To the editor:
Silent cerebral infarcts (SCIs) are the most commonly recognized cause of neurologic injury in patients with sickle cell anemia (SCA), identified in ≥20% of children. In children with SCA, SCIs are associated with an average 5 full-scale IQ point decrement,1 poor academic performance,2 and future overt strokes.3 Recently, Bernaudin et al4 reported that in children with SCA, the cumulative risk for SCI was 19.2% by age 8 years, 32.4% by age 14 years, and 39.1% by age 18 years. Very little is known about the prevalence of SCI in adults with SCA. We tested the hypothesis that the prevalence of SCI was significantly >39% in patients >18 years old with SCA.
Due to the high age-dependent prevalence of SCI in children and adolescents with SCA, associated deficits in neuropsychometric performance,5 the association of SCI with future overt stroke,5 and the impact of these associated morbidities on health care outcomes in adults with SCA, including adherence to complex instructions associated with management of chronic illness, we elected to obtain, as part of routine clinical practice, magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) scans of the brain in adults with SCA. If SCI or cerebral vasculopathy is found via neuroimaging, we refer these adults for cognitive testing, neurology consultation, or both. We completed a retrospective chart review of adults with SCA (hemoglobin SS or hemoglobin S β zero [Sβ0] thalassemia) followed in the comprehensive sickle cell disease clinic, with surveillance MRI and MRA of the brain performed as part of standard-of-care assessment for SCI from 2011 to 2013. The cerebral MRIs and MRAs were rereviewed by 2 board-certified neuroradiologists for the purpose of this study. Consensus findings were recorded including presence of cerebral infarcts (≥3 mm on T2-weighted images in 2 imaging planes),6 intracerebral hemorrhage, aneurysms,7,8 and cerebral vasculopathy9,10 according to prior established criteria for MRI and MRA. If no neurologic concerns were reported in the medical record and the neurologic examination was normal, infarcts were judged to be silent. STATA version 13 (StataCorporation, College Station, TX) was used for all analyses. Patient characteristics were described by median and interquartile range (IQR) for continuous measures and by number and percent for categorical measures. A 2-sided P ≤ .05 was considered statistically significant. This study was approved by the Vanderbilt University Institutional Review Board as a retrospective cohort analysis.
The study population included 121 adults with SCA (hemoglobin SS or Sβ0 thalassemia) out of ∼200 individuals with sickle cell disease that were followed in the clinic. Of these 121 individuals, 69 unselected adults underwent neuroimaging with brain MRI. Nine adults with SCA and overt strokes were excluded. The final study population included 60 adults with SCA and neuroimaging. Of note, a total of 52 individuals were excluded because they did not have neuroimaging, primarily because of failure to keep scheduled appointments during the study time period, insurance coverage issues, and implanted devices that were contraindications for MRI. There were no significant differences between those with and without neuroimaging with regard to age, sex, genotype, median hemoglobin, reticulocyte count, or number of hospitalizations. However, those without neuroimaging were more likely to be on hydroxyurea compared with the imaging group (81% vs 63% on hydroxyurea; P = .043), but were not more likely to be receiving chronic transfusion therapy (16% vs 19%; P = .668). Of the 60 adults with imaging, 48% were male, median age was 30 years (interquartile range: 22-35 years), and all had MRI and MRA scans of the brain. SCIs were present in 53% (n = 32) of the adults with SCA; 37% with SCI (22 of 60) had >1 lesion. Compared with the Bernaudin report of 39% with SCI by age 18,4 our finding of a prevalence of 53% SCI in an unselected adult cohort with SCA, at a median age of 30 years, is significantly different than the younger cohort (χ2 = 3.75; P = .05).
MRA of the brain was of adequate quality to assess for vascular disease or aneurysm in 55 patients. Incidental findings include microhemorrhages in 5 participants (9%); of these 5, 4 also had SCIs present on MRI. Unruptured saccular aneurysms were identified in 9% (5 of 55 patients). The aneurysms were <5 mm in size (Table 1). Significant cerebral vasculopathy was not identified. We note that of the adults excluded due to the presence of overt stroke, 5 of 9 had moyamoya-type cerebral vasculopathy.
Cerebral aneurysms in adults with SCA
| Hemoglobin genotype . | Age (years) . | Aneurysm size(s) (mm) . | Aneurysm location(s) . | Aneurysm treatment(s) . |
|---|---|---|---|---|
| SS | 30 | 3 | ICA (near PCOM) | Observation |
| SS | 21 | 2 | Basilar artery | Observation |
| SS | 35 | 3 | ICA (distal to ophthalmic artery) | Observation |
| Sβ0 | 42 | 2 | ICA terminus | Observation |
| SS | 33 | 3.5, 2 | Right and left ophthalmic arteries | Observation |
| Hemoglobin genotype . | Age (years) . | Aneurysm size(s) (mm) . | Aneurysm location(s) . | Aneurysm treatment(s) . |
|---|---|---|---|---|
| SS | 30 | 3 | ICA (near PCOM) | Observation |
| SS | 21 | 2 | Basilar artery | Observation |
| SS | 35 | 3 | ICA (distal to ophthalmic artery) | Observation |
| Sβ0 | 42 | 2 | ICA terminus | Observation |
| SS | 33 | 3.5, 2 | Right and left ophthalmic arteries | Observation |
ICA, internal carotid artery; PCOM, posterior communicating artery; SS, hemoglobin SS; Sβ0, hemoglobin S β zero thalassemia.
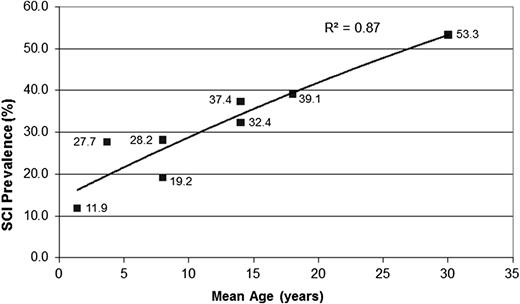
For the first time, we described the prevalence of SCI in an unselected group of adults with SCA, occurring in ≥50% of patients. Based on prior work by Bernaudin et al,4 we anticipated the rate of SCI to be ∼40%, but our recent findings support the hypothesis that the incidence of new SCI in patients with SCA does not attenuate in adulthood (Figure 1).11-15
Prevalence of SCI in children and young adults with SCA. The figure displays the cumulative prevalence of SCI in children and young adults based on 4 cross-sectional studies,11-14 the current cross-sectional study, and 1 longitudinal study.4 The cumulative prevalence of SCI suggests that the incidence of SCI does not plateau in young adulthood to ≥30 years of age. Figure adapted from our review of SCI in children.15
Prevalence of SCI in children and young adults with SCA. The figure displays the cumulative prevalence of SCI in children and young adults based on 4 cross-sectional studies,11-14 the current cross-sectional study, and 1 longitudinal study.4 The cumulative prevalence of SCI suggests that the incidence of SCI does not plateau in young adulthood to ≥30 years of age. Figure adapted from our review of SCI in children.15
The saccular aneurysm prevalence of 9% in a relatively young adult cohort (median age, 30 years) confirms that adults with SCA may have a higher prevalence of cerebral aneurysms7 than the general population (reported between 1.8%16 and 3.2%17 ). The natural history of saccular aneurysm in SCA is unknown; however, in the general population, cigarette smoking, size of the unruptured intracranial aneurysm, hypertension, aneurysm location, and age have been implicated in aneurysmal rupture.18,19 No consensus exists on the influence of these reported risk factors.
The high prevalence of SCI (53%) and saccular aneurysms (9%) in adults with SCA provide evidence of both a morbid and premorbid condition that may warrant medical and/or neurosurgical intervention in this high-risk population. The impact of SCI on cognitive function in children is well recognized, and the recently reported association of anemia and age with poor cognitive performance in adults with SCA20 may underlie some of the cognitive challenges observed in adults with SCA, such as low employment21,22 or changes in treatment patterns after transition to adult care.23 Vichinsky et al20 evaluated cognition in 141 young adults with SCA; 13% had lacunar infarcts defined as ≥5 mm hyperintensity on the T2-weighted and proton density–weighted images, with corresponding hypointensity on T1-weighted images. The study of Vichinsky et al excluded adults with overt strokes and those with known comorbidities associated with SCI, such as poor cognition2 or hypertension.3 However, similar to prior pediatric studies describing the prevalence of SCI,6 our study only excluded those with overt strokes. Perhaps the inclusion of a selected, less severely affected adult SCA population in Vichinsky et al, along with the different imaging criteria for SCI, explains the striking difference in SCI prevalence between this study and ours.
The current study is limited by the lack of cervical MRA to assess the extracranial internal carotid artery, as stenoses have been reported to be a risk factor for SCI in children.4 Also, in this retrospective study, accurate assessment of the duration of hydroxyurea and chronic transfusion use was not possible. Therefore, we were not able to assess whether these therapies were protective against the development of SCI or aneurysm. Further study is warranted to assess optimal strategies for primary and secondary prevention of SCI in adult SCA. Also given the higher prevalence of hemorrhagic stroke,24 and the association of subarachnoid hemorrhage in adults with SCA and intracranial aneurysm,25 further investigation is also needed to determine whether monitoring for aneurysms is warranted. There appears to be an age-dependent increase in neurologic and neurovascular morbidity in adults with SCA.
Authorship
Contribution: S.P. and M.D. reviewed the neuroimaging; M.R. analyzed results and made the figures; M.C.G. and M.A.B. collected and entered the data and critically revised the manuscript; A.A.K., M.R.D., and L.C.J. designed the research and wrote the paper; and all authors reviewed and critically revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lori C. Jordan, Division of Pediatric Neurology, Department of Pediatrics, Vanderbilt University School of Medicine, 2200 Children’s Way, DOT 11212, Nashville, TN 37232-9559; e-mail: lori.jordan@vanderbilt.edu.