the approval of the University of Minnesota institutional animal care board.
BMT model for acute GVHD and treatment of recipients
Bone marrow (BM) transplantation (BMT) experiments were performed as previously described.13,14 Recipients (BALB/c) were irradiated 2 times with 4.5 Gy in an interval of 4 hours before receiving 5 × 106 BM cells from C57BL/6 mice IV. To induce acute GVHD, CD4 and CD8 T cells (Tc) were isolated from C57BL/6 spleens and enriched by positive selection with the MACS cell separation system (Miltenyi Biotec) using anti-CD4 and anti-CD8 MicroBeads. Regulatory T cells (Tregs) were isolated using the CD4+CD25+ Regulatory T-Cell Isolation Kit (Miltenyi Biotec). CD4/CD8 T cells (5 × 105) were injected. For the transplantation with only CD4 or CD8 or Perf−/− T cells or Tregs, the following numbers of cells were used: 7.5 × 105 CD4, 1 × 106 CD8, 8 × 105Perf−/−, 3 × 105 Tregs. T-cell purity was >90% as assessed by flow cytometry (data not shown). C57BL/6 recipients were irradiated twice with 5 Gy with an interval of 4 hours before receiving 5 × 106 BM cells and 1 × 106 CD4 T cells from a BALB.B donor. Bm12 recipients were sublethally irradiated by exposing mice to 6 Gy total body irradiation (TBI) from a 137Cs source at a dose rate of 85 cGy per minute. Four to six hours later, 3 × 104 or 1 × 105 freshly isolated C57BL/6 T cells (WT or Jak3−/−) were injected into the lateral tail vein. Recipients were monitored for survival, hematocrit, and weight loss. Mice were treated with either 30 mg/kg anti-mouse CD132 IgG1 mAb, generated by Novartis (J.K., manuscript in preparation), or isotype control IgG antibody from day −1 until day 15, 3 times per week with intraperitoneal (i.p.) injections. BMT experiments that included the transfer of luciferase transgenic A20 lymphoma cells are described in the supplemental Methods.
BMT for chronic GVHD induction and therapeutic intervention
B10.BR recipients were conditioned with cyclophosphamide (Sigma) on days −3 and −2 (120 mg/kg per day i.p.). On day −1, recipients were irradiated by x-ray (8.3 Gy). B6 donor BM was T-cell depleted with anti-Thy1.2 mAb followed by rabbit complement. T cells were purified from spleens by incubation with biotin-labeled anti-CD19 mAb (eBioscience) followed by EasySep streptavidin rapidspheres, and then depletion on a magnetic column (StemCell Technologies). On day 0, recipients received 1 × 107 T-cell–depleted BM cells with or without purified splenic T cells (1 × 105). Weights of individual mice were recorded weekly. Where indicated, recipients in chronic GVHD groups were given anti-CD132 mAb (30 mg/kg, 3 times per week; clone 9B5mm) or isotype control antibody.
Acute GVHD histopathology
Slides of small intestine, large intestine, and liver samples collected on day 10 after allo-HCT were stained with hematoxylin/eosin (H/E) and scored by an experienced pathologist (A.S.-G.) blinded to the treatment groups according to a previously published histopathology scoring system.15
PFTs
Pulmonary function tests (PFTs) were performed as previously described.16 Briefly, anesthetized mice were weighed and lung function was assessed by whole-body plethysmography using the Flexivent system (Scireq). Data were analyzed using the Flexivent software version 5.1.
Frozen tissue preparation
All organs harvested were embedded in optimal cutting temperature (OCT) compound, snap-frozen in liquid nitrogen, and stored at −80°C. Lungs were inflated by infusing 1 mL of OCT: phosphate-buffered saline (3:1) intratracheally prior to harvest.
Trichrome staining
Cryosections (6 µm) were fixed and stained with the Masson trichrome staining kit (Sigma) for the detection of collagen deposition, which was quantified on trichrome-stained sections as a ratio of blue staining area to total staining area using the Adobe Photoshop CS3 analysis tool (Adobe Systems) as previously described.16
Immunofluorescence
For immunoglobulin (Ig) deposition, 6-µm cryosections were fixed with acetone, blocked with the horse serum and the streptavidin-biotin blocking kit (Vector), and then stained with fluorescein isothiocyanate (FITC)-labeled anti-mouse-Ig (BD Pharmingen). For germinal center detection, fixed 6-µm spleen cryosections were stained with rhodamine-peanut agglutinin (PNA; Vector Laboratories). Confocal images were acquired on an Olympus FluoView500 Confocal Laser Scanning Microscope at ×200, analyzed using FluoView3.2 software (Olympus), and processed with Adobe Photoshop CS3 (version 9.0.2).
Flow cytometry
All antibodies were purchased from BD Biosciences, BioLegend, and eBioscience, and used as FITC, phycoerythrin (PE), Alexa 647, or Pacific Blue conjugates. The following mAbs against murine antigens were used for flow cytometric analysis: CD4 (GK 1.5/RM4-5), CD8 (53-6.7), granzyme B (NGZB), IL-10 (JES5-16E3), IL-2 (JES6-5H4), IL-17 (TC11-18H10.1), IL-4 (11B11), and interferon-γ (IFN-γ; XMG1.2), anti-Phospho-STAT5 (Y694)–Alexa Fluor 647 (clone: 47; Becton Dickinson). Staining of human samples was performed with the following mAbs: CD8 (HIT8a), perforin (dG9), and granzyme B (GB11).
Intracellular staining for cytokines, granzyme B, and perforin was performed as previously described.17 Data were acquired with a CyanADP (Beckman Coulter) and then analyzed with FlowJo 7/8 software (TreeStar).
Cytokine measurements
The levels of IL-6, MCP-1, TNF-α, and IFN-γ were analyzed from serum on day 7 after allo-HCT with the flow cytometry–based CBA inflammation kit (BD Biosciences).
Generation of BM-DCs
BM–dendritic cells (BM-DCs) were prepared as previously described.18 BM cells were cultured at 5 × 105 cells per mL in the presence of 40 ng/mL granulocyte macrophage–colony-stimulating factor (GM-CSF; supernatant from producerline X63-Hybridom) in 10 mL of RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, and 100 µg/mL penicillin-streptomycin (Gibco) in 10-cm Petri dishes (Greiner). On day 3, 10 mL of fresh medium containing 40 ng/mL GM-CSF was added. On day 5, 10 mL of medium was replaced with fresh GM-CSF medium. BM-DCs were used on days 7 to 9.
Stimulation of CD8 T cells
T cells were isolated from the spleens of C57BL/6 mice using anti-CD8 MicroBeads. T cells (2 × 105) were incubated with 1 × 105 BM-DCs or 5 µL of Dynabeads Mouse T-Activator CD3/CD28 (Life Technologies) and the indicated concentrations of common γ-chain cytokines in 200 µL of RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, 100 µg/mL penicillin-streptomycin, and 2.5 × 10−5 M β-mercaptoethanol (Gibco) for 48 hours. Granzyme B expression was assessed by flow cytometry after gating on CD8+ cells.
Phospho-flow for pSTAT5
T cells were isolated from the spleens of C57BL/6 mice using a CD3 isolation kit (Miltenyi Biotec). Briefly, cells were incubated with serial dilutions of anti-CD132 mAb for 30 minutes at 37°C prior to the addition of 10 µL of the concentrated cytokine solution. After an incubation of 15 minutes at 37°C, the samples were fixed with a 2% paraformaldehyde solution and cell pellets resuspended in ice-cold 90% methanol. After storage of at least 2 hours at −20°C, the cells were processed for surface/intracellular staining with the fluorescence labeled antibodies according to standard protocols, after which the level of pSTAT per cell was analyzed by flow cytometry.
Western blot
For western blot analysis, protein was isolated from culture-derived homogenized T cells. Equal amounts of protein were loaded. The following antibodies were used: STAT5a (E289, abcam), phospho-STAT5 (Y694, E208, abcam), JAK3 (D7B12), phospho-JAK3 (Tyr980/981, D44E3), pospho-p38 MAPK (Thr180/Tyr182), p38 MAPK, and β-actin (13E5) (all from Cell Signaling).
Microarray analysis
Splenic CD8+ T cells (C57BL/6) were stimulated with CD3/CD28 beads and IL-2 (50 ng/mL) in 96-well plates. After 48 hours, T cells were collected and RNA was isolated from the purified T cells using the RNeasy Mini Kit (Qiagen) and the sample quality was assessed using a Fragment Analyzer (Advanced Analytical Technologies, Inc). Microarray was performed using Affymetrix WT Mouse Gene 1.0 ST arrays and data analysis used Gene Set Enrichment Analysis (GSEA),19 as further described in the supplemental Methods.
Statistical analysis
Data are reported as mean values ± standard error of the mean (SEM). Comparisons were made using an unpaired, 2-tailed Student t test with Welch correction. Differences in animal survival were analyzed by a Mantel-Cox log-rank test. A P value < .05 was considered statistically significant.
All other methods are described in supplemental Methods.
Results
Anti-CD132 mAb reduces acute GVHD and proinflammatory cytokine production
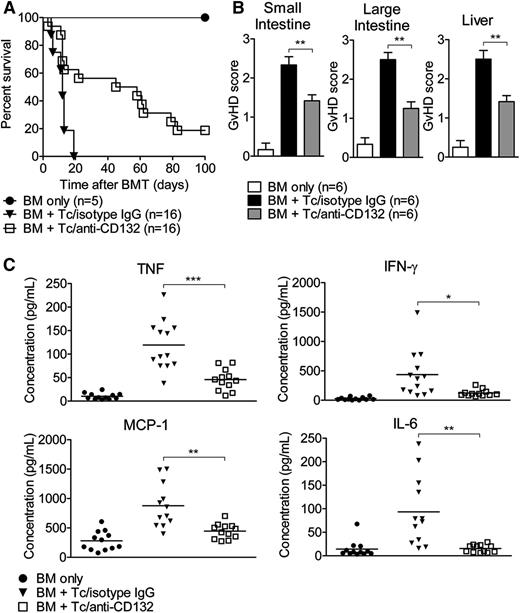
To test the hypothesis that blocking the common γ chain by anti-CD132 mAb could reduce acute GVHD, mice received anti-CD132 mAb or unspecific IgG. Mice transplanted with only BM survived long-term. We observed improved survival of mice treated with an anti-CD132 mAb compared with unspecific IgG (P = .0001; Figure 1A). Compatible with this improved survival, the histopathological severity of acute GVHD in the liver and the small and large intestines, determined as previously described,15 was significantly reduced in allo-HCT recipients treated with anti-CD132 mAb compared with the isotype control (Figure 1B). Because proinflammatory cytokine production is a hallmark of GVHD, we next evaluated the levels of TNF, IFN-γ, IL-6, and MCP-1 on day 7 in the serum of mice in the different groups. We found that all of these proinflammatory cytokines were reduced in allo-HCT recipients treated with the anti-CD132 mAb compared with unspecific IgG (Figure 1C). Overall, these findings indicate that a blockade of the common γ chain has a notable inhibitory effect on acute GVHD. However, this effect was only partial because the survival curve, the GVHD histopathology scores, and TNF levels provided evidence for residual disease activity.
Blockade of CD132 reduces acute GVHD. (A) Survival of transplanted mice receiving isotype control IgG (30 mg/kg) or anti-CD132 (30mg/kg) mAb from day −1 to +15, 3 times per week. The anti-CD132 mAb-treated group lived longer than the isotype-treated group (P < .0001). (B) Seven days after allo-HCT, mice from the indicated groups were sacrificed and the small intestine, large intestine, and liver were analyzed for histological signs of GVHD according to a published15 scoring system (**P < .01). (C) Serum was collected on day 7 after allo-HCT and analyzed for the indicated cytokines. Data are pooled from 2 experiments, representing 12 animals per group (*P < .05, **P < .01, ***P < .001).
Blockade of CD132 reduces acute GVHD. (A) Survival of transplanted mice receiving isotype control IgG (30 mg/kg) or anti-CD132 (30mg/kg) mAb from day −1 to +15, 3 times per week. The anti-CD132 mAb-treated group lived longer than the isotype-treated group (P < .0001). (B) Seven days after allo-HCT, mice from the indicated groups were sacrificed and the small intestine, large intestine, and liver were analyzed for histological signs of GVHD according to a published15 scoring system (**P < .01). (C) Serum was collected on day 7 after allo-HCT and analyzed for the indicated cytokines. Data are pooled from 2 experiments, representing 12 animals per group (*P < .05, **P < .01, ***P < .001).
Protection by anti-CD132 mAb treatment is mainly via CD8- as opposed to CD4-mediated acute GVHD
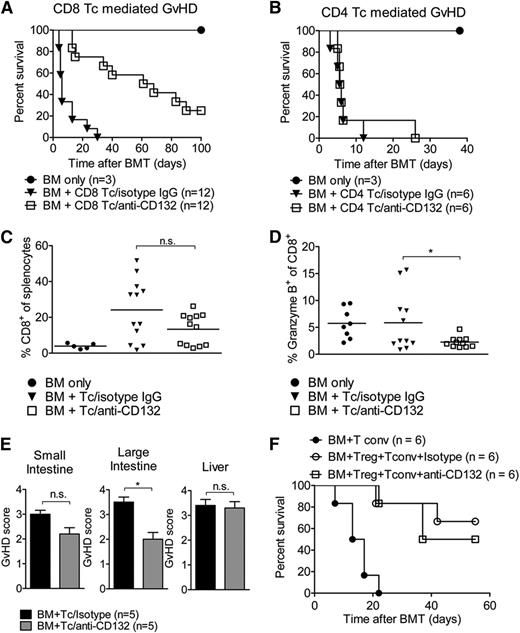
We then aimed to determine whether the protective effects of anti-CD132 mAb against GVHD were due to inhibition of CD4 or CD8 T cells, or both. Using GVHD models in which we transplanted either highly enriched (>95% purity) CD4 or CD8 T cells, we observed that when GVHD was dependent on CD8 T cells, anti-CD132 mAb treatment was protective (Figure 2A). Conversely, no protection was apparent when only CD4 T cells were transplanted to induce GVHD (Figure 2B). This reduction of GVHD severity was not dependent on a reduced relative (Figure 2C) or absolute number (not shown) of CD8 T cells in the spleen, as these values were comparable in allo-HCT recipients treated with the anti-CD132 and unspecific IgG antibodies. However, the frequency of granzyme B–positive cells within the CD8 T-cell compartment was significantly reduced in allo-HCT recipients treated with an anti-CD132 mAb compared with the isotype control (Figure 2D). When using a minor mismatch CD4-dependent model (BALB.B into C57BL/6),20 anti-CD132 did not reduce the histological GVHD severity with respect to liver and small intestine but with respect to colon GVHD (Figure 2E). The protective effect of Tregs against GVHD was not blocked by anti-CD132 treatment (Figure 2F). These data indicate that blocking CD132 has a significant effect on granzyme B production by CD8 T cells and minor effects on CD-mediated GVHD.
Granzyme B production of CD8 T cells is reduced by anti-CD132 treatment. (A) Survival of BALB/c mice which received BM and CD8 T cells (1 × 106) after TBI. Pooled survival of 2 experiments is shown. The group receiving anti-CD132 mAb survived significantly longer than the group receiving the isotype control (P < .0001). (B) Survival of BALB/c mice which received BM and CD4 T cells (7.5 × 105) after TBI. The difference in survival between the anti-CD132 mAb and isotype groups was not significant at the 95% confidence level. (C) Spleens were removed from transplanted mice on day 7 after BMT and analyzed for the percentage of CD8+ cells. Data are shown for 12 animals per group. (D) Granzyme B levels were analyzed in CD8 cells. Data are pooled from 2 experiments representing 12 animals per group (*P < .05). (E) GVHD histopathology scores for liver, small intestine, and large intestine are displayed. Mice underwent allo-HCT (BALB.B into C57BL/6) and treatment with anti-CD132 or unspecific IgG. Allo-HCT recipients were sacrificed on day 12 after transplantation (*P < .05). (F) Survival of mice undergoing allo-HCT (day 0) and transfer of conventional T cells (day 2) alone or in combination with purified CD4+/CD25+ Treg (day 0) and anti-CD132 antibody, or Treg (day 0) and unspecific IgG treatment. No significant difference was observed between the groups receiving Treg cells, independent of CD132 treatment.
Granzyme B production of CD8 T cells is reduced by anti-CD132 treatment. (A) Survival of BALB/c mice which received BM and CD8 T cells (1 × 106) after TBI. Pooled survival of 2 experiments is shown. The group receiving anti-CD132 mAb survived significantly longer than the group receiving the isotype control (P < .0001). (B) Survival of BALB/c mice which received BM and CD4 T cells (7.5 × 105) after TBI. The difference in survival between the anti-CD132 mAb and isotype groups was not significant at the 95% confidence level. (C) Spleens were removed from transplanted mice on day 7 after BMT and analyzed for the percentage of CD8+ cells. Data are shown for 12 animals per group. (D) Granzyme B levels were analyzed in CD8 cells. Data are pooled from 2 experiments representing 12 animals per group (*P < .05). (E) GVHD histopathology scores for liver, small intestine, and large intestine are displayed. Mice underwent allo-HCT (BALB.B into C57BL/6) and treatment with anti-CD132 or unspecific IgG. Allo-HCT recipients were sacrificed on day 12 after transplantation (*P < .05). (F) Survival of mice undergoing allo-HCT (day 0) and transfer of conventional T cells (day 2) alone or in combination with purified CD4+/CD25+ Treg (day 0) and anti-CD132 antibody, or Treg (day 0) and unspecific IgG treatment. No significant difference was observed between the groups receiving Treg cells, independent of CD132 treatment.
Common γ-chain cytokines induce granzyme B production in CD8 T cells, which is functionally linked to the protective effect of anti-CD132 mAb in GVHD
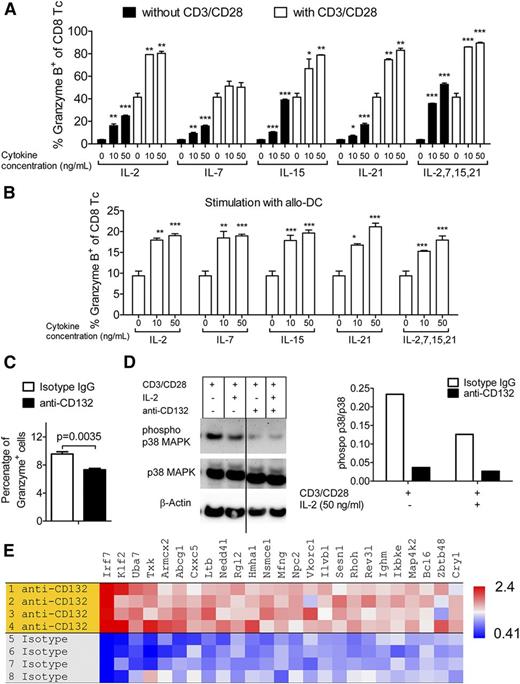
To clarify the connection between common γ-chain cytokines that depend on CD132 and the induction of granzyme B in CD8 T cells, we exposed CD8 T cells to varying concentrations of IL-2, IL-7, IL-15, and IL-21, or all 4 cytokines together. Granzyme B production by CD8 T cells increased when stimulated with CD3 and CD28 beads compared with unstimulated cells (Figure 3A). Granzyme B production by CD8 T cells increased in proportion to the concentration of the cytokines without additional stimulation or when simultaneous stimulation with CD3 and CD28 beads was included (Figure 3A). To test these findings in an allogeneic setting, we activated CD8 T cells (H-2b) with allogeneic BM-DCs (H-2d) and again observed an increase in granzyme B production by the CD8 T cells when exposed to the same panel of cytokines at each respective concentration (Figure 3B). In addition, we observed that stimulation with the allo-DCs led to increased granzyme B production independent of the cytokines (Figure 3B). The combination of the cytokines IL-2, IL-7, IL-15, and IL-21 did not lead to a further increase of granzyme B production over single common γ-chain cytokines (Figure 3A-B). Because IL-21 is a common γ-chain cytokine that was shown to be critical in a humanized mouse model21 and that may have relevance for human GVHD, we next evaluated whether anti-CD132 had an effect on IL-21–induced granzyme B production. Addition of the anti-CD132 antibody reduced granzyme B production in IL-21–stimulated T cells (Figure 3C). As it was previously shown that the MAPK pathway is functionally involved in granzyme B production by NK cells,22,23 we also evaluated p38 MAPK activity. We observed a reduction in the phospho-p38 MAPK/total p38 MAPK ratio when anti-CD132 mAb was included in the culture under different conditions, including stimulation with anti-CD3/CD28 beads alone or in combination with IL-2 (Figure 3D).
Granzyme B is induced by common γ-chain cytokines. (A) CD8 T cells were incubated with the indicated cytokines and stimulated with anti-CD3/CD28 beads for 48 hours. Cells were stained against intracellular granzyme B and analyzed by flow cytometry. One representative of 3 experiments is shown. Stimulation with CD3/CD28 beads led to a significant increase of granzyme B levels compared with the respective sample without CD3/CD28 stimulation (P < .05 in all cases). The granzyme B levels increased over baseline with a stimulation with 10 ng/mL or 50 ng/mL of any of the applied cytokines (**P < .01). (B) CD8 T cells were incubated with the indicated cytokines and stimulated with allogeneic DCs for 48 hours. Cells were stained against intracellular granzyme B and analyzed by flow cytometry. One representative of 3 experiments is shown. Stimulation with the allo-DCs led to increased granzyme B production independent of the cytokines (P < .05 in all cases). The granzyme B levels increased over baseline with a stimulation of 10 ng/mL or 50 ng/mL of any of the applied cytokines (*P < .05, **P < .01, ***P < .001). (C) T cells were incubated for 48 hours with IL-21 (10 ng/mL), anti-CD3/CD28 beads and anti-CD132 (100 nM), or isotype IgG when indicated. Cells were stained against intracellular granzyme B and analyzed by flow cytometry. (D) Western blot analysis is shown for phospho-p38 MAPK (pp38), total p38 MAPK, and β-actin on the protein derived from splenic CD4 and CD8 T cells (C57BL/6) and enriched by magnetic-activated cell sorting (MACS) which had been exposed for 48 hours to CD3/CD28 beads, IL-2 (50 ng/mL), and anti-CD132 (100 nM) when indicated. Left panel, One representative western blot is shown; right panel, quantification of the phospho-p38 MAPK (pp38)/total p38 MAPK ratio is shown. (E) Microarray-based gene expression analysis was performed on the RNA isolated from T cells following CD3/CD28 and IL-2 stimulation and treatment with anti-CD132 mAb or isotype IgG. GSEA identified a naive T-cell signature in cells receiving anti-CD132 mAb (normalized enrichment score = 1.73; nominal P value < .001). Genes upregulated in the anti-CD132 group with respect to the isotype have a high enrichment ratio (red).
Granzyme B is induced by common γ-chain cytokines. (A) CD8 T cells were incubated with the indicated cytokines and stimulated with anti-CD3/CD28 beads for 48 hours. Cells were stained against intracellular granzyme B and analyzed by flow cytometry. One representative of 3 experiments is shown. Stimulation with CD3/CD28 beads led to a significant increase of granzyme B levels compared with the respective sample without CD3/CD28 stimulation (P < .05 in all cases). The granzyme B levels increased over baseline with a stimulation with 10 ng/mL or 50 ng/mL of any of the applied cytokines (**P < .01). (B) CD8 T cells were incubated with the indicated cytokines and stimulated with allogeneic DCs for 48 hours. Cells were stained against intracellular granzyme B and analyzed by flow cytometry. One representative of 3 experiments is shown. Stimulation with the allo-DCs led to increased granzyme B production independent of the cytokines (P < .05 in all cases). The granzyme B levels increased over baseline with a stimulation of 10 ng/mL or 50 ng/mL of any of the applied cytokines (*P < .05, **P < .01, ***P < .001). (C) T cells were incubated for 48 hours with IL-21 (10 ng/mL), anti-CD3/CD28 beads and anti-CD132 (100 nM), or isotype IgG when indicated. Cells were stained against intracellular granzyme B and analyzed by flow cytometry. (D) Western blot analysis is shown for phospho-p38 MAPK (pp38), total p38 MAPK, and β-actin on the protein derived from splenic CD4 and CD8 T cells (C57BL/6) and enriched by magnetic-activated cell sorting (MACS) which had been exposed for 48 hours to CD3/CD28 beads, IL-2 (50 ng/mL), and anti-CD132 (100 nM) when indicated. Left panel, One representative western blot is shown; right panel, quantification of the phospho-p38 MAPK (pp38)/total p38 MAPK ratio is shown. (E) Microarray-based gene expression analysis was performed on the RNA isolated from T cells following CD3/CD28 and IL-2 stimulation and treatment with anti-CD132 mAb or isotype IgG. GSEA identified a naive T-cell signature in cells receiving anti-CD132 mAb (normalized enrichment score = 1.73; nominal P value < .001). Genes upregulated in the anti-CD132 group with respect to the isotype have a high enrichment ratio (red).
To determine whether the protective effect of anti-CD132 treatment was dependent on perforin/granzyme B–mediated cytotoxicity, we next isolated CD8 T cells from perforin-deficient donors based on previous reports that GVHD could be induced under these conditions.24-26 In contrast to one of the studies using a haploidentical model (parent into F1),25 we used a major mismatch model. We applied the Perf−/− T cells to induce GVHD that was not dependent on perforin. Because it was previously shown that multiple effects of granzyme B are dependent on perforin,27 a lack of perforin would also reduce the function of granzyme B. For example, perforin creates pores within the cell membranes, through which the granzymes can enter and induce apoptosis.28 Conversely, perforin-independent functions for granzyme B will remain intact in Perf−/− T cells. We found that treatment with the anti-CD132 mAb was not protective, as judged by survival and histopathology, when GVHD was induced independent of perforin (supplemental Figure 1A-B). However, because GVHD in humans typically does not develop in the absence of perforin, this model differs from the situation seen in the clinic. Because cytotoxicity is important for graft-versus-leukemia (GVL) activity, we next analyzed whether anti-CD132 treatment would still allow for activity of CD8 T cells against A20 lymphoma cells. We found that there was no difference in the bioluminescence signal of luciferase transgenic A20 lymphoma cells in mice undergoing allo-HCT and T-cell transfer, whether they were treated with anti-CD132 or unspecific IgG over a series of time points (supplemental Figure 2A-B). Taken together, these findings are congruent with the hypothesis that common γ-chain cytokines induce granzyme B production in CD8 T cells, and that the protective effect of anti-CD132 mAb is partly based on reduced cytotoxic activity of CD8 T cells.
Common γ-chain blockade causes a naive T-cell gene signature upon activation, and reduces phosphorylation of JAK3, which is required for the ability of T cells to induce GVHD
To better understand which pathways and genes are impacted in T cells by the anti-CD132 antibody, T cells were stimulated with IL-2 and CD3/CD28 beads in the presence vs the absence of anti-CD132. Gene expression analysis was followed by GSEA to compare our data to published gene signatures. Gene expression in CD8 T cells treated with anti-CD132 mAb overlapped strongly with a naive CD8 T-cell signature,29 as judged by a normalized enrichment score of 1.73 and a nominal P value < .001 (Figure 3E).
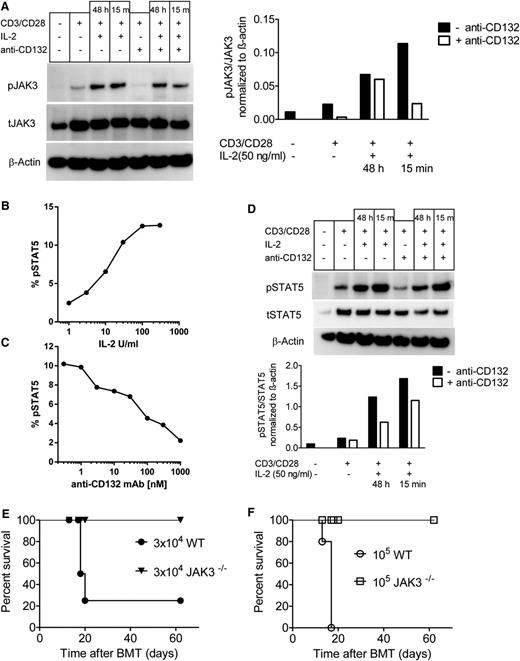
Because JAK3 and STAT5 are downstream of the common γ chain, we next evaluated whether anti-CD132 treatment blocked activation of T cells in response to CD3/CD28 beads and IL-2. We observed a reduction in the phospho (p) JAK3/total (t) JAK ratio when anti-CD132 mAb was included in the culture under different conditions (Figure 4A). pSTAT5 levels in T cells increased in a dose-dependent manner when the T cells were stimulated with increasing concentrations of IL-2 (Figure 4B). Conversely, STAT5 phosphorylation in T cells exposed to IL-2 (100 IU/mL) decreased when increasing concentrations of anti-CD132 were included in the culture, as determined by phospho-flow analysis (Figure 4C). Consistent with the phospho-flow analysis pSTAT5/tSTAT5 ratios in T cells stimulated with CD3/CD28 and IL-2 decreased when anti-CD132 was included in the culture (Figure 4D). To assess the importance of the JAK3 pathway in donor T cells for the development of GVHD, T cells from Jak3−/− mice or WT mice were infused into irradiated recipients. Whereas 105 donor WT T cells uniformly caused lethality in 5 of 5 recipients, and 3 × 104 donor WT T cells caused lethality in 4 of 5 recipient mice, neither 3 × 104 nor 105 donor Jak3−/− T cells caused lethality in any recipient (Figure 4E-F). Because this model of GVHD is characterized by BM aplasia,30 hematocrits were assessed in recipient mice. Recipients of Jak3−/− T cells had normal hematocrit values (>40%) at both 14 and 28 days posttransplant, whereas hematocrit values in recipients of 3 × 104 or 105 WT cells were reduced by >49% or 67% on day 14 posttransplant, respectively (supplemental Table 2). These data indicate that inhibition of the common γ chain with anti-CD132 antibody reduced JAK3 activation, and that Jak3−/− T cells are functionally unresponsive during GVHD induction.
CD132 blockade reduces JAK3 and STAT5 phosphorylation in activated T cells, and JAK3 deficiency in donor T cells reduces GVHD. (A) western blot analysis is shown for pJAK3, tJAK3, and β-actin on the protein derived from splenic CD4 and CD8 T cells (C57BL/6) and enriched by MACS that had been exposed to CD3/CD28 beads, IL-2 (50 ng/mL), and anti-CD132 (100 nm) for 48 hours when indicated. Left panel, One representative western blot is shown; right panel, quantification of the pJAK3/tJAK3 ratio is shown. (B) Flow cytometry–based analysis is shown for pSTAT5 (y-axis), in splenic CD4 T cells (C57BL/6) that were exposed to increasing levels of IL-2 for 15 minutes (x-axis). (C) Flow cytometry–based analysis is shown for pSTAT5 (y-axis), in splenic CD4 T cells (C57BL/6) that were exposed to IL-2 (100 IU/mL) for 15 minutes and increasing levels of anti-CD132 (x-axis). (D) Western blot analysis is shown for pSTAT5, tSTAT5, and β-actin on the protein derived from splenic CD4 and CD8 T cells (C57BL/6) enriched by MACS that had been exposed to CD3/CD28 beads, IL-2 (50 ng/mL), and anti-CD132 (100 nm) for 48 hours when indicated. Top panel, One representative western blot is shown; lower panel, quantification of the pSTAT5/tSTAT5 ratio is shown. (E-F) Sublethally irradiated bm12 recipient mice received IV infusions of either 3 × 104 (E) or 105 (F) T cells derived from Jak3−/− or WT (both C57BL/6 background) mice (n = 5 per group) and survival was measured over time. Survival was improved in the groups receiving T cells derived from Jak3−/− compared with WT mice at both T-cell dosages (P < .001).
CD132 blockade reduces JAK3 and STAT5 phosphorylation in activated T cells, and JAK3 deficiency in donor T cells reduces GVHD. (A) western blot analysis is shown for pJAK3, tJAK3, and β-actin on the protein derived from splenic CD4 and CD8 T cells (C57BL/6) and enriched by MACS that had been exposed to CD3/CD28 beads, IL-2 (50 ng/mL), and anti-CD132 (100 nm) for 48 hours when indicated. Left panel, One representative western blot is shown; right panel, quantification of the pJAK3/tJAK3 ratio is shown. (B) Flow cytometry–based analysis is shown for pSTAT5 (y-axis), in splenic CD4 T cells (C57BL/6) that were exposed to increasing levels of IL-2 for 15 minutes (x-axis). (C) Flow cytometry–based analysis is shown for pSTAT5 (y-axis), in splenic CD4 T cells (C57BL/6) that were exposed to IL-2 (100 IU/mL) for 15 minutes and increasing levels of anti-CD132 (x-axis). (D) Western blot analysis is shown for pSTAT5, tSTAT5, and β-actin on the protein derived from splenic CD4 and CD8 T cells (C57BL/6) enriched by MACS that had been exposed to CD3/CD28 beads, IL-2 (50 ng/mL), and anti-CD132 (100 nm) for 48 hours when indicated. Top panel, One representative western blot is shown; lower panel, quantification of the pSTAT5/tSTAT5 ratio is shown. (E-F) Sublethally irradiated bm12 recipient mice received IV infusions of either 3 × 104 (E) or 105 (F) T cells derived from Jak3−/− or WT (both C57BL/6 background) mice (n = 5 per group) and survival was measured over time. Survival was improved in the groups receiving T cells derived from Jak3−/− compared with WT mice at both T-cell dosages (P < .001).
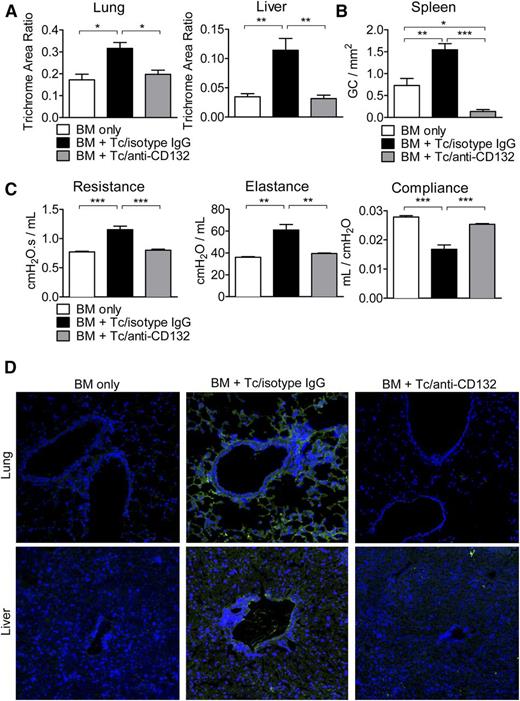
Chronic GVHD is improved by anti-CD132 mAb treatment
We next used an established chronic GVHD model to determine whether protection was restricted to acute GVHD or could also be seen in chronic disease.16 One hallmark of chronic GVHD is fibrosis due to collagen deposition in different tissues. Using trichrome staining, we quantified collagen accumulation in the liver and lungs and found that in both organs, collagen deposition was reduced when mice were treated with anti-CD132 mAb compared with mice treated with the isotype control (Figure 5A). Chronic GVHD is also typically accompanied by B-cell hyperplasia in germinal centers. It was recently shown that during chronic disease, T follicular helper cells support germinal center formation, causing B-cell numbers there to increase.16 We found that CD132 mAb treatment combated this hyperplasia by reducing splenic germinal center formation significantly compared with isotype IgG/T cell and even BM alone recipients (Figure 5B). In addition, we found reduced percentages of CD19+ B cells in the spleens of mice treated with anti-CD132 compared with isotype IgG early after allo-HCT (day 7) (supplemental Figure 2F). Expanding B-cell numbers, in turn, typically results in Ig deposition in chronic GVHD target organs, such as the lung and liver, which is associated with fibrosis and pulmonary dysfunction characteristic of bronchiolitis obliterans. As one feature of this particular chronic GVHD model is bronchiolitis obliterans, we performed PFTs and found significantly reduced lung resistance and elastance in anti-CD132 mAb-treated allo-HCT recipients as compared with mice treated with isotype control antibody (Figure 5C). Conversely, the compliance of the anti-CD132 mAb-treated chronic GVHD recipients was increased in relation to mice treated with the isotype control (Figure 5C). Finally, high levels of Ig deposition in the lung and liver of chronic GVHD recipients treated with the isotype could be visualized using immunofluorescence microscopy. However, Ig deposition was eliminated in allo-HCT recipients administered with anti-CD132 mAb (Figure 5D).
CD132 mAb treatment attenuates characteristics of chronic GVHD. (A) Collagen deposition was quantified by trichrome staining in the lungs and liver. In both organs, the accumulated collagen was reduced when mice were treated with anti-CD132 mAb compared with mice treated with the isotype (*P < .05, **P < .01). The trichrome area ratio equals (trichrome area)/(total area). (B) Germinal center B-cell hyperplasia was assessed in the spleen using a PNA stain (*P < .05, **P < .01, ***P < .001). (C) PFTs were performed in mice developing chronic GVHD to observe lung resistance, elastance, and compliance (**P < .01, ***P < .001). (D) Ig deposition (green) is shown for representative sections of the lungs and liver in the different groups. Nuclear stain with 4′,6-diamidino-2-phenylindole in blue. One representative experiment of 3 is shown.
CD132 mAb treatment attenuates characteristics of chronic GVHD. (A) Collagen deposition was quantified by trichrome staining in the lungs and liver. In both organs, the accumulated collagen was reduced when mice were treated with anti-CD132 mAb compared with mice treated with the isotype (*P < .05, **P < .01). The trichrome area ratio equals (trichrome area)/(total area). (B) Germinal center B-cell hyperplasia was assessed in the spleen using a PNA stain (*P < .05, **P < .01, ***P < .001). (C) PFTs were performed in mice developing chronic GVHD to observe lung resistance, elastance, and compliance (**P < .01, ***P < .001). (D) Ig deposition (green) is shown for representative sections of the lungs and liver in the different groups. Nuclear stain with 4′,6-diamidino-2-phenylindole in blue. One representative experiment of 3 is shown.
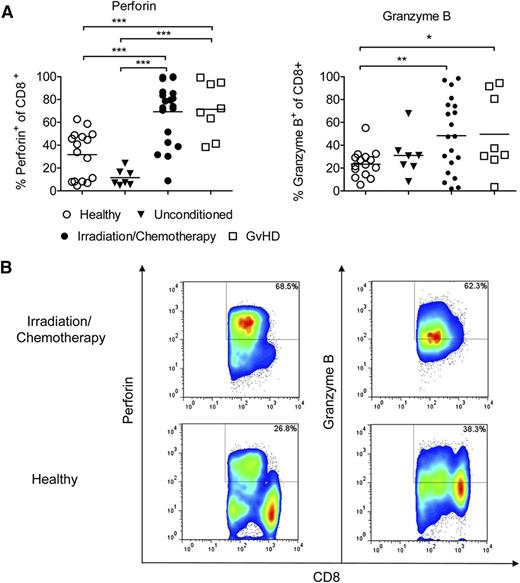
Perforin and granzyme B are upregulated in CD8 T cells in patients undergoing conditioning prior to allo-HCT
To understand whether the therapeutic principle of anti-CD132 mAb treatment could be applicable in patients undergoing allo-HCT, we next determined the expression levels of perforin and granzyme B in CD8+ T cells of healthy individuals, unconditioned patients, patients undergoing conditioning therapy for allo-HCT, and patients with acute GVHD (supplemental Table 1). We observed that both perforin and granzyme B were increased in peripheral blood CD8 T cells obtained from patients receiving preparative conditioning for allo-HCT compared with healthy individuals (Figure 6). Furthermore, the percentage of perforin+ CD8+ T cells was significantly higher in patients with GVHD compared with unconditioned patients or healthy volunteers (Figure 6A). These findings suggest that increased CD8 T-cell cytotoxicity is a potentially targetable factor in patients that receive such allo-HCT conditioning to prevent GVHD.
Perforin/granzyme B is upregulated in human CD8 T cells after conditioning treatment and GVHD. Blood samples from healthy donors (n = 16), patients receiving chemotherapy or irradiation (n = 20) in preparation for allo-HCT, GVHD patients (n = 8) or patients before conditioning (n = 7). Cells were stained against CD8 and perforin or granzyme B, and analyzed by flow cytometry. (A) Quantification of the flow cytometry data. Values are the percentage of total cells identified as both CD8+ and perforin+ or granzyme B+ (***P < .001). (B) Representative flow cytometry data for CD8+ perforin+ or CD8+ granzyme B+ T cells are shown.
Perforin/granzyme B is upregulated in human CD8 T cells after conditioning treatment and GVHD. Blood samples from healthy donors (n = 16), patients receiving chemotherapy or irradiation (n = 20) in preparation for allo-HCT, GVHD patients (n = 8) or patients before conditioning (n = 7). Cells were stained against CD8 and perforin or granzyme B, and analyzed by flow cytometry. (A) Quantification of the flow cytometry data. Values are the percentage of total cells identified as both CD8+ and perforin+ or granzyme B+ (***P < .001). (B) Representative flow cytometry data for CD8+ perforin+ or CD8+ granzyme B+ T cells are shown.
Discussion
Several studies have shown the role of cytokines such as IL-6, TNF, or IL-1β in acute GVHD. In particular, it was shown in different mouse models of acute GVHD that the disease was associated with a proinflammatory “cytokine storm”.3 Whereas in the mouse model this cytokine release is at least partly caused by irradiation-induced tissue damage, in humans GVHD is also observed late after conditioning, after transfer of donor lymphocyte infusions, or following withdrawal of immunosuppression in the absence of obvious tissue damage and despite reduced-intensity conditioning. However, the importance of different common γ-chain cytokines has been repeatedly shown in human and murine GVHD. In particular, the roles of IL-2,31,32 IL-7,33,34 IL-15,35-38 and IL-2121,39 in GVHD have been well documented. Although most studies were performed in the isolated murine setting, IL-21 has not only been shown to be a key mediator of murine acute GVHD39,40 but also of xenogeneic GVHD induced by human lymphocytes.21,41
Here, we show that a blockade of the common γ chain, which is required for the function of the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21, reduced acute GVHD significantly in mice with respect to survival, inflammatory cytokine levels, and histopathological GVHD severity. In line with this potent reduction of acute GVHD in the mouse model, in patients, the plasma levels of IL-7 and IL-15 measured 30 days after both myeloablative35 and nonmyeloablative allo-HCT42 were highly predictive biomarkers of acute GVHD. In a mouse model of GVHD, exogenous IL-15 exacerbated GVHD rates.37 An additional study also showed that IL-15 caused an effector-memory CD8 T-cell expansion in acute GVHD, but was not required for GVL effects.38 IL-7–deficient recipients developed less GVHD compared with WT mice.34 In humans, the serum L-7 levels at day +14 correlated with the severity of acute GVHD.33 In contrast to these studies on single common γ-chain cytokines in GVHD, we blocked multiple cytokines with an antagonistic antibody directed against CD132. This intervention reduced acute GVHD severity in a CD8 T-cell–dependent GVHD model, while having no protective effects when only CD4 T cells were transferred. In addition, anti-CD132 mAb reduced the histopathological GVHD severity in the colon but not the liver in a minor mismatch CD4-dependent model. We delineated the role of CD132 in CD8 T-cell–mediated GVHD in more detail by demonstrating that different common γ-chain cytokines induced granzyme B production in a dose-dependent manner, and that the granzyme B production was strongly reduced when CD132 was blocked. The cytotoxic molecule granzyme B is required for CD8 T-cell function and its reduction could affect antitumor immunity. Although we found that GVL activity against A20 lymphoma cells was not abrogated, the reduction in granzyme B production by CD8 T cells observed upon anti-CD132 treatment could affect GVL effects in other tumor models. Therefore, anti-CD132 treatment would be best suited in situations where GVHD develops in patients undergoing allo-HCT for nonmalignant disorders1,43 or when the malignant disease is in complete remission.
However, the effects of anti-CD132 on T cells were not only limited exclusively to granzyme B production but also affected the gene expression of activated T cells. T cells exposed to anti-CD132 while activated with CD3/CD28 and IL-2 exhibited a distinct naive T-cell signature, indicating reduced activation. The intersection of the naive T-cell signature with the signaling cascade and transcription control clusters helped us to identify several potentially relevant genes upregulated in T cells after anti-CD132 mAb treatment. Several of these were gene products known to have inhibitory effects on nuclear factor “kappa-light-chain-enhancer” of activated B cells (NF-κB), a central molecule during T-cell activation, including RhoH and IκB kinase (IKK)-ε. RhoH interferes with other Rho GTPases to block NF-κB and p38 activation,44 IKK-ε interferes with RelA, and diminishes IFN-β production.45 Another gene identified in this analysis, Trim28, plays a key role in immune tolerance.46 Thus, anti-CD132 mAb modulates a variety of pathways and factors in the cell which are linked to reduced T-cell activation.
Besides these differences in transcriptional levels of T cells exposed to anti-CD132, we also found reduced JAK3 phosphorylation in response to activating stimuli. This is of particular interest because JAK3 is a signaling molecule with a reported role in GVHD.47,48 Reduced JAK3 phosphorylation upon inhibition of the common γ chain by anti-CD132 is consistent with JAK3 being downstream of multiple common γ-chain cytokine receptors. Genetic deficiency of Jak3 leads to abrogation of signal transduction through the common γ chain,49 and we found that Jak3−/− T cells caused less severe GVHD compared with WT T cells, which is consistent with previous studies using JAK3 inhibitors to interfere with GVHD.47,48
Importantly, our anti-CD132 mAb approach was not only limited to acute GVHD, but also reduced the severity of chronic GVHD with respect to pulmonary function, collagen deposition, and Ig accumulation in GVHD target organs. Mechanistically, it is conceivable that anti-CD132 mAb targets T follicular helper cells that produce IL-21, which is essential for germinal center B-cell proliferation and germinal center formation.16 This may have important clinical implications because steroid refractory chronic GVHD is still an unsolved problem. Also, a potential clinical advantage of anti-CD132 treatment could be that it can target both late-onset acute GVHD and chronic inflammatory GVHD, 2 entities of GVHD that are clinically often difficult to differentiate from each other.
In summary, treatment with a neutralizing antibody against CD132 mAb reduced acute GVHD, which was linked to reduced granzyme B levels in CD8 T cells, induction of a naive T-cell phenotype gene signature, and a reduction in JAK3 and p38 MAPK phosphorylation upon activation. The granzyme B produced by CD8 T cells activated with common γ-chain cytokines provides a novel link between a group of inflammatory ILs and GVHD-related end-organ damage. Most importantly, we found that the CD132 blocking approach reduced disease severity of acute and chronic GVHD in 2 independent laboratories, thereby underscoring its high potential for clinical use.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Novartis AG for valuable suggestions and for providing the anti-CD132 mAb. There was no financial support provided by Novartis. Novartis has reviewed the publication, and the views and opinions described in the work do not necessarily reflect those of Novartis.
This work was supported by the Deutsche Forschungsgemeinschaft, Germany (Heisenberg Professorship ZE 872/3-1 [R.Z.], and individual grant ZE872/1-2 [R.Z.]), the Wilhelm Sander Stiftung (2008.046.3), the Else Kröner Fresenius Stiftung (grant 2013_A04), the Fulbright Grant, USA (B.A.H.S.), and the National Institutes of Health, National Institute of Allergy and Infectious Diseases (R01 AI 112613 and P01 AI 056299 [B.R.B.]), National Cancer Institute (P01 CA 142106 [B.R.B.]), and National Heart, Lung, and Blood Institute (T32 HL00706237 [R.F.]).
Authorship
Contribution: A.-K.H., B.A.H.S., R.F., K.H., C.M.-H., D.P., and B.H. helped design and perform experiments, analyzed data, and assisted in the writing of the manuscript; P.A.T., F.L., G.P., and H.D. helped with mouse experiments and flow cytometry; A.S.-G. performed histologic analysis; J.K. helped to design experiments and provided the anti-CD132 mAb; and B.R.B. and R.Z. developed the overall concept, designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: J.K. is employed by Novartis. The remaining authors declare no competing financial interests.
Correspondence: Robert Zeiser, Department of Hematology and Oncology, Freiburg University Medical Center, Albert Ludwigs University, Hugstetterstrasse 55, 79106 Freiburg, Germany; e-mail: robert.zeiser@uniklinik-freiburg.de
References
Author notes
A.-K.H., B.A.H.S., R.F., and K.H. are co-first authors.
B.R.B. and R.Z. are co-senior authors.