In this issue of Blood, Bernaudin et al have identified the rate of acute anemic events (AAEs) and extracranial internal carotid artery (ICA) stenosis as risk factors for silent cerebral infarcts (SCIs) in children with sickle cell anemia (SCA).1 SCIs refer to permanent brain lesions, usually small, that do not produce obvious focal neurologic deficits. These smaller strokes are often not “silent” and can cause neurocognitive impairment and poor academic performance, as well as portend overt stroke. SCIs occur as early as the first year of life, and their prevalence increases with age. About 40% of adolescents with SCA have SCIs. What causes this most frequent form of neurologic injury in SCA? Overt stroke in SCA is often preceded by occlusive cerebral arteriopathy of the large intracranial arteries, so SCIs could be caused by an arteriopathy of small vessels, but this has not been demonstrated. Vasoocclusion of small arteries and arterioles in the brain has also been proposed but has not been confirmed in humans. Critical hypoperfusion events might also cause SCIs, given that SCIs are often found in watershed regions of the brain ipsilateral to occlusive cerebral arteriopathy.
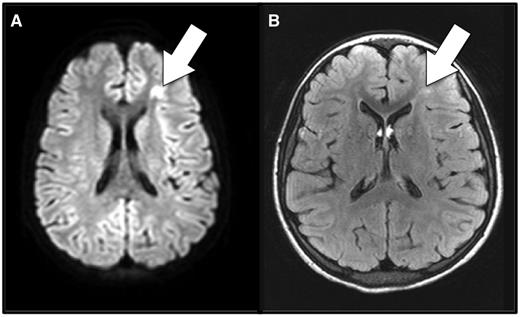
Anemia and cerebral ischemia. Shown here is magnetic resonance imaging of the brain during (A) and 7 months after (B) an acute anemic event in a patient with sickle cell anemia who had no focal neurologic signs corresponding to the brain lesion. A focus of restricted diffusion on diffusion-weighted imaging (A) indicates this is an acutely ischemic lesion. (B) Typical, T2-hyperintense, permanent silent cerebral infarct corresponding to the focus of the acute ischemia detected during the acute anemic event. The imaging was obtained for a screening clinical research study, not for clinical suspicion of stroke.
Anemia and cerebral ischemia. Shown here is magnetic resonance imaging of the brain during (A) and 7 months after (B) an acute anemic event in a patient with sickle cell anemia who had no focal neurologic signs corresponding to the brain lesion. A focus of restricted diffusion on diffusion-weighted imaging (A) indicates this is an acutely ischemic lesion. (B) Typical, T2-hyperintense, permanent silent cerebral infarct corresponding to the focus of the acute ischemia detected during the acute anemic event. The imaging was obtained for a screening clinical research study, not for clinical suspicion of stroke.
Identifying the risk factors for SCIs can help reveal their cause. Recently, several groups have identified a clear association between SCIs and degree of baseline anemia. A US group showed that lower rates of pain and acute chest syndrome, lower baseline hemoglobin (Hb) concentration, and intracranial arterial stenosis were risk factors for SCIs.2 A group in France found that lower baseline Hb concentration was the only independent predictor of SCIs.3 Most recently, an international study group identified lower baseline Hb concentration, higher baseline systolic blood pressure, and male sex as risk factors.4 So, degree of baseline anemia does appear to be important in the genesis of SCIs, but we also need to know when SCIs occur to understand their causes better.
Three reports have shown that SCIs are detectable during the acutely ischemic phase (see figure). The first was a case series of 7 patients who had “acute SCI” or acute silent cerebral ischemic events (ASCIEs) during complications of SCA, 4 of which were AAEs.5 A prospective study showed that ASCIEs occurred in nearly 20% of children with SCA hospitalized for AAEs, defined as a Hb level of <5.5 g/dL and 30% or more lower than baseline.6 A multicenter study showed that ASCIEs could also be detected in asymptomatic, clinically well children undergoing screening magnetic resonance imaging of the brain.7 Compared with the baseline rate of initial SCIs, the rate of ASCIEs was 40-fold higher in children who already had a “remote” SCI, whereas AAE increased the incidence of ASCIEs 600-fold.6-8 Some ASCIEs appear to be reversible and leave no detectable lesion, whereas others become permanent SCIs.
Bernaudin et al hypothesized that common clinical complications associated with hypoperfusion and hypoxemia, such as AAEs and acute chest syndrome, as well as extracranial ICA stenosis, were associated with SCIs.1 Recall, the risk for overt stroke is routinely assessed in SCA by ultrasonography of intracranial arteries. The study population was a contemporary (1992-2010) and aggressively treated cohort of children with SCA followed longitudinally by magnetic resonance imaging and angiography. None had overt stroke. The study confirmed that degree of baseline anemia, defined here as a Hb concentration lower than 7g/dL before age 3 years, was a risk factor for SCIs. Moreover, the yearly rate of AAEs experienced by the patient, or the frequency of acute exacerbations of chronic anemia, and isolated extracranial ICA stenosis were identified as novel risk factors for SCIs, but not rate of pain or acute chest syndrome.
The degree of chronic anemia and the occurrence and frequency of AAEs, therefore, are important risk factors for SCIs. Also, considering their association with arterial stenosis, SCIs are likely the consequence of impaired oxygen delivery to the brain resulting from the combined limitation of blood flow (stenosis) and oxygen-carrying capacity (anemia). This is compounded by the impairment of cerebrovascular autoregulation, near-maximal cerebral oxygen extraction, and possibly increased cerebral metabolic rate in SCA. ASCIEs that occur during the baseline state probably reflect ongoing ischemic injury resulting from chronic anemia and arterial stenosis. Such ASCIEs are often small and potentially reversible, but some do progress to SCIs. AAEs amplify this pathology and dramatically increase the incidence of ASCIEs6 (the acute lesion) and SCIs (the remote lesion).1 Certainly other factors are involved, such as sickling and vasoocclusion resulting from Hb desaturation. However, ASCIEs also occur in children with AAEs who do not have SCA, so sickle Hb is not necessarily required for their formation.6 Moreover, similar brain lesions occur in very well-transfused patients with thalassemia,9 so perhaps platelet activation and prothrombotic potential play additional roles that may be more important in thalassemia than SCA.
How does the clear association of SCIs with acute and chronic anemia inform the care of patients with SCA? Although there are no randomized trials to show that hydroxyurea decreases the incidence of SCIs, widespread use of this prophylactic therapy to decrease the degree of chronic anemia is reasonable (among other benefits). For patients who already have SCIs, chronic transfusion therapy decreases recurrent cerebral ischemia.10 In addition to dilution of sickle Hb, chronic transfusions might prevent SCIs because chronic anemia is decreased, and a higher baseline Hb concentration can mitigate the severity of AAEs. Given the association of AAEs with cerebral ischemia, we may need to be more liberal in our transfusion practices for acute complications of SCA. Transfusion to correct at least the acute component of anemia might be neuroprotective during AAEs, especially because some ASCIEs appear to be reversible. Many patients with SCA tolerate mild to moderate exacerbations of anemia reasonably well, but this perception primarily is based on cardiopulmonary signs and symptoms, which is how we traditionally define “symptomatic anemia.” But ASCIEs and SCIs are “silent” by definition, so how low can you (safely) go? The answer is not entirely clear, but waiting for the appearance of “symptomatic anemia” may be too late for the brain.