To the editor:
Mantle cell lymphoma (MCL) is incurable using current standard therapeutic regimens.1 There are relatively few relevant cell lines and mouse models. To evaluate new drugs and develop rational combination therapies, we have established and thoroughly characterized a new MCL cell line, CCMCL1, and demonstrated its utility as a novel preclinical model for MCL.
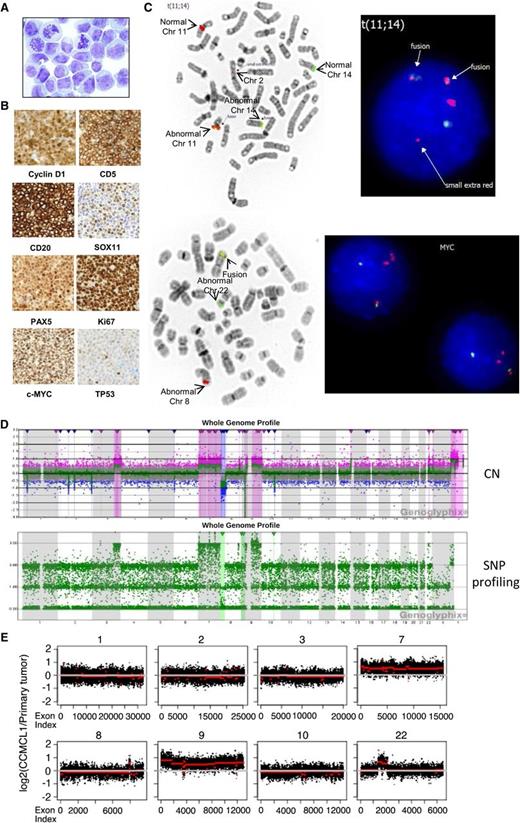
Primary MCL cells (1 × 107) from a 58-year-old man with progressive MCL (patient clinical information provided in supplemental Table 1, available on the Blood Web site) and hyperleukocytosis (white blood cell count 851.9 × 109/L; >99% lymphoma cells) were engrafted in NOD-SCID-γ (NSG) mice via tail vein injection (supplemental Figure 1A-B). Splenocytes from a rapidly engrafted mouse were cultured in RPMI 1640 medium with 10% fetal bovine serum over 12 months as a cell line, CCMCL1, which had a blastoid nuclear morphology (Figure 1A). CCMCL1-engrafted NSG mice displayed disseminated disease including splenomegaly. CCMCL1 expressed human cyclin D1, CD5, CD20, SOX11, PAX5, Ki67 (95%), c-MYC, BCL2, BCL2L1, MCL1, constitutively phosphorylated AKT and BTK, and a low level of TP53 (Figure 1B and supplemental Table 2). CCMCL1 retained an immunophenotype similar to the primary MCL cells and was Epstein-Barr virus–negative (supplemental Table 3 and Figure 1C).
Characterization of CCMCL1 cells. (A) Wright staining (original magnification ×1000) of CCMCL1 cells. (B) An NSG mouse was injected with 10 million first passage CCMCL1 cells via tail vein and sacrificed 5 weeks later. The spleen was collected and fixed in 10% formalin overnight, and a paraffin block was prepared. Immunostaining was performed using an automated stainer. (C) FISH of CCND1 (upper panels) and MYC (lower panels). FISH assay was performed on metaphases with an IGH/CCND1 dual-color, dual-fusion probe set (Abbott Molecular, Des Plaines, IL). Rearrangement of the MYC locus (8q24) was tested with the Break Apart probe from Abbott Molecular. FISH data were analyzed with ASI software. (D) Comparative genomic hybridization (CGH) + single nucleotide polymorphism (SNP) array (detailed information available in supplemental Methods, found on the Blood Web site). Upper panel: chromosome copy number (CN); lower panel: SNP profiling. The log ratio and allele difference plots are shown on the y-axis. (E) Whole-exome sequencing and CNV analysis were performed as described elsewhere.2 The duplication or amplification of specific chromosome regions in CCMCL1 cells (red line) compared with primary tumor cells (white line) was shown. The complete CNV analysis is shown in supplemental Figure 3. Chr, chromosome.
Characterization of CCMCL1 cells. (A) Wright staining (original magnification ×1000) of CCMCL1 cells. (B) An NSG mouse was injected with 10 million first passage CCMCL1 cells via tail vein and sacrificed 5 weeks later. The spleen was collected and fixed in 10% formalin overnight, and a paraffin block was prepared. Immunostaining was performed using an automated stainer. (C) FISH of CCND1 (upper panels) and MYC (lower panels). FISH assay was performed on metaphases with an IGH/CCND1 dual-color, dual-fusion probe set (Abbott Molecular, Des Plaines, IL). Rearrangement of the MYC locus (8q24) was tested with the Break Apart probe from Abbott Molecular. FISH data were analyzed with ASI software. (D) Comparative genomic hybridization (CGH) + single nucleotide polymorphism (SNP) array (detailed information available in supplemental Methods, found on the Blood Web site). Upper panel: chromosome copy number (CN); lower panel: SNP profiling. The log ratio and allele difference plots are shown on the y-axis. (E) Whole-exome sequencing and CNV analysis were performed as described elsewhere.2 The duplication or amplification of specific chromosome regions in CCMCL1 cells (red line) compared with primary tumor cells (white line) was shown. The complete CNV analysis is shown in supplemental Figure 3. Chr, chromosome.
CCMCL1 had a complex karyotype based on classical cytogenetics and spectral karyotyping (supplemental Figure 2), and IGH/CCND1 fusion and MYC rearrangement t(8;22) according to fluorescence in situ hybridization (FISH) (Figure 1C). The karyotype is 49,XY,dup(X)(q25q28),+t(Y;12)(p35;q12),ins(1;2)(p35;p13),t(2;11)(p13;q12),t(2;5)(q35;q13),+7,der(8)t(3;8)(q24;p21),t(8;22)(q24;q11.2),+9,der(9)(9;10)(p21;q23),der(11)ins(11;14)(q12;q24)[20], which is similar to that of the primary cells (supplemental Figure 2). CCMCL1 and primary cells had identical IGH-VDJ gene usage (VH3-7*01/DH3-3*02/JH4*02), with 97.3% homology to the IGHV3-7 germ line sequence. Genomic single nucleotide polymorphism (SNP) microarray (Figure 1D) confirmed the karyotypic data and refined the cytogenetically identified breakpoints. Together, they showed complex chromosomal changes, including gains/duplication of chromosomes X, Y, 3, 7, 9, and 22; loss/deletions of chromosomes 1, 2, 8, and 10; and loss of heterozygosity for the short arm of chromosome 9. Whole-exome sequencing (WES) and copy number variation (CNV) analysis2 demonstrated substantial conservation of the genome from the primary tumor to the cell line, including the deletions on chromosomes 1, 2, 8, and 10 observed by SNP array. The only exceptions were amplification of chromosomes 7 and 9, which may have conferred a selective advantage for CCMCL1 cells in culture, and gain on chromosome 22 (Figure 1E). CCMCL1 had no mutations or deletions commonly found in MCL, such as deletion of ATM or mutation in TP53, the E3 ligase UBR5, cyclin D1, or BIRC3.3-5
The clinically active agents ibrutinib and bortezomib induced apoptosis in CCMCL1 cells6 (supplemental Figure 4A-B), supporting CCMCL1 as a clinically relevant MCL model. CCMCL1 was refractory to rituximab’s direct cytotoxicity (supplemental Figure 4C); however, rituximab kills by multiple mechanisms. The cells grow as a localized tumor after subcutaneous injection and as disseminated disease after intravenous injection in NSG mice (supplemental Figure 4D-E).
A recent Mantle Cell Consortium study7 reported only 7 available well-characterized fully human MCL lines. Basic immunophenotypic, karyotypic, and growth characteristics were described; however, none has been extensively characterized at the molecular level as CCMCL1, nor have they been compared with the primary tumor cells. CCMCL1 has the IGH-CCND1 genetic hallmark of MCL and closely resembles the terminal-phase leukemic MCL cells as evidenced by immunophenotyping, karyotyping, IGHV sequence, and WES. The primary cells also had a MYC translocation, a known albeit unusual abnormality in MCL progression, typically seen in blastoid MCL.8 Although the morphology of the primary leukemic cells was not blastoid (supplemental Figure 5), it was similarly aggressive biologically.
This study represents the first longitudinal genome analysis of a primary tumor and the resulting cell line. WES provides unbiased, direct evidence for conservation of the MCL genome in CCMCL1 at single-nucleotide resolution. It also provides a genomic database for interrogation of mutation, deletion, and CNV of any gene of interest under specific experimental conditions. For example, WES detected no mutation in genes of the B-cell receptor or phosphatidylinositol 3-kinase pathway, including BTK and AKT. However, CCMCL1 constitutively expressed phosphorylated AKT and BTK, suggesting CCMCL1 is an appropriate model for investigating therapeutic targeting of these 2 pathways in MCL. Because other MCL cell lines have mutations or deletions in either ATM or TP53,9,10 CCMCL1 also offers an opportunity for MCL studies without the interference of ATM or TP53 loss.
In summary, CCMCL1 represents a new well-characterized, aggressive MCL cell line. It will likely contribute to preclinical evaluation of novel agents, especially for targeting MCL at relapse, which is an unmet clinical need.
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank Daniel Lindner and Yvonne Parker for their technical support in handling NSG mice.
This work was supported in part by Pilot Project Award from the Robert J. Tomsich Pathology and Laboratory Medicine Institute, Cleveland Clinic (X.Z.), and a Special Initiative Grant from the Lymphoma Research Foundation (S.C.-K.).
Contribution: X.Z., S.C.-K., S.S., M.R.S., and E.D.H. contributed the conception and design of this study; X.Z., S.S., J.B., and L.D. performed the experiments; S.C.-K., M.D.L. ran WES; K.E. and O.E. analyzed WES data; and X.Z., S.C.-K., S.S., O.E., M.R.S., and E.D.H. coordinated the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eric D. Hsi, Department of Laboratory Medicine, Robert J. Tomsich Pathology and Laboratory Medicine Institute, Cleveland Clinic, L-11, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: hsie@ccf.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal