In this issue of Blood, Hechinger and colleagues investigate the efficacy of the blockade of the common γ-chain receptor (CD132) as a novel approach in the management of both acute and chronic graft-versus-host disease (GVHD).1
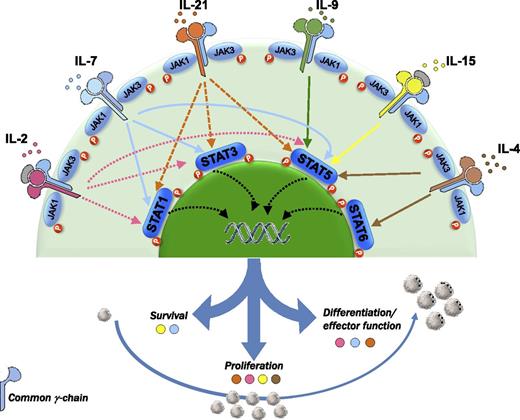
The common cytokine receptor γ-chain family includes IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. This receptor acts mainly through the JAK-STAT pathway. The blockade of these cytokines with an antagonistic antibody directed against CD132 inhibits proliferation and differentiation of T cells and, in turn, might also inhibit GVHD.
The common cytokine receptor γ-chain family includes IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21. This receptor acts mainly through the JAK-STAT pathway. The blockade of these cytokines with an antagonistic antibody directed against CD132 inhibits proliferation and differentiation of T cells and, in turn, might also inhibit GVHD.
The common cytokine receptor γ-chain family consists of interleukin-2 (IL-2), IL-4, IL-7, IL-9, IL-15, and IL-21, and is so called because the receptors for these cytokines share the same γ chain.2 The gene encoding the γ chain (IL2RG) is mutated in humans with X-linked severe combined immunodeficiency (XSCID), and these patients lack T cells and natural killer (NK) cells, which indicates that the γ chain is crucial for the development of these cells.
Acute GVHD has been described as a “cytokine storm” involving a 3-step disease process3 : (1) conditioning regimen–associated inflammation, (2) donor T-cell priming, and (3) an effector phase mediated by cytokines and cellular effectors. In this regard, numerous studies have correlated the levels of cytokines with the risk of GVHD. More specifically, the plasma levels of IL-7 and IL-15 measured along the first month posttransplant have been identified as highly predictive biomarkers for acute GVHD.4 All of these data have prompted the development of clinical trials evaluating the efficacy of cytokine inhibitors either for the prophylaxis or for the treatment of GVHD.5 Although some studies have reported promising results, most have failed to demonstrate a significantly favorable impact on outcome. In contrast to these studies, the blockade of multiple cytokines with an antagonistic antibody directed against CD132 might avoid the redundant effect of most cytokines, which might underlie the poor results reported when a single cytokine is inhibited, and, furthermore, this strategy might be useful both in the acute and in the chronic setting. In this regard, although early studies supported the paradigm of acute GVHD being a T-helper cell 1 (Th1) and chronic GVHD being a Th2 process,6 recent data suggest a specific cytokine’s signature depending on the targeted organ, both in acute and in chronic GVHD. For example, Th2 differentiation, characterized by secretion of IL-4, may be involved in liver and skin GVHD. Similarly, chronic lichenoid GVHD shows a mixed Th1/Th17 signature. Interestingly, treatment of established chronic GVHD using anti-CD132 monoclonal antibody (mAb) reversed liver and lung fibrosis and prevented immunoglobulin deposition. It is worth mentioning that aberrant B-cell function and alloantibody deposition have been shown to play a key role in lung and liver chronic GVHD.7 Furthermore, from a pathophysiological point of view, chronic GVHD is the result of a highly complex network involving both B cells and T cells, this interaction taking place in the germinal center. By targeting T follicular helper cells that produce IL-21, anti-CD132 mAb might also inhibit germinal center B-cell proliferation.8 Moreover, IL-21 favors B-cell differentiation into antibody-secreting plasma cells through the Janus kinase–signal transducer and activator of transcription 3 (JAK-STAT3) pathway, which is inhibited by anti-CD132. Consistent with the fact that the γ-chain family cytokines signal through the JAK-STAT pathway, T cells exposed to anti-CD132 mAb showed reduced JAK3 phosphorylation upon activation. This is of particular interest considering the role of JAK3 in lymphocyte activation and, therefore, in GVHD, as shown in several studies demonstrating a decreased risk of GVHD upon exposure to JAK inhibitors, both in preclinical and in clinical models.9
Finally, the perforin-granzyme pathway plays a key role in CD8+ T-cell–mediated GVHD. In addition to the effect of anti-CD132 mAb on cytokines, Hechinger and colleagues elegantly show that the blockade of the common γ chain inhibits granzyme B production by CD8 T cells.1 Remarkably, the cytotoxic molecule granzyme B is required for CD8 T-cell function and, therefore, its reduction could affect antitumor immunity. Nevertheless, recent studies suggest that GzmB deficiency significantly enhances the ability of donor CD8+ T cells to control tumor growth in the hosts via other mechanisms.10 Thus, although further studies will be required to elucidate this issue, this strategy might decrease GVHD while maintaining a graft-versus-leukemia effect.
In summary, the common γ-chain receptor is a key player in survival, proliferation, activation, and differentiation of T cells. Furthermore, it also affects other subpopulations such as dendritic cells, B lymphocytes, and regulatory T cells. Not surprisingly, its blockade dramatically modifies the immune response after transplantation and opens a potential new route for the management of GVHD.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal