Key Points
Elderly patients with myeloma are heterogeneous and assessment strategies are needed to define the frailty profile.
The proposed frailty score aims to better assess patients and provide them with more suitable therapies.
Abstract
We conducted a pooled analysis of 869 individual newly diagnosed elderly patient data from 3 prospective trials. At diagnosis, a geriatric assessment had been performed. An additive scoring system (range 0-5), based on age, comorbidities, and cognitive and physical conditions, was developed to identify 3 groups: fit (score = 0, 39%), intermediate fitness (score = 1, 31%), and frail (score ≥2, 30%). The 3-year overall survival was 84% in fit, 76% in intermediate-fitness (hazard ratio [HR], 1.61; P = .042), and 57% in frail (HR, 3.57; P < .001) patients. The cumulative incidence of grade ≥3 nonhematologic adverse events at 12 months was 22.2% in fit, 26.4% in intermediate-fitness (HR, 1.23; P = .217), and 34.0% in frail (HR, 1.74; P < .001) patients. The cumulative incidence of treatment discontinuation at 12 months was 16.5% in fit, 20.8% in intermediate-fitness (HR, 1.41; P = .052), and 31.2% in frail (HR, 2.21; P < .001) patients. Our frailty score predicts mortality and the risk of toxicity in elderly myeloma patients. The International Myeloma Working group proposes this score for the measurement of frailty in designing future clinical trials. These trials are registered at www.clinicaltrials.gov as #NCT01093196 (EMN01), #NCT01190787 (26866138MMY2069), and #NCT01346787 (IST-CAR-506).
Introduction
Multiple myeloma (MM) is a neoplastic disease which predominantly affects elderly patients,1 with >60% of diagnoses and nearly 75% of deaths occurring in those over 65 years of age.2 Although novel agents have substantially improved MM outcome,3-7 patients over 70 years of age benefit less from new treatments,8 probably due to an increased treatment-related toxicity and worse biology.3,5,9-12 The well-known biologic and genetic prognostic factors, as well as age per se, are insufficient to explain this difference.13-16 The elderly population is highly heterogeneous and assessment strategies are needed to define the frailty profile. Frail patients are underrepresented in clinical trials, and the role of new drugs in these patients is relatively unknown.17 This prompted the European Medicines Agency to require postmarketing safety studies in the older population.18 To date, the choice of MM treatment is primarily based on chronologic age and performance status.19 However, among adults of the same age, physical and cognitive functions can be highly variable. In cancer patients, frailty and Comprehensive Geriatric Assessment (CGA) are being incorporated to guide treatment decisions.20 Frailty is a state of cumulative decline in many physiological systems, resulting in a diminished resistance to stressors, such as cancer and its treatment.21-23 The CGA is a multidisciplinary, interdisciplinary patient evaluation with validated tools that can contribute to definition of the frailty profile.24 In hematology, the CGA is not routinely performed because it is complex and time-consuming. The optimal tools for an appropriate geriatric assessment (GA) need to be established. Recently, 3 international guidelines have recommended the use of a GA to assess the patients’ cognitive and functional status and comorbidities in the context of clinical trials.25-27 To date, no study in MM has prospectively evaluated the predictive value of a GA, which may be more informative than age and performance status and could better discriminate between fit and frail patients.
We assessed the predictive role of a baseline GA in 869 elderly newly diagnosed MM patients, to define a frailty score and assess its impact on clinical outcome and toxicity.
Methods
Patient population and study design
The European Myeloma Network (EMN) and Gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) groups introduced a baseline GA in all trials for newly diagnosed MM patients ineligible for autologous stem cell transplantation due to age or coexisting comorbidities. Three prospective multicenter trials (EMN01-NCT01093196; 26866138MMY2069-NCT01190787; IST-CAR-506-NCT01346787) were included in this analysis.28-30 Besides Italy, the Czech Republic enrolled patients in the EMN01 study, and The Netherlands participated in the 26866138MMY2069 and IST-CAR-506 studies. Briefly, patients in the EMN01 trial were randomized to lenalidomide with either dexamethasone (Rd) or with cyclophosphamide-prednisone or with melphalan-prednisone. Patients enrolled in the 26866138MMY2069 trial received bortezomib with either prednisone or with cyclophosphamide-prednisone or with melphalan-prednisone (VMP). Patients in the IST-CAR-506 trial received carfilzomib with cyclophosphamide-dexamethasone. Inclusion and exclusion criteria are reported in supplemental Table 1 (see supplemental Data available on the Blood Web site). All patients provided written informed consent to participate in the studies, which had been approved by the institutional ethics committees. The studies were conducted in accordance with the Declaration of Helsinki and the principles of good clinical practice.
The primary objective of this analysis was to identify a simple scoring system based on geriatric parameters to predict overall survival (OS). The secondary objectives included the impact evaluation of the frailty scoring system on treatment-related toxicity and progression-free survival (PFS).
Assessment
The GA consisted of 3 tools: the Katz Activity of Daily Living (ADL), the Lawton Instrumental Activity of Daily Living (IADL), and the Charlson Comorbidity Index (CCI) (supplemental Tables 2-3). The ADL and the IADL scales were adopted to assess self-care activities, tasks of household management, and independence status (supplemental Table 2).31 The CCI estimates the number and the severity of comorbidities (supplemental Table 3).32 Performance status, β-2-microglobulin, albumin, International Staging System (ISS),33 and chromosomal abnormalities [t(4:14), t(11:14), t(14;16), del13, and del17p13] were assessed. OS was calculated from the time of treatment start until the date of death for any cause or the date the patient was last known to be alive. PFS was calculated from the time of treatment start until the date of progression, relapse, death for any cause, or the date the patient was last known to be in remission. Adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 or 4.0. Cumulative incidence of grade ≥3 hematologic and nonhematologic events and drug discontinuation were calculated from the time of treatment start until the date of first toxicity or drug discontinuation due to cause other than progression or death, which were considered as competing events. For all of the time-to-event end points, times of observation were censored on April 22, 2013.
Statistical analysis
Patients were analyzed on an intention-to-treat basis. OS and PFS were estimated using the Kaplan-Meier method, whereas cumulative incidence of grade ≥3 toxicity and treatment discontinuation were estimated accounting for competing events using the method of Gooley et al.34 The frailty score was built combining age, ADL and IADL scales, and CCI, evaluating their prognostic role on OS. To define a categorization of these variables, to simplify the score calculation and inspect the potential nonlinear relationship with OS, the variables were previously evaluated in a Cox model using restricted cubic splines transformation. The prognostic role of age, ADL, IADL, and CCI was evaluated in a Cox model including also ISS, unfavorable chromosome profile (defined as t(4;14) or t(14;16) or del17p13), performance status, and type of treatment. Variables included in the final model were identified through a backward selection based on the Akaike Information Criterion (AIC), choosing the final with the lowest AIC. The discrimination ability of the model was evaluated calculating the Harrell C statistic. Group differences according to the final classification were investigated using the Cox proportional hazard model for OS and PFS, accounting for ISS, chromosome abnormalities, type of treatment, and type of regimens, whereas the cumulative incidences of discontinuation and toxicities were calculated using the Fine and Gray model. Finally, as explorative analysis, the CHAID (CHi-squared Automatic Interaction Detector) method was used as an iterative decision tree to determine patient classification based on ISS and frailty score, according to the linear prediction of the Cox model adjusted for chromosome abnormalities and type of treatment.
Results
Cohort characteristics
The 3 trials included 869 newly diagnosed MM patients. The median age was 74 years and 46% of patients were older than 75 years (Table 1). One hundred nineteen patients (14%) had an ADL score ≤ 4, 156 (18%) an IADL score ≤ 5, and 144 (17%) a CCI ≥ 2. The most frequent comorbidities are diabetes without organ damage (13.2%), mild renal failure (7.4%), cardiopulmonary disease (10.4%), and peripheral vascular disease (5.8%). The most frequent parameters that were abnormal in ADL are those linked to the independence in bathing (19.6%), transferring (13.7%), and dressing (12.1%). Similarly, the most frequent parameters that were abnormal in IADL are those related to mode of transportation (38.0%), housekeeping (37.3%), shopping (33.9%), and laundry (31%).
Baseline patient characteristics
| . | No. of patients, N = 869 . | % of patients . | Median (IQR) . |
|---|---|---|---|
| Age, y | 74 (70-78) | ||
| ≤65 | 16 | 2 | |
| 65-74 | 451 | 52 | |
| ≥75 | 402 | 46 | |
| ≥80 | 161 | 19 | |
| Creatinine, mg/dL | 0.98 (0.80-1.22) | ||
| <2 | 802 | 92 | |
| ≥2 | 37 | 5 | |
| Missing | 30 | 3 | |
| ECOG PS | |||
| 0 | 258 | 30 | |
| 1 | 398 | 46 | |
| 2 | 166 | 19 | |
| 3 | 14 | 2 | |
| ISS | |||
| I | 239 | 28 | |
| II | 361 | 42 | |
| III | 269 | 31 | |
| Chromosome abnormalities | |||
| t(4;14) | 80 | 9 | |
| t(14;16) | 22 | 3 | |
| del17p13 | 105 | 12 | |
| Missing | 147 | 17 | |
| Unfavorable profile | 329 | 38 | |
| ADL | 6 (5-6) | ||
| >4 | 750 | 86 | |
| ≤4 | 119 | 14 | |
| IADL | 8 (6-8) | ||
| >5 | 713 | 82 | |
| ≤5 | 156 | 18 | |
| CCI | 0 (0-1) | ||
| ≤1 | 725 | 83 | |
| ≥2 | 144 | 17 | |
| Therapy | |||
| Lenalidomide-containing regimens | 659 | 76 | |
| Proteasome inhibitor-containing regimens | 210 | 24 |
| . | No. of patients, N = 869 . | % of patients . | Median (IQR) . |
|---|---|---|---|
| Age, y | 74 (70-78) | ||
| ≤65 | 16 | 2 | |
| 65-74 | 451 | 52 | |
| ≥75 | 402 | 46 | |
| ≥80 | 161 | 19 | |
| Creatinine, mg/dL | 0.98 (0.80-1.22) | ||
| <2 | 802 | 92 | |
| ≥2 | 37 | 5 | |
| Missing | 30 | 3 | |
| ECOG PS | |||
| 0 | 258 | 30 | |
| 1 | 398 | 46 | |
| 2 | 166 | 19 | |
| 3 | 14 | 2 | |
| ISS | |||
| I | 239 | 28 | |
| II | 361 | 42 | |
| III | 269 | 31 | |
| Chromosome abnormalities | |||
| t(4;14) | 80 | 9 | |
| t(14;16) | 22 | 3 | |
| del17p13 | 105 | 12 | |
| Missing | 147 | 17 | |
| Unfavorable profile | 329 | 38 | |
| ADL | 6 (5-6) | ||
| >4 | 750 | 86 | |
| ≤4 | 119 | 14 | |
| IADL | 8 (6-8) | ||
| >5 | 713 | 82 | |
| ≤5 | 156 | 18 | |
| CCI | 0 (0-1) | ||
| ≤1 | 725 | 83 | |
| ≥2 | 144 | 17 | |
| Therapy | |||
| Lenalidomide-containing regimens | 659 | 76 | |
| Proteasome inhibitor-containing regimens | 210 | 24 |
Unfavorable profile defined as t(4;14) or t(14;16) or del17p13.
ADL, Activity of Daily Living; CCI, Charlson Comorbidity Index; ECOG PS, Eastern Cooperative Oncology Group performance status; IADL, Instrumental Activity of Daily Living; IQR, interquartile range; ISS, International Staging System.
Identification of prognostic variables and frailty score development
For all geriatric components, no strong evidence of linear association was found and their impact on OS was explored using the recorded categorical variables (supplemental Figure 1). Advanced age, functional decline on ADL and IADL, and the presence of comorbidities showed a trend toward a progressive worsening of OS (supplemental Figure 1A). Their impact on OS was investigated in a multivariate Cox regression model (supplemental Figure 1B). A reduced OS was observed in patients aged 75 to 80 years (hazard ratio [HR], 1.35) and was more pronounced in those >80 years (HR, 2.68), in those with ADL ≤ 4 (HR, 1.58) and IADL ≤ 5 (HR, 1.81), and in patients with CCI ≥2 (HR, 1.58). No difference was found with respect to reference category for ADL 5, IADL 6-7, and CCI 1 (P > .500). The final stratification of variables was defined according to the following cutoff: ADL (>4, ≤4), IADL (>5, ≤5), and CCI (<2, ≥2). After the backward selection, performance status was removed from the final model (with Eastern Cooperative Oncology Group performance status [ECOG PS], AIC = 1750.62; without ECOG PS, AIC = 1748.92). In a multivariate analysis, adjusted for ISS, chromosome abnormalities, and type of therapy, a higher risk of death was observed for patients aged 75 to 80 years (HR, 1.13; 95% confidence interval [CI], 0.76-2.40) and >80 years (HR, 2.4; 95% CI, 1.56-3.71), for those with an ADL score ≤ 4 (HR, 1.67; 95% CI, 1.08-2.56), an IADL ≤ 5 (HR, 1.43; 95% CI, 0.96-2.14), and a CCI ≥2 (HR, 1.37; 95% CI, 0.92-2.05) (Table 2). An additive frailty score based on the integer part of HRs (HR = 1-2, score = 1; HR >2.00, score = 2) was then calculated. By combining the risk scores (range, 0-5) for these variables, patients were stratified into 3 distinctive risk groups for OS: fit (score = 0), intermediate fitness (score = 1), and frail (score ≥ 2). Table 3 indicates the proportion of patients in each risk group, their OS, and their treatment discontinuation and toxicities requiring dose modifications. Among the 260 frail patients, 123 (47%) were older than 80 years and only 50 (19%) were categorized as frail only for age.
The final Cox regression model
| . | HR (95% CI) . | P . | Score . |
|---|---|---|---|
| Age, y | |||
| ≤75 | 1 | — | 0 |
| 76-80 | 1.13 (0.76-1.69) | .549 | 1 |
| >80 | 2.40 (1.56-3.71) | <.001 | 2 |
| ADL | |||
| >4 | 1 | — | 0 |
| ≤4 | 1.67 (1.08-2.56) | .020 | 1 |
| IADL | |||
| >5 | 1 | — | 0 |
| ≤5 | 1.43 (0.96-2.14) | .078 | 1 |
| CCI | |||
| ≤1 | 1 | — | 0 |
| ≥2 | 1.37 (0.92-2.05) | .125 | 1 |
| ISS | |||
| I | 1 | — | — |
| II | 2.37 (1.38-4.09) | .002 | — |
| III | 3.21 (1.85-5.58) | <.001 | — |
| Chromosome abnormalities | |||
| Favorable | 1 | — | — |
| Unfavorable | 1.79 (1.23-2.60) | .002 | — |
| Missing | 1.13 (0.69-1.83) | .036 | — |
| Therapy | |||
| Proteasome inhibitors | 1 | — | — |
| Lenalidomide | 0.74 (0.50-1.11) | .142 | — |
| . | HR (95% CI) . | P . | Score . |
|---|---|---|---|
| Age, y | |||
| ≤75 | 1 | — | 0 |
| 76-80 | 1.13 (0.76-1.69) | .549 | 1 |
| >80 | 2.40 (1.56-3.71) | <.001 | 2 |
| ADL | |||
| >4 | 1 | — | 0 |
| ≤4 | 1.67 (1.08-2.56) | .020 | 1 |
| IADL | |||
| >5 | 1 | — | 0 |
| ≤5 | 1.43 (0.96-2.14) | .078 | 1 |
| CCI | |||
| ≤1 | 1 | — | 0 |
| ≥2 | 1.37 (0.92-2.05) | .125 | 1 |
| ISS | |||
| I | 1 | — | — |
| II | 2.37 (1.38-4.09) | .002 | — |
| III | 3.21 (1.85-5.58) | <.001 | — |
| Chromosome abnormalities | |||
| Favorable | 1 | — | — |
| Unfavorable | 1.79 (1.23-2.60) | .002 | — |
| Missing | 1.13 (0.69-1.83) | .036 | — |
| Therapy | |||
| Proteasome inhibitors | 1 | — | — |
| Lenalidomide | 0.74 (0.50-1.11) | .142 | — |
HRs and relative risks are for OS in patients with the factors as compared with those without the factors. The model was adjusted for ISS, chromosome abnormalities, and therapy. Unfavorable profile defined as t(4;14) or t(14;16) or del17p13.
AIC = 1748.918; Harrell C index = 0.7069.
Additive total score and related rate of OS and PFS at 3 years
| Additive total score . | Patient status . | No. of patients (%) . | % (95% CI) . | Cumulative incidence at 12 mo, % . | ||
|---|---|---|---|---|---|---|
| OS . | PFS . | Treatment discontinuation . | Grade 3-4 nonhematologic AEs . | |||
| 0 | Fit | 340 (39) | 84 (78-89) | 48 (41-56) | 16 | 22 |
| 1 | Intermediate-fitness | 269 (31) | 76 (67-82) | 41 (32-49) | 21 | 26 |
| ≥2 | Frail | 260 (30) | 57 (45-68) | 33 (25-41) | 31 | 34 |
| Additive total score . | Patient status . | No. of patients (%) . | % (95% CI) . | Cumulative incidence at 12 mo, % . | ||
|---|---|---|---|---|---|---|
| OS . | PFS . | Treatment discontinuation . | Grade 3-4 nonhematologic AEs . | |||
| 0 | Fit | 340 (39) | 84 (78-89) | 48 (41-56) | 16 | 22 |
| 1 | Intermediate-fitness | 269 (31) | 76 (67-82) | 41 (32-49) | 21 | 26 |
| ≥2 | Frail | 260 (30) | 57 (45-68) | 33 (25-41) | 31 | 34 |
In the univariate Cox model, the Harrell C index = 0.6608 and the AIC = 1766.077. In the multivariate Cox model, the Harrell C index = 0.7092 and the AIC = 1743.353.
Prognostic characteristics of the frailty score
The median follow-up was 18 months (interquartile range [IQR], 11-28 months). By applying the proposed frailty score, the 3-year OS was 84% in fit, 76% in intermediate-fitness (HR, 1.61; 95% CI, 1.02-2.56; P = .042), and 57% in frail (HR, 3.57; 95% CI, 2.37-5.39; P < .001) patients (Figure 1A). In the multivariate analysis, frailty profiles were associated with a shorter OS, regardless of staging and treatment administered (Table 4). One hundred forty-three (16%) of the 869 patients died, 34 (10%) in the fit, 39 (14%) in the intermediate-fitness, and 70 (27%) in the frail group. The causes of death were: disease progression [18 (5%) in fit, 22 (8%) in intermediate-fitness, and 35 (13%) in frail patients] and toxicity [11 (3%), 10 (4%), and 21 (8%), respectively]. The higher risk of death for disease progression, especially in frail patients, was related with the lower dose intensity as a consequence of the higher rate of drug discontinuation and/or dose reduction (supplemental Table 4). The most frequent reasons of toxicity-related death were cardiac events [3 (1%) in fit, 2 (1%) in intermediate-fitness, 11 (4%) in frail patients] and infections [2 (1%), 2 (1%), and 8 (3%), respectively]. The prognostic impact of the frailty profile on OS was similar in different subgroups defined by ISS, chromosomal abnormalities, type of treatment (lenalidomide-based and proteasome inhibitor-based therapies), and type of regimens (doublet and triplet regimens) (supplemental Figure 2).
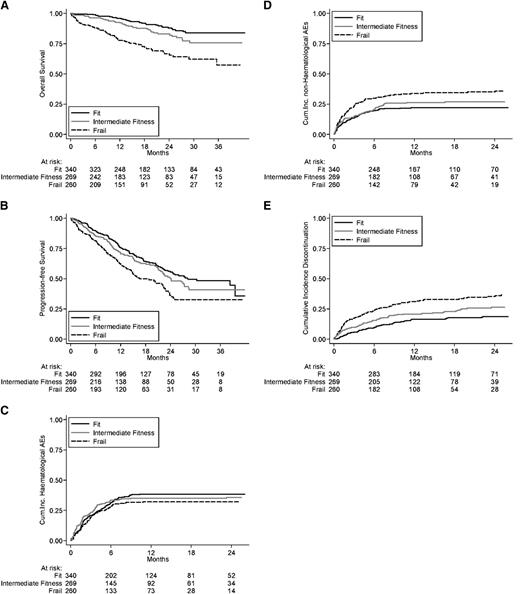
Long-term outcomes. (A) OS, (B) PFS, and (C) cumulative incidence of hematologic adverse events, (D) nonhematologic adverse events, and (E) discontinuation in the intention-to-treat population.
Long-term outcomes. (A) OS, (B) PFS, and (C) cumulative incidence of hematologic adverse events, (D) nonhematologic adverse events, and (E) discontinuation in the intention-to-treat population.
Univariate and multivariate analysis of the impact of the frailty profile of patients on OS, PFS, discontinuation rate, and incidence of grade 3 or higher toxicity
| . | OS . | PFS . | Discontinuation . | Grade ≥3 hematologic toxicity . | Grade ≥3 nonhematologic toxicity . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Crude | ||||||||||
| Fit | 1 | — | 1 | — | 1 | — | 1 | — | 1 | — |
| Intermediate fitness | 1.61 (1.02-2.56) | .042 | 1.18 (0.91-1.53) | .211 | 1.48 (1.05-2.10) | .026 | 0.97 (0.74-1.27) | .808 | 1.23 (0.89-1.71) | .217 |
| Frail | 3.57 (2.37-5.39) | <.001 | 1.68 (1.31-2.15) | <.001 | 2.27 (1.64-3.14) | <.001 | 0.83 (0.62-1.09) | .181 | 1.74 (1.28-2.38) | <.001 |
| Adjusted* | ||||||||||
| Fit | 1 | — | 1 | — | 1 | — | 1 | — | 1 | — |
| Intermediate fitness | 1.37 (0.86-2.18) | .181 | 1.08 (0.83-1.40) | .583 | 1.41 (1.00-2.01) | .052 | 0.97 (0.74-1.28) | .831 | 1.13 (0.81-1.58) | .462 |
| Frail | 2.88 (1.88-4.40) | <.001 | 1.48 (1.15-1.92) | .003 | 2.21 (1.57-3.09) | <.001 | 0.94 (0.71-1.26) | .698 | 1.57 (1.12-2.19) | .008 |
| . | OS . | PFS . | Discontinuation . | Grade ≥3 hematologic toxicity . | Grade ≥3 nonhematologic toxicity . | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Crude | ||||||||||
| Fit | 1 | — | 1 | — | 1 | — | 1 | — | 1 | — |
| Intermediate fitness | 1.61 (1.02-2.56) | .042 | 1.18 (0.91-1.53) | .211 | 1.48 (1.05-2.10) | .026 | 0.97 (0.74-1.27) | .808 | 1.23 (0.89-1.71) | .217 |
| Frail | 3.57 (2.37-5.39) | <.001 | 1.68 (1.31-2.15) | <.001 | 2.27 (1.64-3.14) | <.001 | 0.83 (0.62-1.09) | .181 | 1.74 (1.28-2.38) | <.001 |
| Adjusted* | ||||||||||
| Fit | 1 | — | 1 | — | 1 | — | 1 | — | 1 | — |
| Intermediate fitness | 1.37 (0.86-2.18) | .181 | 1.08 (0.83-1.40) | .583 | 1.41 (1.00-2.01) | .052 | 0.97 (0.74-1.28) | .831 | 1.13 (0.81-1.58) | .462 |
| Frail | 2.88 (1.88-4.40) | <.001 | 1.48 (1.15-1.92) | .003 | 2.21 (1.57-3.09) | <.001 | 0.94 (0.71-1.26) | .698 | 1.57 (1.12-2.19) | .008 |
Adjusted for ISS, chromosome abnormalities, and therapy.
By applying the proposed frailty score, the 3-year PFS was 48% in fit, 41% in intermediate-fitness (HR, 1.18; 95% CI, 0.91-1.53; P = .211), and 33% in frail (HR, 1.68; 95% CI, 1.31-2.15; P < .001) patients (Figure 1B). These data were confirmed in a Cox model (Table 4).
Grade ≥3 hematologic AEs were documented in 130 (38%) fit, 94 (35%) intermediate-fitness, and 79 (30%) frail patients. The cumulative incidences of grade ≥3 hematologic AEs were 24.1% in fit, 29.3% in intermediate-fitness, and 23.2% in frail patients at 4 months, and 38.4% in fit, 35.1% in intermediate-fitness, and 32.2% in frail patients at 12 months (Figure 1C). The risk of grade ≥3 hematologic AE was not significantly higher in intermediate-fitness (HR, 0.97; 95% CI, 0.74-1-28; P = .831) and in frail (HR, 0.94; 95% CI, 0.71-1.26; P = .698) patients compared with fit ones (Table 4).
Grade ≥3 nonhematologic AEs were reported in 62 (18%) fit, 60 (22%) intermediate-fitness, and 77 (30%) frail patients. The cumulative incidences of nonhematologic grade ≥3 AEs were 16.6% in fit, 16.7% in intermediate-fitness, and 26.5% in frail patients at 4 months, and 22.2% in fit, 26.4% in intermediate-fitness, and 34.0% in frail patients at 12 months (Figure 1D, Table 3). The risk of grade ≥3 nonhematologic AE was slightly increased in intermediate-fitness (HR, 1.13; 95% CI, 0.81-1.58; P = .462) and significantly increased in frail patients (HR, 1.57; 95% CI, 1.12-2.19; P = .008) compared with fit ones (Table 4).
Drug discontinuation for any cause, excluding progression and death, was reported in 58 (17%) fit, 58 (22%) intermediate-fitness, and 66 (25%) frail patients. The cumulative incidence of treatment discontinuation was 7.4% in fit, 11.9% in intermediate-fitness, and 19.2% in frail patients at 4 months, and 16.5% in fit, 20.8% in intermediate-fitness, and 31.2% in frail patients at 12 months (Figure 1E, Table 3). The risk of drug discontinuation was significantly higher in intermediate-fitness (HR, 1.41; 95% CI, 1.00-2.01; P = .052) and in frail (HR, 2.21; 95% CI, 1.57-3.09; P < .001) patients compared with fit ones (Table 4).
Integration of the frailty score into the ISS
Combining the frailty score with the ISS stage, 6 groups were identified: (1) 128 (14.7%) fit patients with ISS stage I; (2) 212 (24.4%) fit patients with ISS stage II or III; (3) 177 (20.4%) intermediate-fitness patients with ISS stage I or II; (4) 92 (10.6%) intermediate-fitness patients with ISS stage III; (5) 161 (18.5%) frail patients with ISS stage I or II; and (6) 99 (11.4%) frail patients with ISS stage III (supplemental Figure 3). Survival curves for these 6 categories are shown in supplemental Figure 4: 11% were considered very-high-risk patients, with a 3-year OS of 55%; 19% were high-risk patients, with a 3-year OS of 61%; 55% were considered intermediate-risk patients, with a 3-year OS of 75% to 77%; and 15% were low-risk patients, with a 3-year OS of 94%.
Discussion
This analysis showed that a frailty score that combines age, functional status, and comorbidities can predict survival and toxicity, and is useful to determine the feasibility of a treatment regimen. The frailty profile was associated with an increased risk of death, progression, nonhematologic AEs, and treatment discontinuation, regardless of ISS stage, chromosome abnormalities, and type of treatment.
The global population is rapidly aging, the number of people >80 years is expected to quadruple between 2000 and 2050.35 The standard approved treatments for newly diagnosed elderly MM include nine 6-week cycles of VMP with twice-weekly bortezomib and twelve 6-week cycles of melphalan-prednisone-thalidomide (MPT) with 200 mg per day thalidomide. No changes in dose and schedule are approved according to age or performance status. Unfortunately, these standard schedules induced a high rate of grade 3-4 nonhematologic AEs (91% with VMP and 75% with MPT),3,9 with survival benefit inferior in patients >75 years. Most clinical trials include fit patients, whereas the majority of frail patients are excluded. In these studies, ∼10% of patients are >75 years of age. In contrast, ∼40% of patients who receive treatment of malignancies are frail.18
In a community-based population randomized phase 3b study comparing VMP with bortezomib-thalidomide-dexamethasone and bortezomib-dexamethasone,36 no difference was detected between doublet and triplet regimens. Similarly, continuous treatment with oral Rd significantly improved outcome and reduced the toxicities compared with the standard MPT.6 These data indirectly suggest that, when frail patients are adequately represented in clinical trials, doublet regimens can be as effective as triplet combinations with a better safety profile.
Although this analysis is based on patients enrolled in clinical trials, the less strict inclusion/exclusion criteria allowed 30% of frail patients to be treated. In our analysis, the 3-year OS rate was 84% in fit, 76% in intermediate-fitness, and 57% in frail patients. The OS for fit patients compares favorably with the standard treatments3,4 ; similarly, the survival of frail patients is comparable to that of the community-based population previously reported.36 A significantly higher cumulative incidence of nonhematologic toxicities and drug discontinuation was reported in frail compared with fit patients, and severe nonhematologic AEs and drug discontinuation induced a shorter survival.12 Unexpectedly, the performance status did not affect OS, whereas the frailty status increased the risk of death by approximately threefold, thus confirming the need for a more sophisticated evaluation of elderly patients before starting therapy. Our findings suggest that the cutoff age of 80 years instead of 75 years should be used for the definition of frail conditions. Indeed, the risk of death is only slightly increased in patients 75 to 80 years of age, whereas it is 2.4 times higher in patients >80 years. Besides age, the most common reasons for an increase in frailty were losing independence in self-care activities, household management, and transferring/transportation.
By combining the frailty score with the established ISS, the 3-year OS rate was 55% in the frail-ISS 3 group, and 94% in the fit-ISS 1 group. The combination of these 2 independent parameters significantly improved the prognostic value of the single ones, therefore, this is an important strategy in the future for predicting outcome.
Chronologic age, performance status, and physician’s clinical judgment are not sufficient to characterize the frail population. The GA is a more sensitive predictor of clinical outcomes, and the proposed score may be adopted as a valid new standard to evaluate patients’ frailty. It could be used in everyday clinical practice as well as in the context of research to ensure an adequate representation of elderly patients and to allow more precise cross-trial comparisons. Although evidence-based GA-tailored treatments are still lacking, fit patients could receive full-dose, triplet therapies or even more intensive approach including stem cell transplant. Intermediate-fitness patients may benefit from doublet treatments or less intense triplets.37 Frail patients could benefit from a gentler, reduced-dose doublet approach or even a palliative/supportive treatment because the benefits of low toxicity on survival should be considered carefully, especially in the very frail. Future trials comparing full-dose therapy and adjusted schedules in elderly patients will support these recommendations and validate our approach.
The GA is a time and manpower-consuming procedure. To overcome this limitation, an information and communication technology (ICT) application for computers may significantly reduce the time required to perform the GA to only 5 to 7 minutes. Of note, the time invested in this procedure should be balanced against the advantage of reducing the subsequent risk of severe AEs by approximately one-third.
The strength of this analysis lies in the large, broadly representative and fairly homogenous set of data provided by 72 European institutions. The applicability of the frailty score in a multicenter setting is a prerequisite for its use in the clinical practice. Furthermore, the GA was prospectively obtained prior to initiation of chemotherapy and reflects the patient’s baseline health rather than the toxicities induced by the therapy.
The major limitation of this study is the absence of an independent validation cohort of patients because the GA is not routinely performed in an external cohort of patients, and our sample size is inadequate for an internal validation. The presence of patients exclusively enrolled in experimental trials may be another limitation, yet this allows more homogeneous treatment, thus avoiding the bias of different treatments. Furthermore, although population-based data also include the most frail patients and consequently may give the opportunity to investigate the role of frailty in the population, such databases typically lack the level of detail captured in clinical trials, limiting the possibility to conduct a risk factor analysis.
In summary, this study supports the systematic, prospective use of a GA as important additional tool in the clinical evaluation. Our findings point out some relevant issues of patients’ functional and health status that have a prognostic importance similar to that of myeloma-related risk factors, such as ISS and chromosomal abnormalities. Prospective studies to validate our findings as well as a unique score reflecting both the reserve capacity of patients and established disease-specific risk factors are needed to provide comprehensive algorithms for therapeutic decision-making.
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all the patients who participated in the source studies, the nurses Verbale Michela and Puccio Loredana, the data managers Antonella Fiorillo, Jessica Mastrovito and Marta Santoro, and the editorial assistant Giorgio Schirripa from Torino.
The study was promoted by Fondazione Neoplasie Sangue Onlus, but no funding was received for this study. The promoter had no role in the design and conduct of the study, interpretation of the data, or review and approval of the manuscript.
Authorship
Contribution: All of the International Myeloma Working Group (IMWG) authors, M.-V.M., T.F., S.K.K., P. McCarthy, S.L., S.Z., E.T., A.B., R.H., H.L., A.K.S., P. Moreau, K.A., H.E., B.G.M.D., M.A.D., O.L., J.F.S.M., P.R., P.S., and S.V.R., in collaboration with the Italian authors, A.P., S.B., A.L., M.O., A.E., and P. Musto, had full access to all of the data in the study, designed the analyses, interpreted the data, made comments and suggestions to improve the manuscript, approved the final manuscript, and take responsibility for the integrity of the data and the accuracy of the data analysis; A.P., S.B., and A.L. supervised the study; A.P., S.B., A.L., M.O., S.Z., P. Musto, R.H., and P.S. provided study material and data; S.B. and A.L. collected and assembled the data; A.E. performed the statistical analysis; and A.P., S.B., and A.L. wrote the first draft of the manuscript.
Conflict-of-interest disclosure: A.P. has received honoraria from Amgen, Array BioPharma, Bristol-Myers Squibb, Genmab A/S, Celgene, Janssen-Cilag, Millennium Pharmaceuticals Inc, Onyx Pharmaceuticals, and Sanofi Aventis, and consultancy fees from Amgen, Bristol-Myers Squibb, Genmab A/S, Celgene, Janssen-Cilag, Millennium Pharmaceuticals Inc, and Onyx Pharmaceuticals. S.B. has received honoraria from Celgene, Janssen-Cilag, and Novartis, consultancy fees from Onyx, and has served on the advisory committee of Merck Sharp & Dohme. M.-V.M. has received honoraria from Celgene and Janssen-Cilag, and served on the speakers bureau of Celgene, Millennium, and Ortho-Biotech. A.L. has received honoraria from Celgene and Janssen-Cilag. T.F. has received honoraria from Celgene and Janssen. S.K.K. has received institutional clinical trial funding from Celgene, Novartis, Onyx, Millennium, Cephalon, Merck, and Abbott. M.O. has received honoraria from Celgene and Janssen. P. McCarthy has served on the advisory board and received honoraria from Celgene, Millennium, and Janssen. S.L. is a consultant for Millennium, Celgene, Novartis, BMS, Onyx, and Janssen. S.Z. has served on the advisory board and received a research grant from Celgene, Janssen-Cilag, and Millennium. P. Musto has received research funds from Celgene and honoraria from Celgene, Janssen, Sanofi, and Novartis. E.T. has received honoraria from Novartis, Amgen, Celgene, and Onyx, advisory fees from Amgen, has been on the Steering Committee of Amgen and Janssen-Cilag, and received educational grants from Amgen, Janssen-Cilag, and Celgene. R.H. has received consultancy fees from Celgene, Janssen, and Merck. A.K.S. has received consultancy fees and honoraria from Celgene, Array Pharmaceuticals, Millennium Pharmaceuticals, and Novartis. P. Moreau has served on the advisory board of Janssen, Millennium, Onyx, and Celgene, and received honoraria from Janssen, Celgene, and Mundipharma. K.A. has served on the advisory board of Millennium, Gilead, Sanofi Aventis, and Onyx. H.E. has received consultancy fees and honoraria from Celgene and Janssen-Cilag. B.G.M.D. has received honoraria from Celgene. M.A.D. has received honoraria from Celgene, Ortho-Biotech, and Onyx. J.F.S.M. has received consultancy fees and honoraria from Janssen-Cilag, Millennium Pharmaceuticals, Celgene, Onyx Pharmaceuticals, and Novartis. P.R. is a member of the advisory board for Celgene Corporation. P.S. has received research support from Celgene, Janssen, Onyx, and Millennium. The remaining authors declare no competing financial interests.
A complete list of the members of the International Myeloma Working Group appears in the online data supplement.
Correspondence: Antonio Palumbo, Dipartimento di Oncologia ed Ematologia, Azienda Ospedaliera Città della Salute e della Scienza di Torino, San Giovanni Battista, Via Genova, 3-10126 Torino, Italy; e-mail: appalumbo@yahoo.com.