Key Points
Baseline hemoglobin levels lower than 7 g/dL, acute anemia, and extracranial internal carotid stenosis are significant and independent risk factors for SCI in SCA.
Abstract
Early transcranial Doppler (TCD) screening of the Créteil sickle cell anemia (SCA)-newborn cohort, and rapid initiation of transfusion programs, resulted in successful prevention of overt strokes, but a high cumulative risk of silent cerebral infarcts (SCI) remained, suggesting that TCD screening does not identify all patients with SCA at risk for SCI. We hypothesized that episodes of hypoperfusion/hypoxia, as observed during acute chest syndromes or acute anemic events (AAE), and extracranial internal carotid artery (eICA) stenoses, detectable via submandibular Doppler sonography and cervical magnetic resonance angiography (MRA), could also be risk factors for SCI. This study includes 189 stroke-free patients with SCA from the Créteil newborn cohort (1992-2010) followed longitudinally by magnetic resonance imaging/MRA, including cervical MRA at the last assessment. All patients with abnormal TCD and/or intracranial stenoses were placed on a transfusion program. Mean follow-up was 9.9 years (range, 2.2-19.9 years; 1844 patient-years). Annual rates of clinical events were calculated. The cumulative risk for SCI was 39.1% (95% confidence interval [CI], 23.5%-54.7%) by age 18 years, with no plateau. We confirm that baseline hemoglobin level lower than 7 g/dL before age 3 years is a highly significant predictive risk factor for SCI (hazard ratio, 2.97; 95% CI, 1.43-6.17; P = .004). Furthermore, we show that AAE rate (odds ratio, 2.64 per unit increase; 95% CI, 1.09-6.38; P = .031) and isolated eICA stenosis (odds ratio, 3.19; 95% CI, 1.18-8.70; P = .023) are significant and independent risk factors for SCI.
Introduction
Strokes are an early and devastating complication in patients with SCA.1 They are most often associated with arterial stenoses of the large arteries of the Circle of Willis, which can be detected early by nonimaging transcranial Doppler (TCD)2 or by TCD imaging (TCDI),3,4 allowing primary stroke prevention through the initiation of chronic transfusions.5 We previously reported in the longitudinal Créteil Newborn Cohort the benefits of screening patients early with TCD and promptly initiating a transfusion program in patients with abnormal intracranial velocities, as the risk for stroke by age 18 years was significantly decreased to only 1.9%6 from the previously reported 11%.1,7 However, despite successful prevention of overt strokes, a high cumulative risk for silent cerebral infarcts (SCI) was still observed in this cohort by age 14 years (37.1%; 95% confidence interval [CI], 26.3%-50.7%),6 especially in patients with no history of abnormal TCD and no transfusion program, suggesting that TCD screening does not detect all patients with SCA at risk for SCI and highlighting the need to identify additional risk factors for better prevention of cerebrovascular disease in SCA.
Most SCI are already present by 6 years of age,8-12 and even as soon as 2 years of age, as the Pediatric Hydroxyurea Phase 3 Clinical Trial (BABY HUG) study reported the presence of SCI (13%) at the mean age of 13.7 months.13 However, we showed in the Créteil Newborn Cohort that the cumulative incidence for SCI did not reach “a plateau,”6 indicating SCI can also occur later.
Several risk factors for SCI have already been identified, which include low pain event rate, history of seizure, leukocyte count higher than 11.8 × 109/L, and the SEN βS globin gene haplotype (Cooperative Study of Sickle Cell Disease cohort),9 low baseline hemoglobin level (Créteil6 and Philadelphia cohorts,12 and the Silent Infarct Transfusion [SIT] Trial),14,15 and the presence of intracranial stenosis (Créteil6,16 and Philadelphia cohorts12 ).
Since then, 2 important findings have been reported: first, acute anemic events (AAE), defined as an hemoglobin concentration of 5.5 g/dL or less, regardless of etiology, with at least a 30% decrease from the patient’s clinically established baseline,17 may be responsible for acute silent cerebral ischemic events, which are identified using diffusion-weighted magnetic resonance imaging (MRI) and can be reversible or result in SCI,17,18 suggesting that the rate of AAE could be a risk factor for SCI in patients with SCA. Second, despite the apparently predominant involvement of the Circle of Willis in SCA cerebral vasculopathy, the extracranial portion of the internal carotid artery (eICA) can be the site of stenosis and/or occlusion and is also responsible for overt strokes and SCI.19-23 Contrary to intracranial stenosis in the Circle of Willis, detectable by TCD via a temporal window and by intracranial magnetic resonance angiography (MRA), assessing eICA requires using a new submandibular approach21 and cervical MRA.19-24 We recently reported in 2 large cohorts of stroke-free SCA children that eICA velocities of 160 cm/s or higher were highly predictive of eICA stenosis.24,25
We hypothesized that in addition to low baseline hemoglobin level, the rate of AAE and the presence of eICA stenosis could be additional risk factors for SCI. Thus, to determine the association between eICA stenosis and SCI, we chose to study this issue in a longitudinal cohort, the Créteil Newborn Cohort, as patients are systematically assessed by MRI/MRA.
Patients and methods
The present study includes overt stroke-free patients from the SCA-Créteil cohort (patients with homozygous sickle cell anemia, sickle cell/β0-thalassemia, sickle-cell/D-Punjab, and sickle-cell/O-Arab), born between November 1992 and December 2010, regularly followed at the Créteil-SCD-center from November 1992 to December 2013 until the age of 18 to 20 years. Patients with SC and sickle cell/β+-thalassemia were excluded from this study, as were allografted patients and those specifically referred to the center because of cerebral vasculopathy. Patients were clinically evaluated every 3 months and had a complete check-up every year.
TCDI via temporal window was systematically performed once a year from the age of 12 to 18 months. TCDI3,4 data, recorded without angle correction, were classified according to the Stroke Prevention Trial in Sickle Cell Anemia (STOP) study as normal (time-averaged mean of the maximum velocity < 170 cm/s), conditional (170-199 cm/s), abnormal (≥200 cm/s), or inadequate (unavailable temporal windows). Any patient with conditional TCDI was then evaluated every 3 months. Moreover, since June 2011, all patients with SCA had eICA assessment via a submandibular approach with the same low-frequency (2 Mhz) TCDI transducer, placed below the angle of the jaw and aimed cephalad.24
Patients underwent cerebral MRI/MRA every 2 years after age 5 years to avoid the need for sedation or after 2 transfusions for those requiring a transfusion program. MRI/MRA protocol included fluid-attenuated inversion recovery, T1, T2, diffusion-weighted sequences and 3-dimensional time-of-flight angiography of the Circle of Willis, and the same expert (S.V.) reviewed all MRI/MRA imaging. Since June 2011, an additional 3-dimensional time-of-flight multislab MRA (noncontrasted) sequence exploring extracranial ICA, and carotid bifurcations were systematically added to the routine procedure. Before analysis of the data, stenosis was defined by MRA as at least 20% decrease in the lumen of middle cerebral artery, anterior cerebral artery, intracranial ICA, and eICA. Evidence of ischemic lesions of at least 3 mm by MRI in patients without a history of cerebrovascular events was considered SCI.
Hemoglobin levels were measured the same day as the TCDI assessment. In case of unusually low hemoglobin, as observed during acute events such as splenic sequestrations or erythroblastopenia, the patient was treated with a single transfusion, and the TCDI was then repeated 3 months later. Patients with abnormal TCDI without acute event and those with stenosis were placed on transfusion program.
α-genes, β-globin haplotypes, and glucose-6-phosphate dehydrogenase (G6PD) enzymatic activity were determined. Baseline blood parameters were recorded (Table 1) between 1 and 3 years of age a minimum of 3 months away from a transfusion, 1 month from a painful episode, and before any intensive therapy (hydroxyurea [HU], chronic transfusion program ≥4 months, or stem cell transplantation).
Patients’ clinical and biological characteristics
| Characteristics . | SCA patients (n = 189) . |
|---|---|
| Sex | |
| Female | 101 (53.4%) |
| Male | 88 (46.6%) |
| G6PD | |
| Deficiency | 18 (10.6%) |
| Normal | 152 (88.8%) |
| α genes | |
| 2 | 15 (8.3%) |
| 3 | 61 (33.9%) |
| 4 | 102 (56.7%) |
| 5 | 2 (1.1%) |
| α-thalassemia | |
| Absent | 104 (57.8%) |
| Present | 76 (42.2%) |
| β haplotype | |
| Car/Car | 65 (39.6%) |
| Ben/Ben | 33 (20.1%) |
| Sen/Sen | 21 (12.8%) |
| Others | 45 (27.4%) |
| Parameters at baseline, n (median [Q1-Q3]) | |
| White blood cell count, 109/L | 171 (13.900 [11.400-17.200]) |
| Neutrophil count, 109/L | 164 (5.075 [4.015-7.372]) |
| Platelet count, 109/L | 169 (337.000 [269.500-438.000]) |
| Hemoglobin level, g/dL | 171 (8.1 [7.4-8.9]) |
| Hematocrit, % | 169 (24.6 [22.1-27.9]) |
| Mean corpuscular volume, fL | 168 (78.1 [71.6-85.1]) |
| Reticulocyte count, 109/L | 163 (273.700 [205.800-367.000]) |
| Fetal hemoglobin, % | 157 (15.0 [9.0-22.1]) |
| SpO2, % | 72 (98 [96-100]) |
| LDH, IU/L | 130 (732 [508-1028]) |
| Characteristics . | SCA patients (n = 189) . |
|---|---|
| Sex | |
| Female | 101 (53.4%) |
| Male | 88 (46.6%) |
| G6PD | |
| Deficiency | 18 (10.6%) |
| Normal | 152 (88.8%) |
| α genes | |
| 2 | 15 (8.3%) |
| 3 | 61 (33.9%) |
| 4 | 102 (56.7%) |
| 5 | 2 (1.1%) |
| α-thalassemia | |
| Absent | 104 (57.8%) |
| Present | 76 (42.2%) |
| β haplotype | |
| Car/Car | 65 (39.6%) |
| Ben/Ben | 33 (20.1%) |
| Sen/Sen | 21 (12.8%) |
| Others | 45 (27.4%) |
| Parameters at baseline, n (median [Q1-Q3]) | |
| White blood cell count, 109/L | 171 (13.900 [11.400-17.200]) |
| Neutrophil count, 109/L | 164 (5.075 [4.015-7.372]) |
| Platelet count, 109/L | 169 (337.000 [269.500-438.000]) |
| Hemoglobin level, g/dL | 171 (8.1 [7.4-8.9]) |
| Hematocrit, % | 169 (24.6 [22.1-27.9]) |
| Mean corpuscular volume, fL | 168 (78.1 [71.6-85.1]) |
| Reticulocyte count, 109/L | 163 (273.700 [205.800-367.000]) |
| Fetal hemoglobin, % | 157 (15.0 [9.0-22.1]) |
| SpO2, % | 72 (98 [96-100]) |
| LDH, IU/L | 130 (732 [508-1028]) |
Average biological parameters were obtained at baseline after the age of 12 months and before the age of 3 years, a minimum of 3 months away from a transfusion, 1 month from a painful episode, and before any intensive therapy (hydroxyurea, transfusion program, or stem cell transplantation). G6PD activity was assessed by reduction of NAD phosphate to reduced NAD phosphate, measured by UV spectrophotometry. LDH range for the institution was 135 to 225 IU/L.
Clinical (weight, height, simultaneous pulse oximetry [SpO2], blood pressure) and biological (hemoglobin/hematocrit, reticulocytes, white blood cell, neutrophils, platelets, lactate dehydrogenase [LDH], hemoglobin S percentage (HbS%), hemoglobin A percentage (HbA%), fetal hemoglobin percentage [HbF%], creatinine, and ferritin) parameters were recorded at least once a year and at the last MRI/MRA with cervical MRA assessment.
HU has been used since 1992 in patients older than 3 years experiencing frequent vasoocclusive crises (VOC) and/or acute chest syndromes (ACS),26,27 and since 2000, it has been used in patients with hemoglobin levels lower than 7 g/dL and normal TCDI.6 Moreover, in a subset of patients with normal MRA16 and an abnormal TCDI history who normalized velocities on transfusion program, HU was recommended to avoid long-term transfusion program.6 A 2-month transfusion overlap occurred at the start of HU, TCDI was controlled every 3 months, and transfusions were immediately reinstated6,16 in the case of abnormal TCDI recurrence or new evidence of stenosis. Stem cell transplantation was recommended to patients with cerebral vasculopathy or frequent VOC/ACS who had an available HLA-identical sibling.6,28
Parental written informed consent was obtained in accordance with the Declaration of Helsinki. All events requiring hospitalization (VOC, ACS, AAE; hemoglobin level ≤ 6 g/dL), regardless of etiology, and all data were prospectively and systematically collected in a clinical database. Use of the database was approved for this project by the Créteil Institutional Review Board.
Statistical analysis
Participant baseline characteristics were summarized through the use of percentages, mean (standard deviation, SD), or median, with 25th and 75th percentiles denoted Q1 to Q3. Ninety-five percent confidence intervals (95% CI) around point estimates were computed. Exact Fisher tests were used to compare proportions and Wilcoxon rank sum tests to compare continuous distributions. Birth date defined entry into the study. For Kaplan-Meier estimates of SCI, participants were censored at the date of first SCI occurrence or at the last MRI/MRA in the absence of SCI. SCI time data curves were compared across baseline groups by the Log-rank test. Cumulative numbers and rates/year of VOC, ACS, and AAE from birth to when censored were calculated.
To assess predictive risk factors for SCI among baseline variables recorded before age 3 years, we used Cox regression analysis with estimated hazard ratios and 95% CI. Association among SCI; rates/year of VOC, ACS, AAE; and concomitant variables measured on cervical MRA was assessed using logistic regression with estimated odds ratio (OR) and 95% CI.
Univariable models were fitted and all variables associated with the outcome at the 10% level were retained for introduction into a multivariable model. All statistical tests were 2-sided, with P values of .05 or less denoting statistical significance. Statistical analysis was performed with SPSS, version 22, and MedCalc (Belgium) software packages.
Results
Cohort characteristics
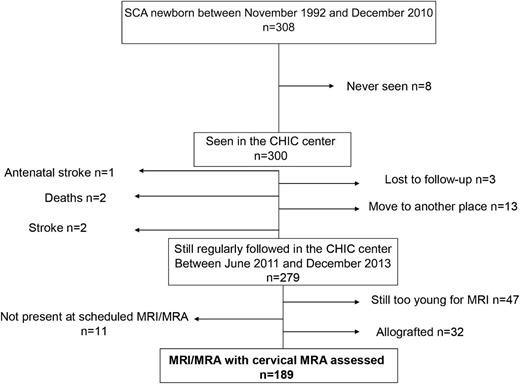
Of 308 newborns with SCA screened at birth in the Créteil area between November 1992 and December 2010 (Figure 1), 8 were never seen at the Centre Hospitalier Intercommunal de Créteil SCD Center, 1 antenatal stroke occurred, 2 had strokes, 2 deaths occurred (one of whom died of the stroke and the other of whom died during a trip to Africa), 3 were lost to follow-up, and 13 moved to another location before June 2011; thus, 279 SCA children were still followed at the Center in June 2011, with 32 successfully transplanted (not included in this study), 47 nontransfused and still too young in December 2013 to be assessed with MRI/MRA without sedation, and 11 no-show to the scheduled MRI. Therefore, 189 stroke-free patients were assessed with cerebral MRI/MRA and cervical MRA and are included in this study.
The main characteristics of the participants, grouped as SCA (n = 189: 182 sickle cell anemia, S β0-thalassemia, and 1 SD-Punjab), are summarized in Table 1. The median age at the first MRI/MRA was 5.4 years. The median number of MRI/MRA per patient was 3 (range, 1-8 scans), and 867 MRI/MRA were performed in the 189 patients. Follow-up between the first visit and the MRI/MRA with cervical MRA provided was 1844 patient-years. The median age for the MRI/MRA with cervical assessment was 8.7 years (range, 2.2-19.9 years).
The intensive therapies received by the 189 patients are described in Figure 2. The total number of patient-years was 280 on HU, 415 on transfusion program (overlap HU/ transfusion program for several patients during several months), and 1199 when no intensive therapy was given.
Patient groups according to initially applied treatment (n = 189). After a period without intensive therapy, 75 of 189 patients were placed on a transfusion program, 51 of them because of cerebral macrovasculopathy (TP1) (abnormal intracranial velocities [n = 49], intracranial stenosis [n = 2, in 1 patient with no available temporal window and in 1 patient with conditional TCD]), and 24 other patients because of frequent other complications in absence of any cerebral macrovasculopathy (TP2) (recurrent splenic sequestrations [n = 7], SCI in patients participating in the SIT Trial and randomized in the transfusion group [n = 3], frequent VOC and/or ACS and age younger than 3 years [n = 5], tricuspid regurgitant jet velocity (TRJV) higher than 2.5 m/s [n = 1], hip osteonecrosis [n = 5], and priapism [n = 3]). Thirty-one patients older than 3 years were treated with HU (n = 31) because of frequent VOC and/or ACS (n = 26) or severe anemia with baseline hemoglobin levels lower than 7 g/dL (n = 5). During follow-up, the transfusion program was stopped postsplenectomy (n = 1) and after normalization of intracranial velocities with absence of intracranial stenosis in patients refusing to take HU (n = 2). The transfusion program was replaced by HU in 31 patients after normalization of intracranial velocities and absence of intracranial (Ic) stenosis (n = 20), Ic stenosis regression (n = 1), history of frequent VOC and/or ACS in patients now older than 3 years (n = 2), postsplenectomy (n = 5), normalized TRJV (n = 1), end of SIT Trial (n = 2). However, 12 of them were placed again on a transfusion program because of HU failure to prevent VOC and/or ACS (n = 5), TRJV higher than 2.5 m/s (n = 3), abnormal TCD relapse, and/or intracranial stenosis occurrence (n = 4). At the end of follow-up (first MRI/MRA with cervical assessment), 86 patients still did not receive intensive therapy, 50 were receiving HU, and 53 were on a transfusion program.
Patient groups according to initially applied treatment (n = 189). After a period without intensive therapy, 75 of 189 patients were placed on a transfusion program, 51 of them because of cerebral macrovasculopathy (TP1) (abnormal intracranial velocities [n = 49], intracranial stenosis [n = 2, in 1 patient with no available temporal window and in 1 patient with conditional TCD]), and 24 other patients because of frequent other complications in absence of any cerebral macrovasculopathy (TP2) (recurrent splenic sequestrations [n = 7], SCI in patients participating in the SIT Trial and randomized in the transfusion group [n = 3], frequent VOC and/or ACS and age younger than 3 years [n = 5], tricuspid regurgitant jet velocity (TRJV) higher than 2.5 m/s [n = 1], hip osteonecrosis [n = 5], and priapism [n = 3]). Thirty-one patients older than 3 years were treated with HU (n = 31) because of frequent VOC and/or ACS (n = 26) or severe anemia with baseline hemoglobin levels lower than 7 g/dL (n = 5). During follow-up, the transfusion program was stopped postsplenectomy (n = 1) and after normalization of intracranial velocities with absence of intracranial stenosis in patients refusing to take HU (n = 2). The transfusion program was replaced by HU in 31 patients after normalization of intracranial velocities and absence of intracranial (Ic) stenosis (n = 20), Ic stenosis regression (n = 1), history of frequent VOC and/or ACS in patients now older than 3 years (n = 2), postsplenectomy (n = 5), normalized TRJV (n = 1), end of SIT Trial (n = 2). However, 12 of them were placed again on a transfusion program because of HU failure to prevent VOC and/or ACS (n = 5), TRJV higher than 2.5 m/s (n = 3), abnormal TCD relapse, and/or intracranial stenosis occurrence (n = 4). At the end of follow-up (first MRI/MRA with cervical assessment), 86 patients still did not receive intensive therapy, 50 were receiving HU, and 53 were on a transfusion program.
Intracranial velocities and stenosis
In this cohort, 62/189 (32.8%) had abnormal intracranial velocities at the median age of 4.1 years (range, 1.3-8.8 years). Kaplan-Meier estimates of the risk for abnormal intracranial velocities by age 18 years were 35.7% (95% CI, 28.3%-43.1%), reaching a plateau by age 9 years.
Intracranial stenosis was observed in 24/189 (12.7%) patients. Kaplan-Meier estimates of the risk for intracranial stenosis by the age of 18 years were 18.1% (95% CI, 10.9%-25.3%), reaching a plateau by the age of 11 years.
Extracranial velocities and stenosis at the MRI/MRA with cervical assessment
High abnormal eICA velocities (≥160 cm/s) were found in 35/189 patients (18.5%), who were significantly younger than those with normal eICA velocities (mean [SD], 7.3 years [3.9] vs 10.3 [4.6]; P = .01). eICA velocities were isolated (without abnormal intracranial velocities) in most patients (30/35), giving a prevalence of 15.9% (30/189). Baseline parameters, including sex, α-thalassemia, G6PD deficiency, β-haplotypes, hemoglobin, reticulocytes, leukocytes, neutrophils, platelets, mean corpuscular volume, HbF%, and LDH, were not significant predictors of abnormal eICA velocities. When analyzing parameters concomitantly recorded at the time of MRI/MRA with cervical MRA (Table 2), the presence of tortuosities (OR, 23.26; 95% CI 5.95-90.91; P < .001) and low hemoglobin level (OR, 2.20; 95% CI, 1.22 - 3.97; P = .009) were significant and independent associated risk factors for eICA velocities (≥160 cm/s) by multivariate logistic regression analysis.
Associated risk factors for isolated eICA of 160 cm/s or more and eICA stenosis (logistic regression analysis)
| Concomitant parameters . | Associated risk factors . | |||
|---|---|---|---|---|
| Isolated eICA ≥160 cm/sec . | Isolated eICA stenosis . | |||
| OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Univariate logistic regression analysis | ||||
| Tortuosities | 27.8 (9.1-83.3) | <.001* | 29.41 (6.45-125) | <.001* |
| Age per 1 y increase | 1.13 (1.02-1.25) | .015* | 1.05 (0.95-1.16) | .363 |
| Leukocyte count per 1 × 109/L increase | 1.14 (1.02-1.28) | .017* | 1.17 (1.04-1.32) | .010* |
| Neutrophil count per 1 × 109/L increase | 1.16 (0.99-1.36) | .051 | 1.17 (1.04-1.32) | .07 |
| Platelet count per 1 × 109/L increase | 1.001 (0.996-1.003) | .903 | 1.000 (0.996-1.004) | .895 |
| Hemoglobin level per 1 g/dL decrease | 2.19 (1.30-3.69) | .003* | 1.38 (0.87-2.19) | .169 |
| Mean corpuscular volume per fL increase | 1.03 (0.97-1.08) | .346 | 1.005 (0.947-1.067) | .868 |
| Reticulocyte count per 1 × 109/L increase | 1.003 (0.998-1.007) | .211 | 1.005 (1.000-1.010) | .051 |
| HbF per 1% increase | 1.02 (0.95-1.09) | .648 | 1.02 (0.94-1.10) | .649 |
| SpO2 per 1% increase | 1.29 (0.99-1.69) | .063 | 1.07 (0.77-1.48) | .696 |
| LDH per IU/mL increase | 30.02 (1.02-885.71) | .049* | 68.9 (1.52-3128) | .030* |
| HbS% per 1% increase | 1.030 (1.003-1.057) | .026* | 1.001 (0.979-1.023) | .962 |
| Absence of intensive therapy (HU or TP) | 5.01 (2.03-12.36) | <.001* | 2.67 (1.02-6.95) | .045* |
| Isolated eICA ≥160 cm/s | 14.49 (5.24-40) | <.001* | ||
| Multivariate analysis | ||||
| Tortuosities | 23.26 (5.95-90.91) | <.001* | 10.31 (2.51-41.67) | .001* |
| Hemoglobin level per 1 g/dL decrease | 2.20 (1.22-3.97) | .009* | ||
| Isolated eICA ≥160 cm/s | 4.39 (1.39-13.9) | .012* | ||
| Concomitant parameters . | Associated risk factors . | |||
|---|---|---|---|---|
| Isolated eICA ≥160 cm/sec . | Isolated eICA stenosis . | |||
| OR (95% CI) . | P . | OR (95% CI) . | P . | |
| Univariate logistic regression analysis | ||||
| Tortuosities | 27.8 (9.1-83.3) | <.001* | 29.41 (6.45-125) | <.001* |
| Age per 1 y increase | 1.13 (1.02-1.25) | .015* | 1.05 (0.95-1.16) | .363 |
| Leukocyte count per 1 × 109/L increase | 1.14 (1.02-1.28) | .017* | 1.17 (1.04-1.32) | .010* |
| Neutrophil count per 1 × 109/L increase | 1.16 (0.99-1.36) | .051 | 1.17 (1.04-1.32) | .07 |
| Platelet count per 1 × 109/L increase | 1.001 (0.996-1.003) | .903 | 1.000 (0.996-1.004) | .895 |
| Hemoglobin level per 1 g/dL decrease | 2.19 (1.30-3.69) | .003* | 1.38 (0.87-2.19) | .169 |
| Mean corpuscular volume per fL increase | 1.03 (0.97-1.08) | .346 | 1.005 (0.947-1.067) | .868 |
| Reticulocyte count per 1 × 109/L increase | 1.003 (0.998-1.007) | .211 | 1.005 (1.000-1.010) | .051 |
| HbF per 1% increase | 1.02 (0.95-1.09) | .648 | 1.02 (0.94-1.10) | .649 |
| SpO2 per 1% increase | 1.29 (0.99-1.69) | .063 | 1.07 (0.77-1.48) | .696 |
| LDH per IU/mL increase | 30.02 (1.02-885.71) | .049* | 68.9 (1.52-3128) | .030* |
| HbS% per 1% increase | 1.030 (1.003-1.057) | .026* | 1.001 (0.979-1.023) | .962 |
| Absence of intensive therapy (HU or TP) | 5.01 (2.03-12.36) | <.001* | 2.67 (1.02-6.95) | .045* |
| Isolated eICA ≥160 cm/s | 14.49 (5.24-40) | <.001* | ||
| Multivariate analysis | ||||
| Tortuosities | 23.26 (5.95-90.91) | <.001* | 10.31 (2.51-41.67) | .001* |
| Hemoglobin level per 1 g/dL decrease | 2.20 (1.22-3.97) | .009* | ||
| Isolated eICA ≥160 cm/s | 4.39 (1.39-13.9) | .012* | ||
Significant tests.
eICA stenoses were present in 23/189 patients (12.2%) and were highly associated with high eICA velocities (≥160 cm/s; 18/35 vs 5/154; P < .001). eICA stenosis was only associated with intracranial stenosis in 2 young patients (3.7 and 5.3 years old), giving a prevalence of isolated eICA stenosis of 11.1% (21/189). eICA stenosis was significantly more frequent in nonintensified patients (16/86; 18.6%) compared with intensified patients (P = .023; 2/50 patients receiving HU and 5/53 patients on a transfusion program).
No baseline biological parameters were significant predictive risk factors for eICA stenosis. The associations of eICA stenosis with concomitant parameters are shown in Table 2. The presence of tortuousities (OR, 10.31; 95% CI, 2.51-41.67;, P = .001) and isolated abnormal high eICA velocities (OR, 4.39; 95% CI, 1.39-13.9; P = .012) were retained as significant and independent associated risk factors for isolated eICA stenosis by multivariate logistic regression analysis.
SCI
The cumulative risk for SCI was 19.2% (95% CI, 12.6%-25.8%) by age 8 years, 32.4% (95% CI, 22.4%-42.4%) by age 14 years, and 39.1% (95% CI, 23.5%-54.7%) by age 18 years, with no plateau. At the first MRI/MRA, SCI were present in 31/189 (16.4%) patients. Seven new patients developed SCI during follow-up: one still had abnormal velocities despite a transfusion program, 2 were nonintensified but developed abnormal velocities in ICA and eICA, and 4 were receiving HU (Figure 2). Thus, 38/189 patients (20.1%) developed SCI at the median age of 6.4 years (range, 1.8-18.0 years). At the last follow-up MRI/MRA (ie, the first with cervical MRA), 34/189 still had SCI, whereas ischemic lesions were no longer visible or of a significant size for 4 of them on a transfusion program.
Predictive risk factors for SCI among baseline parameters.
No predictive risk factor for SCI was found for sex (P = .43), α-thalassemia (P = .41), G6PD deficiency (P = .16), and β-haplotype using Cox regression analyses (Tables 3 and 4).
Comparison of baseline parameters in patients with or without a history of SCI
| Characteristic . | History of SCI . | P . | |
|---|---|---|---|
| Yes (n = 38) . | No (n = 151) . | ||
| Sex, n (%) | |||
| Male (n = 88) | 21 (23.9%) | 67 (76.1%) | .276 |
| Female (n = 101) | 17 (16.8%) | 84 (83.2%) | |
| G6PD, n (%) | |||
| Normal (n = 152) | 31 (20.4%) | 121 (79.6%) | |
| Deficiency (n = 18) | 6 (33.3%) | 12 (66.7%) | .230 |
| α-thalassemia, n (%) | |||
| Present (n = 76) | 14 (18.4%) | 62 (81.6%) | |
| Absent (n = 104) | 24 (23.1%) | 80 (76.9%) | .467 |
| Parameters at baseline, n (mean [SD]) | |||
| White blood cell count, 109/L | 37 (15.1 [4.9]) | 134 (14.3 [4.8]) | NS |
| Neutrophil count, 109/L | 34 (6.5 [3.1]) | 130 (5.8 [3.2]) | NS |
| Platelet count, 109/L | 36 (363.2 [147.6]) | 133 (350.5[128.0]) | NS |
| Hemoglobin level, g/dL | 37 (7.8 [1.1]) | 134 (8.31 [1.21]) | .016* |
| Mean corpuscular volume, fL | 35 (80.7 [7.7]) | 133 (77.1 [9.5]) | .025* |
| Reticulocyte count, 109/L | 35 322.4 (105.6) | 128 (286.3 [122.8]) | NS |
| HbF, % | 3 213.1 (6.9) | 125 (16.5 [8.5]) | .020* |
| SpO2, % | 1 497.0 (3.7) | 58 (98.0 [2.0]) | NS |
| LDH, IU/L | 30 863 (414) | 100 (776 [351]) | NS |
| Characteristic . | History of SCI . | P . | |
|---|---|---|---|
| Yes (n = 38) . | No (n = 151) . | ||
| Sex, n (%) | |||
| Male (n = 88) | 21 (23.9%) | 67 (76.1%) | .276 |
| Female (n = 101) | 17 (16.8%) | 84 (83.2%) | |
| G6PD, n (%) | |||
| Normal (n = 152) | 31 (20.4%) | 121 (79.6%) | |
| Deficiency (n = 18) | 6 (33.3%) | 12 (66.7%) | .230 |
| α-thalassemia, n (%) | |||
| Present (n = 76) | 14 (18.4%) | 62 (81.6%) | |
| Absent (n = 104) | 24 (23.1%) | 80 (76.9%) | .467 |
| Parameters at baseline, n (mean [SD]) | |||
| White blood cell count, 109/L | 37 (15.1 [4.9]) | 134 (14.3 [4.8]) | NS |
| Neutrophil count, 109/L | 34 (6.5 [3.1]) | 130 (5.8 [3.2]) | NS |
| Platelet count, 109/L | 36 (363.2 [147.6]) | 133 (350.5[128.0]) | NS |
| Hemoglobin level, g/dL | 37 (7.8 [1.1]) | 134 (8.31 [1.21]) | .016* |
| Mean corpuscular volume, fL | 35 (80.7 [7.7]) | 133 (77.1 [9.5]) | .025* |
| Reticulocyte count, 109/L | 35 322.4 (105.6) | 128 (286.3 [122.8]) | NS |
| HbF, % | 3 213.1 (6.9) | 125 (16.5 [8.5]) | .020* |
| SpO2, % | 1 497.0 (3.7) | 58 (98.0 [2.0]) | NS |
| LDH, IU/L | 30 863 (414) | 100 (776 [351]) | NS |
Biological parameters were recorded before age 3 years. NS, not significant.
Significant tests.
Predictive risk factors for a history of SCI
| Characteristic . | Cox regression analysis . | |
|---|---|---|
| Hazard ratio (95% CI) . | P . | |
| Sex | ||
| Male | 1.30 (0.68-2.46) | .426 |
| Female | ||
| G6PD | ||
| Normal | ||
| Deficiency | 1.88 (0.78-4.52) | .161 |
| α-thalassemia | ||
| Present | ||
| Absent | 1.32 (0.68-2.55) | .411 |
| Parameters at baseline | ||
| Leukocyte count per 1 × 109/L increase | 1.03 (0.97-1.10) | .365 |
| Neutrophil count per 1 × 109/L increase | 1.02 (0.93-1.12) | .655 |
| Platelet count per 1 × 109/L increase | 1.00 (0.99-1;00) | .985 |
| Hemoglobin level per 1 g/dL decrease | 1.42 (1.03-1.96) | .030* |
| Mean corpuscular volume per fL increase | 1.02 (0.99-1.06) | .233 |
| Reticulocyte count per 1 × 109/L increase | 1.002 (1.000- 1.005) | .095 |
| HbF per 1% increase | 0.97 (0.93-1.02) | .253 |
| SpO2 per 1% increase | 0.94 (0.78-1.15) | .553 |
| LDH per IU/mL increase | 1.03 (0.38-2.83) | .950 |
| Characteristic . | Cox regression analysis . | |
|---|---|---|
| Hazard ratio (95% CI) . | P . | |
| Sex | ||
| Male | 1.30 (0.68-2.46) | .426 |
| Female | ||
| G6PD | ||
| Normal | ||
| Deficiency | 1.88 (0.78-4.52) | .161 |
| α-thalassemia | ||
| Present | ||
| Absent | 1.32 (0.68-2.55) | .411 |
| Parameters at baseline | ||
| Leukocyte count per 1 × 109/L increase | 1.03 (0.97-1.10) | .365 |
| Neutrophil count per 1 × 109/L increase | 1.02 (0.93-1.12) | .655 |
| Platelet count per 1 × 109/L increase | 1.00 (0.99-1;00) | .985 |
| Hemoglobin level per 1 g/dL decrease | 1.42 (1.03-1.96) | .030* |
| Mean corpuscular volume per fL increase | 1.02 (0.99-1.06) | .233 |
| Reticulocyte count per 1 × 109/L increase | 1.002 (1.000- 1.005) | .095 |
| HbF per 1% increase | 0.97 (0.93-1.02) | .253 |
| SpO2 per 1% increase | 0.94 (0.78-1.15) | .553 |
| LDH per IU/mL increase | 1.03 (0.38-2.83) | .950 |
Biological parameters were recorded before age 3 years.
Significant tests.
Baseline blood parameters (ie, leukocyte, neutrophil, reticulocyte and platelet counts, SpO2, and LDH) were not significantly different in patients with or without history of SCI, whereas mean (SD) hemoglobin levels (7.8 [1.1] vs 8.3 [1.2] g/dL; P = .016) and HbF (13.1 [6.9] vs 16.5% [8.5]; P = .020) were significantly lower, and mean (SD) corpuscular volume higher (80.7 [7.7] vs 77.1 [9.5] fL; P = .025) in patients with SCI (Table 3).
Baseline hemoglobin was the only predictive risk factor for SCI retained by univariate Cox regression analysis (hazard ratio per 1 g/dL decrease = 1.42; 95% CI, 1.03-1.96; P = .03) (Table 4). Baseline hemoglobin level lower than 7 g/dL (n = 24) was highly predictive of SCI (hazard ratio, 2.97; 95% CI, 1.43-6.17; P = .004), and those patients had a significantly higher cumulative risk for SCI (log rank; P = .002) (Figure 3).
Probability of SCI occurrence depending of the baseline hemoglobin level before age 3 years.
Probability of SCI occurrence depending of the baseline hemoglobin level before age 3 years.
Association of SCI with rate/year of VOC, ACS, and AAE.
Rates per year of VOC and ACS were not significantly different in patients with a history of SCI than in those without. In contrast, the rate of AAE per year was significantly higher in those who developed SCI (0.32 [0.58] vs 0.15 [0.29]; P = .027) (Table 5). Univariate logistic regression analysis showed a significant association between SCI occurrence and the AAE rate (OR, 2.72 per 1/y increase; 95% CI, 1.13-6.54; P = .025) (Table 6).
Comparison of the the rates per year (VOC, ACS, AAE) in the patients with or without a history of SCI
| Rate per year . | History of SCI . | P . | |||
|---|---|---|---|---|---|
| Yes (n = 38) . | No (n = 151) . | ||||
| VOC | 34 | 0.51 (0.70) | 150 | 0.55 (0.69) | NS |
| ACS | 34 | 0.13 (0.19) | 150 | 0.13 (0.17) | NS |
| AAE | 34 | 0.32 (0.58) | 150 | 0.15 (0.29) | .027 |
| Rate per year . | History of SCI . | P . | |||
|---|---|---|---|---|---|
| Yes (n = 38) . | No (n = 151) . | ||||
| VOC | 34 | 0.51 (0.70) | 150 | 0.55 (0.69) | NS |
| ACS | 34 | 0.13 (0.19) | 150 | 0.13 (0.17) | NS |
| AAE | 34 | 0.32 (0.58) | 150 | 0.15 (0.29) | .027 |
Association of history of SCI with annual rates of events (VOC, ACS, and AAE)
| Rate per year . | Associated risk factors for a history of SCI (logistic regression analysis) . | |
|---|---|---|
| OR (95% CI) . | P . | |
| VOC per 1/y increase | 1.10 (0.61-1.97) | .752 |
| ACS per 1/y increase | 1.21 (0.15-9.86) | .861 |
| AAE per 1/y increase | 2.72 (1.13-6.54) | .025* |
| Rate per year . | Associated risk factors for a history of SCI (logistic regression analysis) . | |
|---|---|---|
| OR (95% CI) . | P . | |
| VOC per 1/y increase | 1.10 (0.61-1.97) | .752 |
| ACS per 1/y increase | 1.21 (0.15-9.86) | .861 |
| AAE per 1/y increase | 2.72 (1.13-6.54) | .025* |
Significant tests.
Association of SCI with intracranial stenosis.
A trend toward significance was observed at the first MRI/MRA for the association between SCI and the presence of intracranial stenosis, as SCI were seen in 6/18 (33.3%) patients with intracranial stenosis vs 25/171 (14.6%) patients without SCI (Pearson χ-square test, P = .041; but Fischer Test, P = .085 NS). The presence of intracranial stenosis was associated with a significantly increased risk for SCI by logistic regression analysis (OR, 2.924; 95% CI, 1.004-8.475; P = .049).
However, no significant association was found between history of SCI and history of intracranial stenosis, probably because all patients with intracranial stenosis had been promptly placed on a transfusion program.
Association of SCI with extracranial ICA stenosis.
A significant association was found between history of SCI and the presence of isolated eICA stenosis (OR, 2.83; (95% CI, 1.08-7.41; P = .035). Isolated eICA stenosis (OR, 3.15; 95% CI, 1.17-8.47; P = .023) and age at MRI/MRA with cervical assessment (OR, 1.082 per year increase; 95% CI, 1.001-1.170; P = .047) were retained as significant and independent associated risk factors for SCI. This suggests a relation between the duration of undetected extracranial stenosis during follow-up and the risk for SCI occurrence.
Comparison of the baseline parameters, AAE rate, eICA stenosis, and SCI among 3 groups of patients.
Table 7 shows that the 51 patients placed on a transfusion program because of cerebral macrovasculopathy (49 abnormal TCD and 2 intracranial stenosis) when compared with the nonintensified patients (less severe) at baseline had significantly lower hemoglobin and HbF levels and higher reticulocyte white blood cell counts and LDH levels compatible with a severe anemia/hemolysis profile and a significantly higher SCI prevalence, whereas those with frequent other complications (VOC, ACS, osteonecrosis, etc.) had only a significantly lower HbF level. It shows also that patients who required intensification had a significantly higher AAE rate and SCI prevalence, whereas nonintensified patients had more prevalent eICA stenosis.
Baseline parameters (recorded before age 3 years), AAE rate, eICA stenosis, and SCI in the 3 different groups of initially applied treatment
| Characteristic . | TP1, mean (SD) . | HU+TP2, mean (SD) . | None, mean (SD) . | Comparison (P) . | ||
|---|---|---|---|---|---|---|
| Intracranial macrovasculopathy (n = 51) . | Frequent VOC/ACS (n = 55) . | Less severe (n = 83) . | TP1 vs none . | HU+TP2 vs none . | Intensified vs none . | |
| White blood cell | 16.5 (5.5) | 14.4 (4.7) | 13.2 (4.1) | .001* | .152 | .002* |
| Hb | 7.7 (0.9) | 8.2 (1.2) | 8.5 (1.3) | <.001* | .124 | .002* |
| Mean corpuscular volume | 80.5 (7.7) | 78.5 (9.3) | 75.9 (9.7) | .004* | .150 | .012* |
| Reticulocyte count | 363 (149) | 290 (100) | 256 (92) | <.001* | .071 | <.001* |
| Platelets | 345 (144) | 372 (153) | 344 (110) | .817 | .288 | .405 |
| LDH | 935 (396) | 826 (390) | 681 (293) | .002* | .058 | .001* |
| HbF | 14.4 (7.8) | 13.9 (7.1) | 17.7 (8.8) | .038* | .014* | .008* |
| AAE rate | 0.24 (0.44) | 0.21 (0.43) | 0.12 (0.26) | .082 | .182 | .044* |
| SCI | 31.4% | 21.8% | 12.0% | .012* | .547 | .017* |
| eICA stenosis | 5.9% | 7.3% | 18.1% | .066 | .082 | .021* |
| Characteristic . | TP1, mean (SD) . | HU+TP2, mean (SD) . | None, mean (SD) . | Comparison (P) . | ||
|---|---|---|---|---|---|---|
| Intracranial macrovasculopathy (n = 51) . | Frequent VOC/ACS (n = 55) . | Less severe (n = 83) . | TP1 vs none . | HU+TP2 vs none . | Intensified vs none . | |
| White blood cell | 16.5 (5.5) | 14.4 (4.7) | 13.2 (4.1) | .001* | .152 | .002* |
| Hb | 7.7 (0.9) | 8.2 (1.2) | 8.5 (1.3) | <.001* | .124 | .002* |
| Mean corpuscular volume | 80.5 (7.7) | 78.5 (9.3) | 75.9 (9.7) | .004* | .150 | .012* |
| Reticulocyte count | 363 (149) | 290 (100) | 256 (92) | <.001* | .071 | <.001* |
| Platelets | 345 (144) | 372 (153) | 344 (110) | .817 | .288 | .405 |
| LDH | 935 (396) | 826 (390) | 681 (293) | .002* | .058 | .001* |
| HbF | 14.4 (7.8) | 13.9 (7.1) | 17.7 (8.8) | .038* | .014* | .008* |
| AAE rate | 0.24 (0.44) | 0.21 (0.43) | 0.12 (0.26) | .082 | .182 | .044* |
| SCI | 31.4% | 21.8% | 12.0% | .012* | .547 | .017* |
| eICA stenosis | 5.9% | 7.3% | 18.1% | .066 | .082 | .021* |
TP1 is the group with cerebral macrovasculopathy: abnormal TCD (n = 49) or intracranial stenosis (n = 2). HU+TP2 is the group treated with HU (n = 31) or TP (n = 24) because frequent VOC/ACS or other complications in absence of cerebral macrovasculopathy. None is the nonintensified group because it has a less severe clinical profile.
Significant tests.
Multivariate analysis.
When AAE rate and isolated eICA stenosis were introduced in the multivariate logistic regression model, both the AAE rate (OR, 2.64 per 1/y increase; 95% CI, 1.09-6.38; P = .031) and the presence of isolated eICA stenosis (OR, 3.19; 95% CI, 1.18-8.70; P = .023) remained significant and independent associated risk factors for SCI. Moreover, when a baseline hemoglobin level lower than 7 g/dL was also introduced in the model, all remained as significant and independent risk factors for SCI: AAE rate (OR, 3.39 per 1/y increase; 95% CI, 1.01-11.34; P = .048), eICA stenosis (OR, 3.11; 95% CI, 1.10-8.85; P = .033), and baseline hemoglobin level lower than 7 g/dL (OR, 2.88; 95% CI, 1.05-7.87; P = .039).
Discussion
SCIs are the most common form of neurological diseases in children with SCA, and they occur in 27% of this population before the age of 6 years12 and in 37% before the age of 14 years.6 Despite this, relatively little is known about the causes of silent infarcts and the optimal preventive therapy. It has been assumed that SCI are caused by small vessel disease; however, most SCI observed in patients with SCA occur in the distal-field territories, that is, the deep white matter, suggesting they could be caused by hypoperfusion or hypoxic events,17 which is favored by an underlying arteriopathy of large vessels.
This study in the Créteil Newborn Cohort (1992-2010) confirms that severe anemia at baseline is a predictive risk factor for SCI. We had previously reported a similar association in the 1988-2007 newborn cohort.6 Others have found that low hemoglobin level is associated with the risk for SCI by univariate analysis in the Cooperative Study of Sickle Cell Disease cohort,9 but the multivariable analysis only retained as significant the low pain event rate, which is known to be associated with low hemoglobin. A significant association between severe anemia and SCI was also found by univariate analysis in the Philadelphia cohort.12 The large multicenter SIT Trial cohort, including selected patients with SCA with no stroke history, no abnormal TCD, and no treatment with HU or transfusion program also showed that low baseline hemoglobin level is a significant associated risk factor for SCI.14 Therefore, all studies are in agreement that severe baseline anemia is a risk factor for SCI in patients with SCA. We can speculate that treatments such as HU, which significantly decreases hemolytic rate and the degree of baseline anemia, could decrease the risk for SCI. In fact, in this latest cohort (1992-2010), patients with severe baseline anemia (hemoglobin < 7 g/dL) had been treated with HU since 2000, which may have contributed to the apparent reduction of the cumulative risk for SCI from 37.1% in the previous cohort (1988-2007)6 to 32.4% by age 14 years in the present one.
A relationship between cerebrovascular events and/or SCI and acute anemic events17,18,29,30 has been previously described, but not systematically evaluated in a large newborn cohort. Cerebrovascular episodes have been reported to be as 58 times higher than expected in the 5-week interval after AAE related to B19 infection.29 A recent study reports the occurrence in 7 patients with SCA of acute SCI, defined as an area of restricted diffusion on diffusion-weighted imaging sequences in the absence of focal neurologic findings lasting longer than 24 hours.30 In 4 of the 7 cases, these were observed in the clinical setting of AAE, suggesting that worsening anemia could be an additional risk factor for SCI. The present study reports a significant association between the AAE rate and the risk for SCI, strengthening the causal relationship previously suggested.17,18,29,30
A trend toward significance for the association between intracranial stenosis and SCI was found at the first MRI/MRA assessment. This is in agreement with data from the Philadelphia young cohort study,12 which assessed 68 (70.8%) of 96 children before age 6 years and found SCI in 18/68 patients, associated with the presence of intracranial stenosis by univariate analysis. In contrast, we found no association between intracranial stenosis and SCI at the last MRI/MRA assessment, but only 7 of 24 patients with a history of intracranial stenosis still had one at the last MRI. It may be that, as all patients with abnormal TCDI and/or intracranial stenosis were promptly placed on a transfusion program, stenosis might have been reduced by a long-term transfusion program, thereby preventing SCI. This suggests a beneficial effect of a long-term transfusion program on stenosis outcome and SCI prevention. This is in accordance with the STOP II trial, which randomly assigned patients who had no evidence of stenosis and a history of abnormal intracranial velocities that had normalized on transfusion program to pursuing vs halting transfusions.31 In the pursuing transfusion group, the number of SCI remained the same (n = 25 vs 24), whereas the number of SCI increased from 27 to 45 in the transfusion-halted group.31 Thus, current data suggest that transfusion program may be beneficial in preventing SCI occurrence in patients with a history of abnormal TCD. For patients with no abnormal intracranial velocities, the SIT Trial, which randomly assigned patients with SCI to transfusions vs simple observation, recently reported that transfusion program significantly reduced the incidence of SCI recurrence.32
The relationship between cerebrovascular events and extracranial ICA stenosis had previously been suspected.20-24 An eICA bulbar occlusion with a contralateral postbulbar eICA stenosis was reported in a 19-year-old man who suffered a stroke.20 Telfer et al23 reported 10 patients with eICA occlusion or stenosis among 67 patients with SCA assessed with MRI/MRA and neck MRA because of history of stroke or abnormal velocities. Gorman et al.,21 using TCD assessment via a submandibular approach and neck MRA, identified 3 stroke-free patients with SCA with eICA stenosis and SCI. We recently reported24 that eICA velocities of 160 cm/s or higher are highly predictive of eICA stenosis, which were more frequently observed in tortuous arteries than in straight arteries, suggesting a causal mechanism similar to that involved in the carotid siphon stenosis. Turbulent flow secondary to increased cardiac and cerebral blood flow resulting from chronic anemia and decreased oxygen supply to the brain may induce shear stress gradients and subsequent endothelial dysfunction, especially in bended segments, leading to narrowing of the arterial lumen. As suggested by Wang,33 chronic anemia in patients with SCA results in increased cerebral blood flow, chronic vasodilatation, and an inadequate vascular response to hypoxic stress as in AAE induced by splenic sequestration or Parvovirus infection,29 leading to parenchymal ischemia, mainly in the distal-field territories. In the present Créteil Newborn Cohort study, in which all patients were systematically assessed by MRI/MRA after age 5 years, with a neck MRA at last assessment, we report for the first time in overt stroke-free children with SCA that SCI are significantly associated with eICA stenosis. This suggests that previously undetected extracranial arteriopathy plays a significant and independent role in SCA cerebral ischemia, along with low baseline hemoglobin level and the rate of AAE. In the present study, eICA stenosis was more frequent in so-called nonintensified patients than in those on a transfusion program or receiving HU, suggesting a positive effect of intensive therapy in reducing the prevalence of eICA stenosis, probably via an improvement of anemia, as low baseline hemoglobin was a significant predictive risk factor for abnormal eICA high velocities and stenosis.
These data show that chronic hypoxia via severe anemia and acute hypoxia via AAE and eICA stenosis are significant and independent risk factors for SCI, which are known to be associated with cognitive impairment.34 Moreover, even in the absence of SCI, severe anemia alone is a risk factor for cognitive impairment35,36 that tends to increase during aging,37,38 suggesting a cumulative effect of SCA on focal and diffuse brain injury.
This study has some limitations, as it is a longitudinal prospective observational cohort study with no control group, and the follow-up ended at the first MRI/MRA with cervical MRA. Thus, determining effectiveness or assessing net benefits cannot be inferred here. Only prospective randomized trials testing the effect of HU, a transfusion program, or simple observation in patients with eICA stenosis could answer these issues. Nevertheless, this study offers the advantages of long-term and accurate data collection in a cohort from a single referral center at which management care and procedures are standardized. Moreover, prospective randomized trials testing intensive therapy vs observation for patients with eICA stenosis could be unethical, as a transfusion program is usually recommended for patients with SCA in the presence of intracranial stenosis. In the present study, the regression of stenosis in 17/24 patients after a mean 7.4 years on a long-term transfusion program suggests a beneficial effect of a transfusion program on intracranial stenosis outcome, in agreement with the STOP trial that has a mean follow-up of 21 months, where a transfusion program could reduce the risk of worsening intracranial stenosis compared with the observational group.39 In contrast, such regression of stenosis has not been described in patients with a history of stroke, with, in fact, frequent worsening of stenosis observed despite a long-term transfusion program,40-44 suggesting the importance of starting a transfusion program as soon as stenosis is detected to provide the maximum chance for regression. HU, which significantly increases hemoglobin level, might prevent eICA stenosis, but a beneficial effect on established stenosis in stroke-free patients seems unlikely. The Stroke with Transfusions Changing to Hydroxyurea trial,43 which randomly assigned patients with stroke history to pursuing a transfusion program with iron chelation vs HU with phlebotomies was prematurely closed because greater stroke recurrence (10% vs 0%) was observed in the HU/phlebotomy group, showing that transfusion program/chelation remains the best way to manage children with SCA and stroke. However, a very plausible hypothesis is that the monthly phlebotomies of 10 mL/kg performed in the Stroke with Transfusions Changing to Hydroxyurea trial for patients receiving HU with hemoglobin levels of 7 g/dL or higher, but with stenotic underlying cerebral vasculopathy, may have been partly responsible for the strokes observed in the HU group. However, HU might be helpful in reducing mild stenosis in stroke-free patients.
We confirm here that baseline severe anemia is a highly significant predictive risk factor for SCI. We report for the first time that the rate of AAE and the presence of previously undetected eICA stenosis are significant and independent associated risk factors for SCI, making eICA assessment combining TCDI and cervical MRA essential for complete cerebral vasculopathy evaluation in patients with SCA. Our data strongly suggest that chronic (via severe anemia) and acute (via AAE and eICA stenosis) hypoxia contribute to the progressive brain injury observed in SCA and support the future design of controlled studies using HU, transfusion programs, and stem cell transplantation to prevent SCA brain injury and preserve cognitive functioning.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the patients, their parents, and all the nurses and physicians at the Centre Hospitalier Intercommunal de Créteil, who all contributed to the management of patient care, and Dr Martine Torres for her critical reading of the manuscript and editorial assistance.
This work was supported in part by an institutional grant “Programme Hospitalier de Recherche Clinique” (IDF05001) from the French Ministry of Health.
Authorship
Contribution: F.B. designed and performed the research, collected the data, performed the statistical analyses, interpreted the data, and wrote the manuscript; S.V. designed the study, performed Doppler ultrasound scans and MRI/MRA, analyzed and interpreted data, and wrote the manuscript; C.P., C.A., and A.K. collected and interpreted data and cowrote the manuscript; F.B., C.P., C.A., A.K., I.H., F.M., C.F., S.B., and R.E. participated in the management of patient care; M.V. and F.K. performed Doppler ultrasound scans and MRI/MRA; and all authors critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Françoise Bernaudin, Pédiatrie, Centre de Référence des Syndromes Drépanocytaires Majeurs, Centre Hospitalier Intercommunal de Créteil, 40 avenue de Verdun, 94010 Créteil, France; e-mail: francoise.bernaudin@chicreteil.fr.

![Figure 2. Patient groups according to initially applied treatment (n = 189). After a period without intensive therapy, 75 of 189 patients were placed on a transfusion program, 51 of them because of cerebral macrovasculopathy (TP1) (abnormal intracranial velocities [n = 49], intracranial stenosis [n = 2, in 1 patient with no available temporal window and in 1 patient with conditional TCD]), and 24 other patients because of frequent other complications in absence of any cerebral macrovasculopathy (TP2) (recurrent splenic sequestrations [n = 7], SCI in patients participating in the SIT Trial and randomized in the transfusion group [n = 3], frequent VOC and/or ACS and age younger than 3 years [n = 5], tricuspid regurgitant jet velocity (TRJV) higher than 2.5 m/s [n = 1], hip osteonecrosis [n = 5], and priapism [n = 3]). Thirty-one patients older than 3 years were treated with HU (n = 31) because of frequent VOC and/or ACS (n = 26) or severe anemia with baseline hemoglobin levels lower than 7 g/dL (n = 5). During follow-up, the transfusion program was stopped postsplenectomy (n = 1) and after normalization of intracranial velocities with absence of intracranial stenosis in patients refusing to take HU (n = 2). The transfusion program was replaced by HU in 31 patients after normalization of intracranial velocities and absence of intracranial (Ic) stenosis (n = 20), Ic stenosis regression (n = 1), history of frequent VOC and/or ACS in patients now older than 3 years (n = 2), postsplenectomy (n = 5), normalized TRJV (n = 1), end of SIT Trial (n = 2). However, 12 of them were placed again on a transfusion program because of HU failure to prevent VOC and/or ACS (n = 5), TRJV higher than 2.5 m/s (n = 3), abnormal TCD relapse, and/or intracranial stenosis occurrence (n = 4). At the end of follow-up (first MRI/MRA with cervical assessment), 86 patients still did not receive intensive therapy, 50 were receiving HU, and 53 were on a transfusion program.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/125/10/10.1182_blood-2014-09-599852/4/m_1653f2.jpeg?Expires=1767829069&Signature=wrsNuAHELWYr46zVSM~FvtLO~MrUQnw95L5UMVP8bYD--w9Ao1ZOYqg3WaYjp2eyoee4wfjZV7dZJZoWaUxs9KWkeXtn8~EXPzMaNRM-jvLRwjC3wMBubdAgmAnON6Xccue~ceoyh6FwN~GJ-VLy4hu68He6lhFqGz1-MYME0B4Nly4eK6yBtlkELw1BhxBLyJBGzuqnws5BBCsQfq~W7zSqf2JTJchA4jbSUtYJI7jbd7jgN9kaBYPL42AXW18pn6t1ZGSN43FhgZnmgcTOuCzTxnAxAyYT917MaUucQutyogF--wy-lEpiahUirYb0G4Qt45TZBWj02qQZR5vIPQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal