To the editor:
The National Heart, Lung, and Blood Institute (NHLBI) community has established a long legacy of research success, resulting in dramatic improvements in longevity and quality of life. To continually advance its mission of research that enhances public health, the NHLBI is embarking on a Strategic Visioning process to identify scientific priorities that will guide the institute’s future directions in heart, lung, blood, and sleep (HLBS) research for next decade. The institute is using a digital crowdsourcing approach to engage its broad community on the scientific opportunities and challenges to research progress that could be addressed through activities and initiatives led by the NHLBI. The result of the Strategic Visioning process will be a complete understanding of the most pressing scientific questions and research barriers that the NHLBI can address today and for 5 to 10 years to come. The resulting scientific priorities will serve as a guide for resource allocation and will be regularly refreshed through an ongoing iterative and dynamic process of scientific engagement with the NHLBI community.
The NHLBI in 2025: what if…
Imagine a world where we are able to prevent and preempt the burden of cardiovascular, lung, and blood diseases; a world where we are able to capture the promise of personalized precision medicine, where each person receives the right treatment, tailored to his or her needs, at the right time. In this new world, what if we were able to realize a stroke-free generation of individuals living with sickle cell disease (SCD); what if we were able to eliminate health inequities (both domestic and global) with effective and rapid uptake of evidence-based practices and tools; and what if we could expand the frontiers of scientific knowledge and revolutionize how we diagnose, prevent, and treat disease by leveraging the power of big scientific data systems? This vision is not merely a collection of idle dreams; the boundless possibilities of this bold new world are well within our reach.
Building upon the legacy of success
The mission of the NHLBI is to enhance public health by transforming research discoveries in HLBS systems into improved disease prevention and treatment. The NHLBI is building upon a history of research excellence by embarking on a Strategic Visioning process to collectively identify the greatest unmet needs in HLBS research. Our aim is to engage all corners of the NHLBI community to identify the most compelling scientific questions of our time, questions that address pressing public health challenges, capitalize on major advances in technology, and take bold new approaches that, because of their scope and complexity, transcend any individual laboratory or organization and require NHLBI leadership (Figure 1). Investigator-initiated research (eg, R01 awards) makes up the majority (∼80%) of the NHLBI extramural budget. We have every intention to maintain our high level of commitment to investigator-initiated fundamental discovery science; it is our top priority. This Strategic Visioning process will largely inform our investments that focus on institute-initiated solicitations (eg, requests for applications; ∼20% of extramural research funds) designed to catalyze the pursuit of investigations into important yet unexplored scientific areas. Although the Strategic Visioning process will guide institute-initiated activities, it may also provide additional insights for individual investigator-initiated programs.
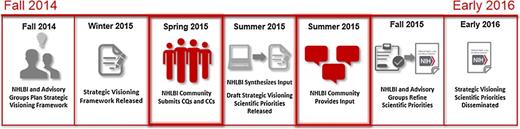
The Strategic Visioning process timeline for community engagement. The National Heart, Lung, and Blood Advisory Council and the Board of External Experts met jointly in September 2014 to create the initial outline for the Strategic Visioning process. The NHLBI staff then developed a full Strategic Visioning framework to elicit and collect scientific CQs and CCs from the NHLBI community. In the spring of 2015, the entire NHLBI community will contribute CQs and CCs. These CQs and CCs will be refined, integrated, and evaluated against the NHLBI research portfolio to develop Draft Strategic Visioning Scientific Priorities that will be released for public comment in the summer of 2015. The NHBLI, working with its scientific advisory groups, will select a final prioritized list of CQs and CCs and publish them as part of a Strategic Visioning Scientific Priorities document by early 2016 that will describe the strategic priorities of the NHLBI.
The Strategic Visioning process timeline for community engagement. The National Heart, Lung, and Blood Advisory Council and the Board of External Experts met jointly in September 2014 to create the initial outline for the Strategic Visioning process. The NHLBI staff then developed a full Strategic Visioning framework to elicit and collect scientific CQs and CCs from the NHLBI community. In the spring of 2015, the entire NHLBI community will contribute CQs and CCs. These CQs and CCs will be refined, integrated, and evaluated against the NHLBI research portfolio to develop Draft Strategic Visioning Scientific Priorities that will be released for public comment in the summer of 2015. The NHBLI, working with its scientific advisory groups, will select a final prioritized list of CQs and CCs and publish them as part of a Strategic Visioning Scientific Priorities document by early 2016 that will describe the strategic priorities of the NHLBI.
For more than 60 years, the NHLBI community has fostered a legacy of research excellence through groundbreaking fundamental discovery science, landmark clinical trials and population-based cohort studies, and innovative health education and dissemination efforts. For example, in the cardiovascular arena, the Framingham Heart Study led to the identification of cholesterol as a major risk factor for heart disease.1 Subsequent NHLBI-supported fundamental discovery science identified pathways of cholesterol synthesis that paved the road for the development of statins,2 a major contributor to the dramatic 75% reduction in coronary heart disease since 1968.3,4 Today, exome sequencing technology is identifying rare genomic variants, such as those in the apolipoprotein C3 (APOC3) gene that result in lower triglyceride levels and lower risk of heart attack, which could lead to the development of next-generation drugs that can more effectively prevent heart disease.5 Exciting new technologies are also changing how we capitalize on these discoveries to arrest, or possibly even reverse, disease pathobiology. For example, researchers have developed high-density lipoprotein nanoparticle carriers that can deliver statins directly to atherosclerotic plaques and inhibit plaque progression or induce plaque regression.6
Similarly, contributions of the lung research community continue to transform scientific advances into better health. Research supported by the NHLBI on chronic obstructive pulmonary disease (COPD), the third-most common cause of death in the United States, has effectively enabled improved quality of life, allowing patients to stay more active and slowing the progression of disease. However, not all patients with similar exposures develop disease, and the variability in disease severity and outcome poses several unanswered questions. The NHLBI is funding a number of studies to better understand the heterogeneity, susceptibility, and progression of disease, analyzing genomic, transcriptomic, imaging, microbiome, comorbidity, and biomarker data from large numbers of subjects with varying levels of disease and Mendelian susceptibility to COPD. For example, investigators have recently demonstrated that high-resolution computed tomography of the lung can facilitate the identification of certain lung pathologies well before the onset of symptoms and irreversible damage has occurred.7 Integrating these data, we are learning about multiple subphenotypes of COPD that appear to be both clinically relevant and, in the case of some clusters, associated with known genetic variants.8 Revealing the genetic and physical characteristics underlying various subtypes of COPD may enable the development of targeted, personalized, and more effective early-stage therapeutic interventions to preempt chronic disease.
Likewise, the blood research community has improved the longevity and quality of life of patients living with hematologic disorders and ensured the safety of the donated blood supply. With the advent of rapid, accurate tests to detect communicable blood diseases, blood transfusions are safe for patients. Research in blood systems has also established an understanding of vascular trauma caused by sepsis, malaria, and traumatic injury. Landmark clinical trials established penicillin, blood transfusions, and hydroxyurea as mainstay therapeutic options for the management of SCD,9 mitigating devastating complications of this disease. Today, with the discovery of genetic regulators of fetal hemoglobin such as BCL11a, gene-editing techniques to correct the SCD-causing mutation, and new approaches to hematopoietic stem cell transplantation, we are on the precipice of breakthroughs offering new therapeutic options or even a cure for SCD.
Despite such major advances, cardiovascular and respiratory diseases still pose a major health and economic burden, accounting for 41% of all deaths and 3 of the 4 leading causes of death in the United States.10,11 Significant health disparities exist across racial/ethnic, age, sex, socioeconomic, and geographic groups in diagnosis, prevention, and treatment.12 Substantial lags exist in the translation of research findings into products and practices that could benefit population health.13 There are, however, many exciting potential approaches to overcoming these challenges, including the development of rigorous dissemination and implementation research approaches to shorten the evidence-to-practice lag in clinical and public health settings; the identification of novel multilevel strategies for improving care in underserved vulnerable communities; advances in reparative biology, gene therapy, and nanotechnology; the expanding application of computational power to perform systems biology analysis; and the implementation of electronic medical records to facilitate the delivery of personalized, predictive, and preemptive clinical care. As public stewards for advancing biomedical research, the NHLBI recognizes the need to be forward looking and proactively leverage these scientific advances, and support the development of others, as ways to tackle challenges and significantly accelerate the pace of research translation for maximum patient-care impact.
Charting our future together: the NHLBI’s Strategic Visioning process
Through a robust and mission-driven portfolio of research, training, and health education programs, the NHLBI community has established the foundation of research excellence that continues to turn discovery science into improved health. The NHLBI Strategic Visioning process will begin the launch of a new research agenda for the next decade by identifying bold and mission-critical questions and challenges in basic, clinical, translational, and population science related to HLBS disorders. We invite you to chart this future with us through a Strategic Visioning process that is open, inclusive, and iterative and draws upon your creativity. This process is designed to build on our existing Strategic Plan14 by inviting the entire NHLBI community to share their ideas for addressing future research and workforce needs.
Provide your perspective and expertise
A major innovative feature of the Strategic Visioning process is the active, iterative, and grassroots engagement of the NHLBI community to identify bold and compelling scientific questions that NHLBI needs to address in order to promote HLBS health of all individuals. As accountable stewards of the public’s investment, we are striving for an inclusive process engaging a broad circle of partners, including scientists, medical professionals, policymakers, patients and patient advocates, professional groups, and the general public. Exercise your imagination and apply your expertise through this Strategic Visioning process (think big, bold, and creative ideas) and know that your voice, and the voices of your colleagues, will be heard. With the benefit of these diverse perspectives that provide insights, solutions, and concerns, the NHLBI will be well positioned to understand the needs of the NHLBI community as it plans for future institute-initiated activities.
The NHLBI’s mission-driven Strategic Goals (Table 1) broadly address basic, clinical, population, and public health research in HLBS systems in health and disease, expedite translational research, and promote the development of a biomedical workforce with skills and tools to pursue these goals, all to advance knowledge that enhances the prevention, treatments, and cures of HLBS disorders. The ideas submitted should be related to the 4 Strategic Goals and identify important gaps in knowledge (referred to as Compelling Questions [CQs]) or new opportunities to overcome major barriers to progress (referred to as Critical Challenges [CCs]) that require facilitation by the NHLBI.
The Strategic Goals supporting the NHLBI mission encompass understanding human health and disease, translating basic research into preventive and therapeutic interventions, and developing a biomedical workforce with the necessary skills and tools for HLBS research
| Goal 1: promote human health | To expand knowledge of the molecular and physiological mechanisms governing the normal function of HLBS systems as essential elements for sustaining human health |
| Goal 2: reduce human disease | To extend our knowledge of the pathobiology of HLBS disorders and enable clinical investigations that advance the prediction, prevention, preemption, treatment, and cures of human diseases |
| Goal 3: advance translational research | To facilitate innovation and accelerate research translation, knowledge dissemination, and implementation science that enhances public health |
| Goal 4: develop workforce and resources | To develop and enable a diverse biomedical workforce equipped with the essential skills and research resources to pursue emerging opportunities in science |
| Goal 1: promote human health | To expand knowledge of the molecular and physiological mechanisms governing the normal function of HLBS systems as essential elements for sustaining human health |
| Goal 2: reduce human disease | To extend our knowledge of the pathobiology of HLBS disorders and enable clinical investigations that advance the prediction, prevention, preemption, treatment, and cures of human diseases |
| Goal 3: advance translational research | To facilitate innovation and accelerate research translation, knowledge dissemination, and implementation science that enhances public health |
| Goal 4: develop workforce and resources | To develop and enable a diverse biomedical workforce equipped with the essential skills and research resources to pursue emerging opportunities in science |
The NHLBI has released a Strategic Visioning Framework that outlines this process in more detail and invites the community to participate.15 The community can now submit CQs and CCs over the coming months using an interactive feature on a new Web site specially dedicated to the Strategic Visioning process (http://strategicvisioning.nhlbi.nih.gov). These questions and challenges will then be reviewed, refined, synthesized, and assessed against the current NHLBI research portfolio to help shape the Draft Strategic Visioning Scientific Priorities, which will be released for public comment in mid-2015. Using public comments and key prioritization considerations, the NHLBI, working with its scientific advisory groups, will prioritize the CQs and CCs within each Strategic Goal. This process will result in the Strategic Visioning Scientific Priorities, which will be released in their final form to the public by early 2016 and will describe the most pressing knowledge gaps and exciting prospects for advances in HLBS health.
The strategic priorities that emerge from this process will serve as a “living GPS guide” for NHLBI activities for the future. Together, let us design the path that will lead to a world with dramatically improved prevention, diagnosis, and treatment of HLBS diseases.
Authorship
Acknowledgments: The authors thank Terrie Squadere, National Institutes of Health, who provided assistance with manuscript preparation. The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, National Institutes of Health, or the US Department of Health and Human Services.
Contribution: W.K.H., J.P.K., M.L., and G.A.M. contributed equally to the authorship of this article; N.L.C., S.C.M., Y.P., and A.P.P. contributed ideas and assisted in the authorship of the article; G.H.G. created the initial topic generation and had final approval of the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: W. Keith Hoots, Director, Division of Blood Diseases and Resources, National Heart, Lung, and Blood Institute, National Institutes of Health, 6701 Rockledge Dr, Room 9136, Bethesda, MD 20892-7950; e-mail: hootswk@nhlbi.nih.gov.