Key Points
Alloreplete iC9-T cells can promote immune recovery posttransplant and protect patients against viral infections.
iC9-T cells can be eliminated from both peripheral blood and CNS by administration of AP1903 leading to a rapid resolution of GVHD.
Abstract
To test the feasibility of a single T-cell manipulation to eliminate alloreactivity while sparing antiviral and antitumor T cells, we infused 12 haploidentical hematopoietic stem cell transplant patients with increasing numbers of alloreplete haploidentical T cells expressing the inducible caspase 9 suicide gene (iC9-T cells). We determined whether the iC9-T cells produced immune reconstitution and if any resultant graft-versus-host disease (GVHD) could be controlled by administration of a chemical inducer of dimerization (CID; AP1903/Rimiducid). All patients receiving >104 alloreplete iC9-T lymphocytes per kilogram achieved rapid reconstitution of immune responses toward 5 major pathogenic viruses and concomitant control of active infections. Four patients received a single AP1903 dose. CID infusion eliminated 85% to 95% of circulating CD3+CD19+ T cells within 30 minutes, with no recurrence of GVHD within 90 days. In one patient, symptoms and signs of GVHD-associated cytokine release syndrome (CRS-hyperpyrexia, high levels of proinflammatory cytokines, and rash) resolved within 2 hours of AP1903 infusion. One patient with varicella zoster virus meningitis and acute GVHD had iC9-T cells present in the cerebrospinal fluid, which were reduced by >90% after CID. Notably, virus-specific T cells recovered even after AP1903 administration and continued to protect against infection. Hence, alloreplete iC9-T cells can reconstitute immunity posttransplant and administration of CID can eliminate them from both peripheral blood and the central nervous system (CNS), leading to rapid resolution of GVHD and CRS. The approach may therefore be useful for the rapid and effective treatment of toxicities associated with infusion of engineered T lymphocytes. This trial was registered at www.clinicaltrials.gov as #NCT01494103.
Introduction
Haploidentical hematopoietic stem cell transplantation (haplo-HSCT) is an effective therapeutic strategy for transplant candidates lacking a major histocompatibility complex (MHC)–matched donor; however, removal of T cells from the graft is required to prevent lethal graft-versus-host disease (GVHD).1-3 Removal of all T cells increases the risk of graft rejection, relapse, and viral and other opportunistic infections.4-6 Consequently, efforts have been made to retain the desired T-cell subsets while selectively depleting alloreactive T cells7-9 or enriching for the cells that are directed to pathogens or malignancies, or that are enriched for GVHD-suppressive regulatory T cells.10-12 Although each of these strategies is feasible, it is difficult to develop a single T-cell manipulation that both eliminates alloreactivity and spares T cells representing all the available antiviral and antitumor specificities in the donor’s peripheral blood (PB).
The expression of a safety or suicide gene in otherwise unselected donor T lymphocytes may preserve broad antigen specificity while eliminating alloreactive T cells should GVHD occur. One such approach introduces the herpes simplex virus thymidine kinase (HSV-tk) gene into donor T cells, allowing the T cells to be ablated by the administration of prodrugs such as ganciclovir.13 Several clinical trials support the feasibility of this approach.14-18 However, HSV-tk is a viral gene and may induce an unwanted immune response against functionally desirable T cells. Activation of the system also requires a clinically useful prodrug (like ganciclovir) to be administered for cell destruction. Moreover, the mechanism of action (incorporation of phosphorylated nucleoside analogs into DNA) is slow and may require relatively prolonged administration of the prodrug, which may still deliver insufficient T-cell destruction.
We developed an alternative approach based on the expression of an inducible human caspase-9 transgene (iC9), which is dimerized and hence activated by the administration of an otherwise bioinert small-molecule drug, AP1903.19-22 Our approach allows patients to receive gancyclovir and related drugs to treat viral infections without T-cell damage. Unlike the HSV-tk–based suicide gene, the iC9 safety switch is human derived and has limited immunogenicity.23 Moreover, activation of iC9 produces up to 99% eradication of iC9-expressing T cells (iC9-T cells) in vitro and in vivo within 2 hours of a single dose of the chemical inducer of dimerization (CID) (AP1903/Rimiducid)24,25 and controls GVHD within 24 to 48 hours. However, the iC9-T cells infused in our earlier study had already been depleted of alloreactive precursors by ex vivo culture with recipient B-cell lymphoblastoid cell lines followed by negative selection of responding (alloreactive) donor T cells. This lengthy process may eliminate helpful tumor-targeted cells and is impractical for patients requiring urgent transplantation. In addition, its complexity makes the process unsuited for scaling to general clinical use. Whether iC9 activation alone is sufficient to produce both rapid and long-term control of GVHD caused by alloreplete haploidentical donor T cells in vivo, or whether these cells could restore beneficial immunity to the recipients, remains unknown.
We hypothesized that activation of the iC9 transgene could produce sufficient in vivo allodepletion of GVHD-inducing T cells for sustained benefit and retention of the donor T lymphocytes’ desired properties. We investigated whether a single dose of CID could effectively control GVHD without the loss of protective immunity against pathogens. Finally, because the small remaining fraction of donor iC9-T cells re-expand after administration of CID, we determined whether these cells were functionally allodepleted in vivo or reinduced GVHD.
Methods
Patients and study design
This phase 1 clinical study (Administration of haploidentical DOnor T cells Transduced with the Inducible caspase-9 suicide gene [DOTTI] trial, IND 13813) was approved by the institutional review board of Baylor College of Medicine and the US Food and Drug Administration (FDA) and was reviewed by the Recombinant DNA Advisory Committee. The study was designed to assess the safety and efficacy of infusing escalating doses of donor-derived iC9-T cells in patients undergoing haplo-HSCT using Clinimacs-selected CD34+ stem cells from an HLA haploidentical donor. Briefly, patients meeting eligibility criteria received iC9-T cells between 30 and 90 days after transplant (>33 days post-Campath) following a dose-escalation protocol: dose level 1 (1 × 104 cells per kilogram), dose level 2 (1 × 105 cells per kilogram), dose level 3 (5 × 105 cells per kilogram), dose level 4 (1 × 106 cells per kilogram), and dose level 5 (5 × 106 cells per kilogram).8,26 Patients who developed acute GVHD grade I or II after the infusion of iC9-T cells received 0.4 mg/kg AP1903 (Bellicum Pharmaceuticals, Inc.) as a 2-hour infusion.27
Generation of iC9-T cells
Cell manipulation was performed under good manufacturing practice conditions at the Center for Cell and Gene Therapy. In brief, PB mononuclear cells (PBMCs) from transplant donor were obtained by Ficoll density before being activated by anti-CD3 antibody. Gene modification with iC9 followed the previously reported procedure.22,24
Flow cytometry analysis
The iC9-T cells were characterized using a panel of fluorochrome-conjugated monoclonal antibodies (BD Biosciences and Beckman Coulter). Cells were acquired on a FACSCalibur flow cytometer. Nontransduced control cells were used to set the negative gate for CD19 expression. Flow cytometry data were analyzed using CellQuest software (Becton Dickinson) and Kaluza software (Beckman Coulter).
Real-time Q-PCR of iC9.T2A.ΔCD19 transgene
Detection of pathogen-specific T cells
Interferon-γ (IFN-γ) release from PBMCs collected after T-cell infusions was evaluated by enzyme-linked immunospot (ELISPOT) as previously described.22,25 Peptide libraries spanning the Epstein-Barr virus (EBV) antigens BZLF1, LMP1, LMP2, EBNA1, EBNA3a, EBNA3b, and EBNA3c, the cytomegalovirus (CMV) antigens pp65 and IE1, human herpes virus 6 (HHV6) antigens U11, U14, U54, and U90, the varicella zoster virus (VZV) antigens IE61, IE62, IE63, ORF10, and ORF68, and BK virus (BKV) antigens LT and VP-1 were used to stimulate the PBMCs. Staphylococcal enterotoxin B was used as a positive control. To evaluate the dimerizer-resistant (CID-R) population, CID was added to the PBMCs 30 minutes prior to the pepmixes. ELISPOTs were independently enumerated by Zellnet Consulting (Fort Lee, NJ).
Monitoring of infections
Viral (BKV, CMV, HHV6, VZV) reactivation or infections were monitored by Q-PCR assays (ViraCor-IBT Laboratories Inc.) on plasma as noted. EBV-DNA viral load was determined by Q-PCR of PBMCs using specific primers and probes targeting the EBER gene.28
Results
Patients, GVHD, and toxicity
Twelve patients received alloreplete iC9-T cells between 30 and 90 days after haplo-HSCT and were followed for >28 days after infusion. The patients’ characteristics are summarized in Table 1. Their median age was 10 years (range, 2-50 years) and they received iC9–T-cell infusions using a dose-escalation schedule from 1 × 104 cells per kilogram to 5 × 106 cells per kilogram, at a median of 42 days posttransplant (range, 31-82 days). Eleven patients received a single T-cell infusion, and 1 patient (no. 7) received an additional T-cell infusion in an effort to eradicate EBV reactivation and persistent mixed chimerism (Table 1). There were no immediate toxicities related to infusion, but 3 patients (nos. 6, 8, 9) subsequently developed GVHD by days 65, 19, and 89, and were treated with 1 dose of the iC9 CID AP1903. All patients responded fully within 6 to 48 hours after AP1903 administration with no recurrence of GVHD within 90 days. A fourth patient (no. 12) had 3 transplants and 6 months of chemotherapy prior to the fourth HSCT. Patient 12 received AP1903 for an encephalopathy considered a possible manifestation of cerebral GVHD (see “Administration of dimerizer drug AP1903 depletes alloreactive T cells in vivo”). Two patients relapsed (nos. 1 and 6) and 4 died of progressive disease (nos. 2, 3, 5, 9), whereas patient 12 with encephalopathy died of progression of this disorder. Six patients are alive (nos. 1, 6, 7, 8, 10, 11) at a median of 476 days after transplant (range, 278-674 days) (Table 1).
Characteristics of the patients
| Pt. . | Sex (age, y) . | Diagnosis . | Disease status at SCT . | Conditioning . | Infused CD34+/kg at SCT . | Infused CD3+/kg at SCT . | Infused T cells/kg (no. of infusions) . | Time to T-cell infusion (day after SCT) . | Time to off study or received other T cells/stem cells (day after T-cell infusion) . | Acute GVHD (grade) . | Virus reactivation . | Immune responses detected from infused CD3+CD19+ T cells . | Current status (day after SCT*) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M (49) | T-cell ALL | Active | Cy-Campath-TBI | 1.31 × 107 | 2.34 × 103 | 1 × 104 (1) | 31 | 315 | None | EBV | N/A | Relapse (D+150), Alive (D+674 after 2nd SCT) |
| 2 | M (4) | ALL (CNS) | CR2 | AraC-Cy-Campath-TBI | 1.56 × 107 | 7.31 × 103 | 1 × 104 (1) | 61 | 81 | None | CMV | N/A | Death from progressive disease (D+142) |
| 3 | M (2) | JMML | Active | AraC-Cy-Campath-TBI | 2.82 × 107 | 2.5 × 104 | 1 × 105 (1) | 32 | 115 | None | EBV, AdV | N/A | Death from progressive disease (D+147) |
| 4 | M (10) | ALL | CR3 | craniospinal XRT+ AraC-Cy-Campath-TBI | 1.58 × 107 | 1.9 × 103 | 1 × 105 (1) | 38 | 31 | None | N/A | N/A | Withdrawal from study (D+166) |
| 5 | F (10) | MDS | CR3 | Flu-Mel-Campath | 2.22 × 107 | 5.21 × 103 | 1 × 105 (1) | 82 | 782 | None | CMV, EBV | EBV | Death from progressive disease (D+864) |
| 6 | M (50) | T-cell ALL | CR3 | Flu-Mel-Campath | 1.4 × 107 | 2.42 × 103 | 5 × 105 (1) | 36 | N/A | Skin I, gut I | VZV, EBV | VZV, EBV | Relapse (D+408), alive (D+674) |
| 7 | F (4) | EBV-LPD | CR1 | Flu-Campath-TBI | 1.31 × 107 | 2.05 × 103 | 5 × 105 (2) | 42 (#1) | 201 | None | EBV, CMV, AdV, HHV6 | EBV, AdV, HHV6 | CD34 top off (D+243 for mixed chimera), alive in CR (D+551) |
| 116 (#2) | |||||||||||||
| 8 | M (15) | MDS | CR3 | Flu-Campath-TBI | 1.05 × 107 | 1.47 × 103 | 1 × 106 (1) | 42 | 59 | Skin II | CMV, EBV, BKV | CMV, EBV | Received another T-cells on D+101, alive in CR (D+400) |
| 9 | M (8) | HLH (CNS) | Extensive prior therapy | Flu-Mel-Campath | 1.62 × 107 | 5.19 × 103 | 1 × 106 (1) | 47 | 248 | Skin II, liver (stage 2 ALT) | CMV, EBV, BKV | CMV, EBV, BKV | Death from zygomycetes infection (D+295) |
| 10 | M (9) | AML | CR2 | Cranial XRT + Bu-Cy-Campath | 1.13 × 107 | 1.26 × 103 | 5 × 106 (1) | 40 | 28 | None | CMV, BKV | CMV | Received another T-cells on D+68, alive (D+278) |
| 11 | F (17) | ALL | CR1 | AraC- Cy-Campath-TBI | 1.01 × 107 | 3.34 × 103 | 5 × 106 (1) | 73 | N/A | None | HHV6, EBV, VZV | HHV6, EBV, VZV | Alive in CR (D+306) |
| 12 | M (19) | AML | CR2 | Flu-Campath-TBI | 4.07 × 106 | 8.41 × 102 | 5 × 106 (1) | 46 | 38 | CNS GVHD? | CMV, BKV | CMV | Death from encephalopathy due to extensive prior treatment including 3 previous transplants (D+84) |
| Pt. . | Sex (age, y) . | Diagnosis . | Disease status at SCT . | Conditioning . | Infused CD34+/kg at SCT . | Infused CD3+/kg at SCT . | Infused T cells/kg (no. of infusions) . | Time to T-cell infusion (day after SCT) . | Time to off study or received other T cells/stem cells (day after T-cell infusion) . | Acute GVHD (grade) . | Virus reactivation . | Immune responses detected from infused CD3+CD19+ T cells . | Current status (day after SCT*) . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M (49) | T-cell ALL | Active | Cy-Campath-TBI | 1.31 × 107 | 2.34 × 103 | 1 × 104 (1) | 31 | 315 | None | EBV | N/A | Relapse (D+150), Alive (D+674 after 2nd SCT) |
| 2 | M (4) | ALL (CNS) | CR2 | AraC-Cy-Campath-TBI | 1.56 × 107 | 7.31 × 103 | 1 × 104 (1) | 61 | 81 | None | CMV | N/A | Death from progressive disease (D+142) |
| 3 | M (2) | JMML | Active | AraC-Cy-Campath-TBI | 2.82 × 107 | 2.5 × 104 | 1 × 105 (1) | 32 | 115 | None | EBV, AdV | N/A | Death from progressive disease (D+147) |
| 4 | M (10) | ALL | CR3 | craniospinal XRT+ AraC-Cy-Campath-TBI | 1.58 × 107 | 1.9 × 103 | 1 × 105 (1) | 38 | 31 | None | N/A | N/A | Withdrawal from study (D+166) |
| 5 | F (10) | MDS | CR3 | Flu-Mel-Campath | 2.22 × 107 | 5.21 × 103 | 1 × 105 (1) | 82 | 782 | None | CMV, EBV | EBV | Death from progressive disease (D+864) |
| 6 | M (50) | T-cell ALL | CR3 | Flu-Mel-Campath | 1.4 × 107 | 2.42 × 103 | 5 × 105 (1) | 36 | N/A | Skin I, gut I | VZV, EBV | VZV, EBV | Relapse (D+408), alive (D+674) |
| 7 | F (4) | EBV-LPD | CR1 | Flu-Campath-TBI | 1.31 × 107 | 2.05 × 103 | 5 × 105 (2) | 42 (#1) | 201 | None | EBV, CMV, AdV, HHV6 | EBV, AdV, HHV6 | CD34 top off (D+243 for mixed chimera), alive in CR (D+551) |
| 116 (#2) | |||||||||||||
| 8 | M (15) | MDS | CR3 | Flu-Campath-TBI | 1.05 × 107 | 1.47 × 103 | 1 × 106 (1) | 42 | 59 | Skin II | CMV, EBV, BKV | CMV, EBV | Received another T-cells on D+101, alive in CR (D+400) |
| 9 | M (8) | HLH (CNS) | Extensive prior therapy | Flu-Mel-Campath | 1.62 × 107 | 5.19 × 103 | 1 × 106 (1) | 47 | 248 | Skin II, liver (stage 2 ALT) | CMV, EBV, BKV | CMV, EBV, BKV | Death from zygomycetes infection (D+295) |
| 10 | M (9) | AML | CR2 | Cranial XRT + Bu-Cy-Campath | 1.13 × 107 | 1.26 × 103 | 5 × 106 (1) | 40 | 28 | None | CMV, BKV | CMV | Received another T-cells on D+68, alive (D+278) |
| 11 | F (17) | ALL | CR1 | AraC- Cy-Campath-TBI | 1.01 × 107 | 3.34 × 103 | 5 × 106 (1) | 73 | N/A | None | HHV6, EBV, VZV | HHV6, EBV, VZV | Alive in CR (D+306) |
| 12 | M (19) | AML | CR2 | Flu-Campath-TBI | 4.07 × 106 | 8.41 × 102 | 5 × 106 (1) | 46 | 38 | CNS GVHD? | CMV, BKV | CMV | Death from encephalopathy due to extensive prior treatment including 3 previous transplants (D+84) |
Conditioning regimen: Patient 1: Cy: 120 mg/kg, Campath: 30 mg, TBI 1200 cGy. Patients 2, 3, 4, and 11: Ara-C: 3 g/m2 per dose for 6 doses; Cy: 45 mg/kg; Campath 5 mg to 10 mg per dose for 3 doses; TBI: 1400 cGy over 4 days. Patients 5 and 6: Flu 150 mg/m2, Campath: 30 mg, Mel: 140 mg/m2. Patients 7 and 8: Flu 120 mg/m2, Campath: 40 mg, TBI: 600 cGy. Patient 9: Flu 150 mg/m2, Campath: 0.86 mg/kg, Mel: 140 mg/m2. Patient 10: Bu: 12.8 mg/kg, Cy: 200 mg/kg, Campath: 40 mg. Patient 12: Flu: 150 mg/m2, Campath: 40 mg, TBI: 600 cGy.
ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; AraC, cytosine arabinoside; AdV, adenovirus; Bu, busulfan; CR, complete remission; Cy, cyclophosphamide; D, day; F, female; Flu, fludarabine; HLH, hemophagocytic lymphohistiocytosis; JMML, juvenile myelomonocytic leukemia; LPD, lymphoproliferative disorder; M, male; MDS, myelodysplastic syndrome; Mel, melphalan; N/A, not applicable; Pt., patient; TBI, total body irradiation; XRT, irradiation.
Follow-up as of December 31, 2014.
Engraftment of haploidentical alloreplete iC9-T cells
The detailed phenotype of the infused T-cell products is provided in supplemental Table 1 (see supplemental Data available on the Blood Web site). These haploidentical alloreplete iC9-T cells were predominantly effector memory cells. Because the infused cells were haploidentical to the recipient and had not been depleted of alloreactive cells, our dose escalation study began with the infusion of just 1 × 104 iC9-T cells per kilogram recipient weight. We chose this dose based on previous studies where infusion of this number of allorepelete haploidentical T cells did not cause GVHD.8 CD3+CD19+ T cells were not detected postinfusion in the 2 patients infused at the lowest dose (patients 1 and 2). In the subsequent 10 patients (from T-cell doses 1 × 105/kg upward), CD3+CD19+ T cells were detected in the PB by flow cytometry as early as 7 days after infusion. Maximal expansion of iC9-T cells was achieved at a median of 1 month after infusion in patients who did not develop GVHD (Figure 1; supplemental Figure 1A), and at 14 to 89 days postinfusion in patients who did. Consistent with our previous study, the early rise of infused CD3+CD19+ T cells was followed rapidly by recovery of CD3+CD19− “endogenous” T cells (supplemental Figure 1B). The mean absolute count of CD3+CD19− T cells was 24 ± 17 cells per microliter at the time of T-cell infusion, increasing to 116 ± 64 cells per microliter and 211 ± 67 cells per microliter at 30 days and 60 days after infusion, respectively (Figure 1A), exceeding 500 per microliter (mean = 530 ± 184 cells per microliter) at 4 months postinfusion (mean = 5.5 months posttransplantation). Haploidentical alloreplete iC9-T cells engrafted long-term; CD3+CD19+ and iC9 transgene–positive cells were detected >12 months after infusion by flow cytometry and Q-PCR, respectively (supplemental Figure 1C).
The kinetics of T-cell subsets after iC9-T-cell infusion in patients not treated with AP1903. Counts of circulating CD3+ (A), CD4+ T cells (B) and CD8+ T cells (C) in 8 patients who did not receive AP1903. Black line with filled circle represents CD19+ T cells, and gray dashed line with diamond represents CD19− T cells. The number of evaluable patients at each point is 8 (from 0 to month 1), 6 (months 2-4), 4 (months 5 and 6), 2 (month 7), and 1 (month 8, 9, and 12). Data show means ± standard error of mean of patients infused with iC9-T cells.
The kinetics of T-cell subsets after iC9-T-cell infusion in patients not treated with AP1903. Counts of circulating CD3+ (A), CD4+ T cells (B) and CD8+ T cells (C) in 8 patients who did not receive AP1903. Black line with filled circle represents CD19+ T cells, and gray dashed line with diamond represents CD19− T cells. The number of evaluable patients at each point is 8 (from 0 to month 1), 6 (months 2-4), 4 (months 5 and 6), 2 (month 7), and 1 (month 8, 9, and 12). Data show means ± standard error of mean of patients infused with iC9-T cells.
Administration of dimerizer drug AP1903 depletes alloreactive T cells in vivo
Three patients (nos. 6, 8, 9) developed acute GVHD at 19 to 89 days after T-cell infusion as manifested by rash, fever, and diarrhea; skin biopsies were consistent with GVHD. Patient 12 had unexplained and worsening encephalopathy with no obvious infectious, drug or vascular-associated etiology, and after discussion was treated with CID for a possible cerebral-localized GVHD. The patients received a single infusion of AP1903 at days 65, 19, 89, and 31 after iC9–T-cell infusion, respectively. Circulating CD3+CD19+ T cells rapidly decreased by 86% to 96% after infusion as detected by flow cytometry (Figure 2A-D), and there was an equivalent decline in iC9 transgene copy numbers by Q-PCR (Figure 2I). Administration of AP1903 did not affect the number of circulating CD3+CD19− T cells in patients 6, 8, and 12 (Figure 2 E,F,H), but caused a transient fall in cell counts in patient 9 that normalized by 48 hours (Figure 2G). There were no other immediate or delayed adverse effects associated with the administration of AP1903 in any patient. GVHD-associated abnormalities of the skin began resolving within 2 hours of the infusion, and all symptoms had disappeared within 24 to 48 hours. The encephalopathy of patient 12 continued to progress even after CID, and he died 1 week later. The etiology of the encephalopathy remains unexplained even after histopathological examination. His frontal lobe cortex had 552 copies per microgram of DNA of the iC9 transgene at day 7 after CID by Q-PCR analysis, indicating the presence of donor T cells, but at low number. Hence, there is no evidence to suggest the encephalopathy was attributable to the infused iC9-T cells or CID.
iC9-T-cell engraftment and in vivo allodepletion by dimerizer drug. Counts of T-cell subsets in 4 patients who received AP1903. CD3+CD19+ T cells (A, Pt. 6; B, Pt. 8; C, Pt. 9; D, Pt. 12) and CD3+CD19− T cells (E, Pt. 6; F, Pt. 8; G, Pt. 9; H, Pt. 12). ●, □, and ▲ represent the CD3+, CD4+, and CD8+ subtypes, respectively. (I) The copy number of the iC9 transgene per microgram of DNA extracted from PBMC, evaluated by Q-PCR. Arrow indicates the time at which the patient was treated with AP1903. Pt., patient.
iC9-T-cell engraftment and in vivo allodepletion by dimerizer drug. Counts of T-cell subsets in 4 patients who received AP1903. CD3+CD19+ T cells (A, Pt. 6; B, Pt. 8; C, Pt. 9; D, Pt. 12) and CD3+CD19− T cells (E, Pt. 6; F, Pt. 8; G, Pt. 9; H, Pt. 12). ●, □, and ▲ represent the CD3+, CD4+, and CD8+ subtypes, respectively. (I) The copy number of the iC9 transgene per microgram of DNA extracted from PBMC, evaluated by Q-PCR. Arrow indicates the time at which the patient was treated with AP1903. Pt., patient.
In the 3 patients who had biopsy-confirmed GVHD and responded to AP1903, residual CD3+CD19+ T cells expanded over the ensuing 14 days without inducing recurrence of GVHD, indicating that AP1903 produces effective in vivo allodepletion of alloreactive T cells.
iC9-T cells contribute to in vivo antiviral activity
We determined whether the infused alloreplete iC9-T cells had antiviral activity in patients with viral infections (Table 1) by sequential measurement of viral load and the number of T cells in PB that were reactive to overlapping peptide libraries derived from EBV, CMV, HHV6, VZV, and BKV antigens. We measured antiviral reactivity in both the CID-sensitive population (CID-S) (CD3+CD19+ iC9-T cells, and thus sensitive to AP1903) and the population resistant to CID (CID-R) (CD3+CD19− endogenous T cells).
We found evidence of antiviral activity from the engrafted CD3+CD19+ T cells in patients receiving doses of 1 × 105 cells per kilogram and above. At the time of T-cell infusion, patient 5 had an EBV load of 31 copies per microgram of DNA, which increased to 1608 copies per microgram of DNA within 10 days of T-cell infusion (Figure 3A). As the CD3+CD19+ T cells engrafted, the viral load rapidly declined (supplemental Figure 2A). The decline corresponded with an increase in circulating EBV-reactive T cells in both the infused (chemical inducer of dimerization-sensitive [CID-S] CD3+CD19+) and endogenous (CID-R CD3+CD19−) populations (Figure 3A). By week 4 after infusion, EBV DNA became undetectable.
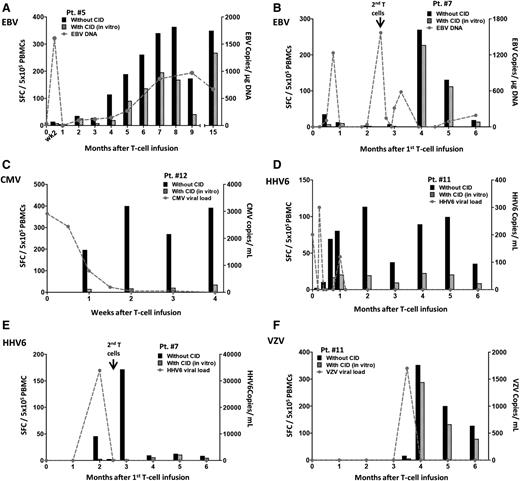
Antiviral immune reconstitution after iC9–T-cell infusion. Quantification of pathogen-specific T cells detected by IFN-γ ELISPOT at multiple time points for each patient who did not receive AP1903. Pts. 5 (A) and 7 (B) had EBV reactivations, Pt. 12 had CMV reactivation (C), and Pt. 11 had HHV6 reactivation (D) before iC9–T-cell infusion. Pt. 7 had HHV6 infection (E), and Pt. 11 had VZV infection (F) after iC9–T-cell infusion. Black histograms represent response from total T cells and striped histograms represent response from endogenous T cells. The value of their difference represents the response from infused iC9-T cells. The gray dashed line indicates the viral load at multiple time points.
Antiviral immune reconstitution after iC9–T-cell infusion. Quantification of pathogen-specific T cells detected by IFN-γ ELISPOT at multiple time points for each patient who did not receive AP1903. Pts. 5 (A) and 7 (B) had EBV reactivations, Pt. 12 had CMV reactivation (C), and Pt. 11 had HHV6 reactivation (D) before iC9–T-cell infusion. Pt. 7 had HHV6 infection (E), and Pt. 11 had VZV infection (F) after iC9–T-cell infusion. Black histograms represent response from total T cells and striped histograms represent response from endogenous T cells. The value of their difference represents the response from infused iC9-T cells. The gray dashed line indicates the viral load at multiple time points.
Similarly, patient 7 had EBV reactivation prior to the T-cell infusion, with an initial increase in viral DNA after infusion and a subsequent fall at the time of iC9–T-cell engraftment (Figure 3B; supplemental Figure 2B). Because the viral DNA again increased 10 weeks after the first infusion, patient 7 received a second dose of 5 × 105 cells per kilogram iC9-T cells. This dose reduced and then cleared the viral load within 10 days, associated with a significant rise in EBV-specific T cells (Figure 3B).
Patient 12 had a 3-week history of CMV antigenemia in PB despite treatment with ganciclovir. CMV viral load declined, becoming undetectable 2 weeks after infusion at the time of maximal expansion of the iC9-T cells (supplemental Figure 2C). CMV-reactive T cells were predominantly (>90%) derived from the CID-S population (Figure 3C; supplemental Figure 2C).
The iC9-T cells also benefited patients with active HHV6 infection. Patient 11 had a 2-week history of HHV6 reactivation. Viral load was 201 copies per milliliter at the time of T-cell infusion, becoming undetectable after 2 weeks (Figure 3D). There was a corresponding increase in HHV6-specific T cells predominantly derived from the CID-S population (Figure 3D; supplemental Figure 2D).
Similar anti-HHV6 immune responses were observed in patient 7, who developed an active HHV6 infection 2 months after T-cell infusion. The elevation in viral load was rapidly controlled, becoming undetectable within 2 weeks concomitantly with a rise in HHV6-specific T cells, of which >96% were in the CID-S population (Figure 3E).
iC9-T cells continue to contribute to in vivo antiviral activity after administration of AP1903
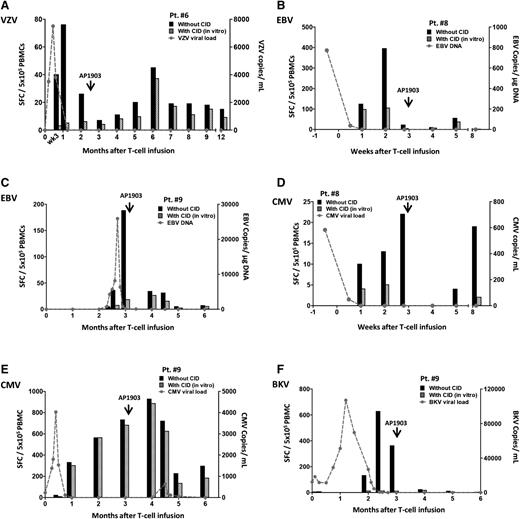
To evaluate whether the beneficial effect of iC9-T cells was retained after GVHD was successfully treated with CID, we measured T-cell responses to VZV, EBV, CMV, and BKV antigens in the CID-S CD3+CD19+ and CID-R CD3+CD19− cell subsets of patients 6, 8, and 9 before and after AP1903 treatment. Prior to receiving the iC9–T-cell infusion, patient 6 had disseminated VZV with shingles and retinitis. At the time of T-cell infusion, the viral load was 3500 copies per milliliter in PB, despite the administration of foscarnet. After an initial rise to >5 million copies, the VZV was cleared as the iC9-T cells engrafted (Figure 2A). The VZV-reactive T cells were predominantly within the CID-S population (>90%, Figure 4A). At week 9 after administration of the iC9-T cells, the patient developed GVHD and received AP1903 (Figure 2A). Although there was an initial decline in VZV-reactive T cells (Figure 4A), the antiviral response subsequently recovered in the CID-S population (Figure 2A).
Virus-specific T cells are retained and remain functional after administration of AP1903. Quantification of pathogen-specific T cells detected by IFN-γ ELISPOT in each patient who received AP1903 to control acute GVHD. Patients had viral infection and/or reactivation for: VZV (A, Pt. 6), EBV (B, Pt. 8 and C, Pt. 9), CMV (D, Pt. 8 and E, Pt. 9), BKV (F, Pt. 9). Black histograms represent response from total T cells and striped histograms represent response from endogenous T cells. The value of their difference represents the response from infused iC9-T cells. The gray dashed line indicates the viral load at multiple time points.
Virus-specific T cells are retained and remain functional after administration of AP1903. Quantification of pathogen-specific T cells detected by IFN-γ ELISPOT in each patient who received AP1903 to control acute GVHD. Patients had viral infection and/or reactivation for: VZV (A, Pt. 6), EBV (B, Pt. 8 and C, Pt. 9), CMV (D, Pt. 8 and E, Pt. 9), BKV (F, Pt. 9). Black histograms represent response from total T cells and striped histograms represent response from endogenous T cells. The value of their difference represents the response from infused iC9-T cells. The gray dashed line indicates the viral load at multiple time points.
Patient 8 had EBV reactivation prior to T-cell infusion. Viral load was cleared as the iC9-T cells engrafted. Seventy-five percent of the EBV-specific T cells detected were CID-S (Figure 4B). CID-S cells remained after the administration of AP1903, and represented 35% of the total EBV-specific T cells after 2 weeks.
Patient 9 had an EBV reactivation 2 months after T-cell infusion that was controlled by a rapid increase in EBV-specific T cells, of which >80% were in the CID-S population. As these EBV-specific T cells increased in number, the EBV DNA returned to baseline (Figure 4C). After 3 months, the patient developed acute GVHD. As shown in Figure 4C, after the administration of AP1903 we observed an initial fall in the number of EBV-specific T cells in the CID-S population, but 6 weeks after CID administration they constituted 52% of the total EBV-specific T cells. There was no resurgence of EBV.
Patients 8 and 9 also had CMV reactivation prior to iC9-T-cell infusion. The virus incompletely responded to >4 weeks’ treatment with ganciclovir and foscarnet (Figure 4D-E). In both subjects, T-cell infusion was followed by an upsurge of CMV-specific T cells and control of CMV viremia. In patient 8, the majority (>70%) of CMV-specific T cells originated from the CID-S cells. When the patients developed GVHD and were treated with AP1903, there was an initial loss of CMV-reactive cells in PB, but they recovered in both the CID-S and CID-R populations (Figure 4D). In patient 9, loss of specificity was accompanied by a modest but transient resurgence of CMV that was rapidly controlled without further treatment (Figure 4E).
Finally, patient 9, who also reactivated BKV prior to iC9–T-cell infusion, had a gradual reduction and ultimately clearance of BKV viremia. The clearance was associated with a significant increase in BKV-reactive T cells in PB, essentially all (>98%) of which were from the CID-S population. After administration of the CID, there was an initial fall in BKV-reactive T cells followed by their recovery. There was no recurrence of BK viremia/viruria (Figure 4F).
Effect of AP1903 on cytokine release syndrome and CNS T-cell infiltration
Rapid expansion and activation of adoptively transferred T cells may cause a cytokine release syndrome (CRS) associated with the release of proinflammatory cytokines and manifested by high fevers and, in severe form, cardiorespiratory failure. GVHD from adoptive transfer of alloreactive T cells rarely produces CRS. However, patient 8 had a fever reaching 106°F on day 18 after infusion of the iC9-T cells, associated with skin rash, diarrhea, and a high level of circulating cytokines including interleukin-6 (IL-6) (Figure 5). Within 2 hours of AP1903 administration, the body temperature normalized (Figure 5A), skin rash improved (Figure 5B) and elevated plasma cytokine levels declined in the absence of additional therapy (Figure 5C). Hence, iC9 activation can rapidly control GVHD-associated CRS.
Administration of AP1903 rapidly resolves GVHD symptoms and reduces cytokine release. In Pt. 8, (A) the highest body temperature to the time of AP1903 infusion. (B) Pictures of the skin rash were taken prior to and 25 minutes after beginning the AP1903 infusion. (C) Cytokine production in plasma measured from samples collected 4 hours prior to, 2.5 hours after beginning, and 48 hours after the infusion of AP1903.
Administration of AP1903 rapidly resolves GVHD symptoms and reduces cytokine release. In Pt. 8, (A) the highest body temperature to the time of AP1903 infusion. (B) Pictures of the skin rash were taken prior to and 25 minutes after beginning the AP1903 infusion. (C) Cytokine production in plasma measured from samples collected 4 hours prior to, 2.5 hours after beginning, and 48 hours after the infusion of AP1903.
We also saw evidence that administration of AP1903 may reduce iC9-T cells in the central nervous system (CNS). As shown in Figure 4A, patient 6 had disseminated VZV at the time of iC9–T-cell infusion and subsequently developed VZV meningitis with >5 million copies of VZV per milliliter of cerebrospinal fluid (CSF). Within 1 week, viral load declined to 6900 copies of VZV per milliliter, before becoming undetectable. Flow cytometry analysis of CSF 55 days after infusion of the iC9-T cells showed a significant population (25%) of CD3+CD19+ T cells, consistent with the presence iC9-T cells in PB (33%) (Figure 6A-B). The patient then developed GVHD and was treated with AP1903. Fourteen days later, we found <1% and <3% CD3+CD19+ T cells in CSF and PB, respectively (Figure 6C-D), indicating the depletion of iC9-T cells in both compartments following activation of the iC9 transgene.
Administration of AP1903 affects iC9-T cells in CSF. Detection of iC9-T cells in CSF and PB by flow cytometry before and after administration of AP1903 (Pt. 6). Nine days before CID treatment in CSF (A) and in PB (B), and 14 days after treatment in CSF (C) and in PB (D). The percentage of CD3+CD19+ T cells and CD3+CD19− T cells was calculated under the gate of CD3+ T cells.
Administration of AP1903 affects iC9-T cells in CSF. Detection of iC9-T cells in CSF and PB by flow cytometry before and after administration of AP1903 (Pt. 6). Nine days before CID treatment in CSF (A) and in PB (B), and 14 days after treatment in CSF (C) and in PB (D). The percentage of CD3+CD19+ T cells and CD3+CD19− T cells was calculated under the gate of CD3+ T cells.
Discussion
We have shown that activation of the iC9 safety system can rapidly, effectively, and sustainably remove alloreactive T cells causing GVHD after haplo-HSCT, even when the infused T cells are alloreplete. iC9-T cells contributed to more rapid immune reconstitution than is reported after haplo-HSCT without adoptive transfer.25 Moreover, effective antiviral immunity persisted even after in vivo depletion of alloreactive cells. Activation of the iC9 transgene also rapidly controlled symptoms and signs related to CRS and depleted iC9-T cells not only in the PB but also in the CNS.
We previously described how the iC9 transgene could be successfully used as a means to deplete haploidentical T cells posttransplant if they caused GVHD. In the initial studies, however, iC9-T cells had been nominally depleted of their alloreactive component ex vivo, using a lengthy and complex process that is not scalable for general clinical use. The current study shows that iC9 allodepletion could be performed in vivo instead, and that >85% of circulating iC9-T cells can be eliminated within 30 minutes of a single AP1903 infusion with resolution of associated signs and symptoms of GVHD within 6 to 48 hours. Importantly, there was no recurrence of GVHD associated with the gradual recovery of iC9-T cells following AP1903 administration. Overall, these findings indicate that iC9 activation is sufficiently potent to promote in vivo allodepletion and abrogate GVHD, even when the infused T cells have not undergone ex vivo allodepletion, and that the resurgent haploidentical iC9-T cells are functionally allodepleted and well tolerated.
Selective in vivo depletion of alloreactive cells by iC9 activation likely reflects the high level of alloreactive iC9–T-cell activation during GVHD, hence their increased expression of iC9 and susceptibility to AP1903. A similar concept involves administering cyclophosphamide (50 mg/kg) to patients for 2 days starting 3 days after haplo-HSCT from T-cell replete donors to eliminate alloreactive T cells while sparing nonalloreactive/quiescent donor T cells.29-33 This selective depletion is simpler than the genetic modification described here, and appears to effectively prevent GVHD. However, because every patient is treated with cyclophosphamide irrespective of GVHD, unnecessary depletion of critical T-cell subsets may occur, potentially delaying immune reconstitution and increasing opportunistic infections. By contrast, only patients who develop GVHD are treated with the apoptosis-inducing prodrug in our suicide system.
We demonstrate that infusion of alloreplete iC9-T cells provides rapid protection from EBV, CMV, HHV6, VZV, and BKV infections and also appears to accelerate the recovery of endogenous T cells. Of note, patients with preexisting CMV infection could continue to be treated with ganciclovir during the period of iC9–T-cell engraftment without attenuating the expansion and function of the CMV-specific iC9-T cells, potentially providing an additional safety margin over the HSV-tk system. Although administration of the CID in patients with GVHD reduces the level of circulating virus-specific iC9-T cells, these cells subsequently recover and in vivo antiviral activity is retained. Even in patient 9, who had active EBV infection at the time of CID infusion, iC9-associated antiviral activity recovered. Hence, in vivo–expanded iC9+ virus-specific T cells may be more resistant to the apoptotic signals generated by iC9 than alloreactive T cells.34-39 Thus, iC9 activation in alloreplete T cells can selectively eliminate the alloreactive component of T cells that cause GVHD, while sparing the virus-reactive subpopulation, even when CID is administered during an active viral infection.
The speed and efficiency with which iC9 activation produces T-cell depletion may have benefit beyond the treatment of GVHD. Following activation and expansion in vivo, adoptively transferred T cells can produce proinflammatory cytokines and induce other cells, including monocytes, to release additional proinflammatory mediators causing life-threatening CRS.40-42 Although severe CRS is an uncommon manifestation of T-cell engraftment after HSCT, patient 8 developed signs and symptoms compatible with GVHD and CRS. Administration of AP1903 not only improved clinical symptoms, but also decreased proinflammatory cytokines including IL-6, which is produced by activated monocytes and other accessory cells. The effect on circulating serum cytokines may have value for other T-cell immunotherapies, such as adoptive transfer of CAR-T cells in which CRS is more prevalent and severe.42-45
Our data also show that AP1903 may control iC9-T cells in the CNS. In patient 6 with VZV meningitis, we detected iC9-T cells in the CSF and found they could be eliminated by a single IV dose of CID. We could not obtain repeat samplings of the CSF at multiple time points and so do not know whether there was direct destruction of iC9-T cells within the CSF following AP1903 entry, or a more gradual reduction of iC9-T cells in the CSF caused by the redistribution of normal T lymphocytes between blood and CSF after AP1903-mediated depletion of iC9-T cells in the PB. The pharmacokinetics and biodistribution of AP1903 within the human CNS are currently unknown, and the prodrug’s short half-life (5 hours in PB) could explain the absence of AP1903 in the CSF collected several days after IV administration. Nonetheless, our observation raises the possibility of using iC9 to treat neurological toxicities caused by adoptive transfer of gene-modified T cells.46
In conclusion, we found that alloreplete iC9-T cells indeed can reconstitute immunity posttransplant and administration of AP1903 eliminates them from both PB and CNS, leading to rapid resolution of GVHD and CRS.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are thankful to the patients and their families for their cooperation, and are grateful to: Catherine Gillespie for editing the manuscript; Melissa Gates, Keli Sharpe, and Sarah Driedger for flow cytometry; Rong Cai and Sita Nookala for follow-up sample support; and Haruko Tashiro for helping with follow-up experiments.
This clinical protocol (I.N.D.13813) was supported by National Institutes of Health National Heart, Lung, and Blood Institute grant U54HL08100, and development of the caspase system by National Cancer Institute grants P01CA094237 and P50CA126752. The clinical trial also received support from the Clinical Research Center at Texas Children’s Hospital, the Institute for Clinical and Translational Research at Baylor College of Medicine, and shared resources of the Dan L. Duncan Cancer Center support grant P30CA125123.
Authorship
Contribution: M.K.B., G.D., H.E.H., and C.M.R. designed the study; X.Z. performed cell manufacturing, experiments, and analyzed data; X.Z., G.D., and M.K.B. wrote the manuscript; H.E.H., C.M.R., and D.M.S. contributed to the preparation of the manuscript; R.A.K., C.A.M., S.N., and R.T.K. enrolled patients and monitored clinical responses; X.Z., A.D.S., B.S., C.M.R., and A.P.G. developed good manufacturing practice protocols; A.G.D. performed flow cytometry on patient samples; O.D. performed Q-PCR; H.L. designed biostatistical analysis for study; Y.-F.L. coordinated the study; and B.J.G. and H.E.H. ensured compliance with regulatory requirements for the clinical trial.
Conflict-of-interest disclosure: D.M.S. is an employee of Bellicum Pharmaceuticals, Inc, which provided the CID AP1903. The Center for Cell and Gene Therapy has a collaborative research agreement with Celgene. The remaining authors declare no competing financial interests.
Correspondence: Xiaoou Zhou, Center for Cell and Gene Therapy, 1102 Bates St, Suite 1640 Feigin Center, Houston, TX 77030; e-mail: xiaoouz@bcm.edu; or Malcolm K. Brenner, Center for Cell and Gene Therapy, 1102 Bates St, Suite 1640 Feigin Center, Houston, TX 77030; e-mail: mbrenner@bcm.edu.