Key Points
Adenosine signaling via ADORA2B induces SphK1 activity in sickle and normal erythrocytes via PKA-mediated ERK1/2 activation.
Lowering adenosine by PEG-ADA or interfering ADORA2B activation by specific antagonist decreases SphK1 activity in normal and sickle RBCs.
Abstract
Erythrocyte possesses high sphingosine kinase 1 (SphK1) activity and is the major cell type supplying plasma sphingosine-1-phosphate, a signaling lipid regulating multiple physiological and pathological functions. Recent studies revealed that erythrocyte SphK1 activity is upregulated in sickle cell disease (SCD) and contributes to sickling and disease progression. However, how erythrocyte SphK1 activity is regulated remains unknown. Here we report that adenosine induces SphK1 activity in human and mouse sickle and normal erythrocytes in vitro. Next, using 4 adenosine receptor-deficient mice and pharmacological approaches, we determined that the A2B adenosine receptor (ADORA2B) is essential for adenosine-induced SphK1 activity in human and mouse normal and sickle erythrocytes in vitro. Subsequently, we provide in vivo genetic evidence that adenosine deaminase (ADA) deficiency leads to excess plasma adenosine and elevated erythrocyte SphK1 activity. Lowering adenosine by ADA enzyme therapy or genetic deletion of ADORA2B significantly reduced excess adenosine-induced erythrocyte SphK1 activity in ADA-deficient mice. Finally, we revealed that protein kinase A-mediated extracellular signal-regulated kinase 1/2 activation functioning downstream of ADORA2B underlies adenosine-induced erythrocyte SphK1 activity. Overall, our findings reveal a novel signaling network regulating erythrocyte SphK1 and highlight innovative mechanisms regulating SphK1 activity in normal and SCD.
Introduction
Sphingosine-1-phosphate (S1P) is a widely produced bioactive signaling lipid. It regulates various cellular and physiological processes via activation of 5 S1P receptors and/or by interaction with key regulatory proteins within cells.1 S1P levels are governed by 2 generating enzymes, sphingosine kinase (SphK)1 and 2, and 3 degrading enzymes, S1P lyase and S1P phosphatase 1 and 2. Due to the high activity of S1P degrading enzymes, S1P levels are generally low in peripheral tissues with values <30 nmol/g.2 Contrary to low tissue S1P levels, the highest S1P concentration in the body is found in blood where concentrations reach ∼200 nM in human plasma and 700 nM in mouse plasma.3,4 The concentration gradient between circulation and peripheral tissues is important for various physiological processes, including lymphocyte trafficking,5 vascular integrity,6 bone homeostasis,7 neo-vascularization,8 and antigen presentation.9
Recent studies strongly suggest that the red blood cell (RBC) is the primary contributor of S1P in plasma5,10,11 because of the unique feature in S1P metabolism: high sphingosine kinase activity and no S1P degrading enzymes.12,13 At 100% hematocrit, human and mouse RBCs contain >2000 nM S1P.11 In addition, due to lack of SphK2, which localizes predominantly to the nucleus,14 only SphK1 is responsible for generating S1P in human erythrocytes.15 Mice with a genetic deficiency of SphK1 have significantly lower serum S1P levels to <50% that of normal mice.16 However, factors regulating erythrocyte S1P production, especially SphK1 activity, remain unknown.
A recent study revealed that erythrocyte SphK1 activity is elevated in both patients and mice with sickle cell disease (SCD), the most prevalent hemolytic genetic disease.17 As such, both humans and mice with SCD contain significantly elevated intraerythrocyte and circulating S1P levels.17 Further studies demonstrated that elevated intracellular S1P due to increased SphK1 activity directly contributes to sickling, a central pathogenesis of the disease.17 Intriguingly, these studies also demonstrated that hypoxia is a previously unrecognized potent stimulus significantly induces erythrocyte SphK1 activity in SCD mice and in human sickle erythrocytes in vitro.17 Thus, it is likely that sickle cells have a higher SphK1 activity than normal erythrocytes in both human and mice with SCD because of anemia-induced hypoxia, and SphK1 activity is further increased in response to hypoxia conditions. In view of these important findings, we sought to identify specific factors and signaling pathways related to hypoxia that contribute to increased SphK1 activity in sickle and normal erythrocytes.
We report here that elevated adenosine, a signaling molecule known to be induced by hypoxia, induces erythrocyte SphK1 activity in normal and sickle erythrocytes. We show that adenosine regulates erythrocyte SphK1 activity by activation of the A2B adenosine receptor (ADORA2B), leading to downstream activation of protein kinase A (PKA) and extracellular signal-regulated kinase (ERK)1/2 signaling pathways. Altogether, our study provides new insights into erythrocyte physiology and pathology by revealing a novel signaling pathway regulating erythrocyte SphK1 activity and highlighting previously unrecognized role of adenosine-ADORA2B signaling in S1P generation in erythrocytes.
Methods
Human subjects
Individuals with sickle cell disease were identified by hematologists on the faculty of the University of Texas Medical School at Houston. Subjects participating in this study had no blood transfusion for ≥6 months before blood samples were collected. Normal human subjects were of African descent and were free of hematological disease. The research protocol, which included informed consent from the subjects, was approved by the Institutional Review Board of The University of Texas Health Science Center at Houston.
Mice
Adenosine deaminase (ADA)-deficient mice (Ada−/−) were generated and genotyped as previously described.18,19 Control mice, designated Ada+/−, were littermates that were heterozygotes for the null Ada allele. Ada−/−/Adora2b−/− mice were generated by crossing Ada−/− mice with Adora2b−/− mice.20 Four adenosine receptor-deficient mice were initially transferred from Dr Michael Blackburn and later bred in our laboratory. Berkley SCD transgenic mice expressing exclusively human sickle hemoglobin were purchased from The Jackson Laboratory.21 All phenotypic comparisons were performed among littermates. Animal care was in accordance with National Institutes of Health guidelines and the Animal Welfare Committee at The University of Texas Health Science Center at Houston.
Polyethyleneglycol-modified ADA treatment
Polyethyleneglycol-modified ADA (PEG)-ADA was generated by the covalent modification of purified bovine ADA with activated PEG as described previously.22-24 Five units of PEG-ADA were delivered weekly by intraperitoneal injection to reduce adenosine levels as indicated in Figures 3A and 5A.
Blood collection and preparation from humans and mice
Approximately 4 mL of blood was withdrawn from forearm veins of normal individuals and patients with SCD and collected in sodium heparin-coated tubes. For mouse, 1 mL of the blood was collected in a 1.5-mL tube containing 17 USP (United States Pharmacopeia) units of sodium heparin. Mouse blood collected for adenosine analysis was collected as previously described.25 RBCs were then purified via Percoll density purification (Sigma) to remove white blood cells as previously described.26
Plasma adenosine analysis
Adenosine concentration in plasma was measured by high-performance liquid chromatography as previously described.25 In brief, adenosine was isolated from 200 μL plasma by sequentially adding perchloric acid, KHCO3/3.6 N KOH, ammonium dihydrogen phosphate (pH 5.1), and phosphoric acid (30%) and centrifuged at 20 000g. Two hundred microliters of the final supernatant was used for high-performance liquid chromatography analysis as described previously.19,27
Isolation of total erythrocytes and treatment of human and mouse erythrocytes in vitro
RBCs were isolated from blood collected with heparin as an anticoagulant. Packed RBCs were purified using Percoll as mentioned above and then washed 3 times with culture media (F-10 nutrients mix; Life Technology) and resuspended to 4% hematocrit. One milliliter of RBCs was added to each well of a 12-well plate and treated with different compounds including 5′-(N-ethyl-carboxamido) adenosine (NECA; R&D Systems), ADORA2B antagonist MRS 1754, PKA inhibitor H89, PKA activator Forskolin, and ERK1/2 inhibitor PD98059 (R&D Systems).
SphK1 activity assay
Erythrocyte SphK1 activity was measured using previously described methods,28,29 with a few modifications. Briefly, RBCs were lysed in a pH 7.4 Tris-HCl buffer containing 1 mM EDTA, 1 mM β-mercaptoethanol, 0.3% Triton X-100, 50% glycerol and protease, and phosphatase inhibitors. Then, the lysates were assayed using 250 µM d-erythro-sphingosine in bovine serum albumin (0.4%) and [γ-32P] adenosine triphosphate (10 μCi, 20 mM) containing 200 mM MgCl2. Lipids were extracted and then resolved by thin-layer chromatography on a silica gel G60 with 1-butanol/methanol/acetic acid/water (80:20:10:20, v/v). The plates were then exposed to phosphor-imaging screening (Bio-Rad) and scanned for radioactive signals as an indication of the amount of S132P synthesized.
Erythrocyte membrane isolation and western blot
Pelleted erythrocytes were frozen and thaw in 20-fold volume of 5 mM phosphate buffer pH 7.4 containing 150 mM NaCl, protease inhibitors (Roche), and phosphatase inhibitors (Sigma), and then centrifuged at 20 000g for 20 min. Supernatant were removed, and pellets were washed 4 times before dissolving in the same buffer with 1% Triton X-100. Fifty micrograms of membrane protein was loaded for western blot detection of membrane-bound SphK1 and serine 225-phosphorylated SphK1 with anti-SphK1 antibody (LifeSpan) and anti-Ser225 p-SphK1 antibody (ECM Bioscience).
Statistics
All data are expressed as the mean ± standard error of the mean (SEM). Data were analyzed for statistical significance using GraphPad Prism 5 software (GraphPad Software). Differences between the means of multiple groups were compared by 1-way analysis of variance, followed by a Turkey’s multiple comparisons test. P < .05 was considered significant.
Results
Adenosine induces SphK1 activity in normal and sickle erythrocytes from both humans and mice in vitro
To identify specific molecules responsible for increased SphK1 activity in sickle erythrocytes, we initially screened a series of molecules able to regulate SphK1 activities in other cell types, including angiotensin II, tumor necrosis factor-α,30 endothelin 1,31,32 and even S1P.33 However, none of them induced SphK1 activity in wild-type (WT) mouse erythrocytes in vitro (Figure 1A). Next, we chose to focus on hypoxia-related molecules because SphK1 activity in sickle erythrocytes is further increased in response to hypoxia.17 Intriguingly, early nonbiased metabolomic screening showed adenosine accumulates in the circulation of SCD mice and contributes to sickling, as does S1P.25 However, whether adenosine induces erythrocyte SphK1 activity remains unknown. To test this possibility, we treated normal erythrocytes isolated from WT mice with NECA, a potent, nonmetabolizable adenosine analog. NECA stimulated a significant increase in SphK1 activity, indicating that adenosine can directly induce SphK1 activity in normal mouse erythrocytes (Figure 1A) in a time- (Figure 1B) and dose- (Figure 1C) dependent manner. Next, to determine whether adenosine can induce SphK1 activity in normal human erythrocytes, we treated erythrocytes isolated from normal individuals with NECA and found that NECA significantly induced SphK1 activities in cultured primary normal human erythrocytes in vitro (Figure 2A).
Test of potential molecules capable of inducing erythrocyte SphK1 activity. (A) SphK1 activity in cultured primary erythrocytes from WT mice treated with endothelin-1 (100 nM), angiotensin II (100 nM), tumor necrosis factor-α (50 ng/mL), sphingosine 1-phosphate (100 nM), and NECA (10 μM). (B) Induction of erythrocyte SphK1 activity by NECA in a time-dependent manner. (C) Dose-dependent erythrocyte SphK1 activation by NECA treatment of 30 min. Values shown represent the mean ± SEM (n = 3∼5 for each group). *P < .05 NECA vs control.
Test of potential molecules capable of inducing erythrocyte SphK1 activity. (A) SphK1 activity in cultured primary erythrocytes from WT mice treated with endothelin-1 (100 nM), angiotensin II (100 nM), tumor necrosis factor-α (50 ng/mL), sphingosine 1-phosphate (100 nM), and NECA (10 μM). (B) Induction of erythrocyte SphK1 activity by NECA in a time-dependent manner. (C) Dose-dependent erythrocyte SphK1 activation by NECA treatment of 30 min. Values shown represent the mean ± SEM (n = 3∼5 for each group). *P < .05 NECA vs control.
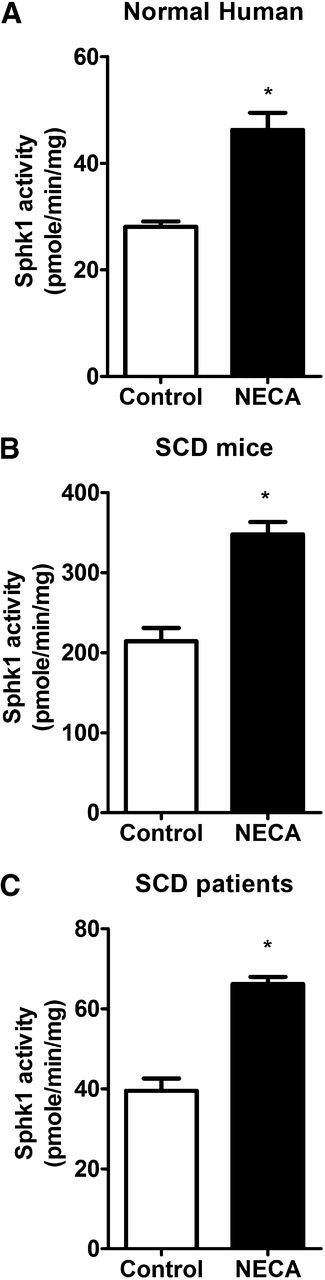
Adenosine directly increases erythrocyte SphK1 activity. SphK1 activity in cultured primary erythrocytes from (A) normal human subjects, (B) SCD transgenic mice, and (C) SCD patients after NECA (10 μM) treatment of 30 min. Values shown represent the mean ± SEM (n = 5 for SCD patients and normal human subjects; n = 6 for SCD transgenic mice and WT mice). *P < .05 NECA vs control.
Adenosine directly increases erythrocyte SphK1 activity. SphK1 activity in cultured primary erythrocytes from (A) normal human subjects, (B) SCD transgenic mice, and (C) SCD patients after NECA (10 μM) treatment of 30 min. Values shown represent the mean ± SEM (n = 5 for SCD patients and normal human subjects; n = 6 for SCD transgenic mice and WT mice). *P < .05 NECA vs control.
Additionally, we extended our study of normal erythrocytes to determine whether adenosine can also induce SphK1 activity in sickle human and mouse erythrocytes. Similar to the normal erythrocytes, we observed that the adenosine analog, NECA, can induce SphK1 activity in both mouse and human sickle erythrocytes (Figure 2B-C). Thus, we determined that adenosine is a common signaling molecule responsible for increased SphK1 activity in normal and sickle erythrocytes from both humans and mice.
Genetic deletion of adenosine deaminase leads to excess plasma adenosine and elevated erythrocyte SphK1 activity in vivo
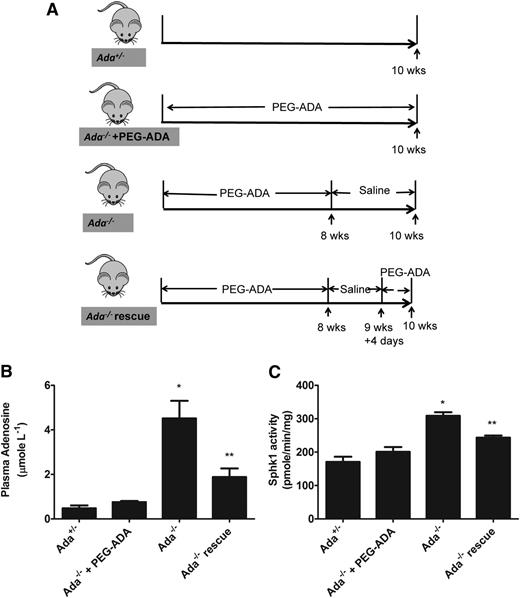
Although our in vitro studies using primary erythrocyte demonstrated that adenosine induces SphK1 activity in normal and sickle erythrocytes, whether in vivo changes of circulating adenosine concentrations can regulate erythrocyte SphK1 activity remain unknown. To address this question, we extended our in vitro study to in vivo using ADA-deficient mice (Ada−/−). ADA catalyzes the irreversible deamination of adenosine to inosine. As a result of ADA deficiency, mice accumulate high levels of adenosine in the circulation and in all tissues examined.18 Human and mice with ADA deficiency are lethal and need to take exogenous PEG-ADA enzyme to maintain a low plasma adenosine level and live normally. Once enzyme therapy is withdrawn, mice accumulate a high amount of plasma adenosine within days.18 Thus, we used PEG-ADA to regulate adenosine levels in Ada−/− mice as a powerful experimental strategy to investigate the role of adenosine on erythrocyte SphK1 activity in vivo. The specific experimental strategy is showed in Figure 3A. First, to allow Ada−/− mice to develop normally to adulthood, we treated the mice with PEG-ADA until they were 8 weeks old. Then the mice were divided into 4 groups as shown in Figure 3A: group 1, control Ada+/− mice; group 2, prevention group, continuously treated with PEG-ADA to prevent adenosine accumulation; group 3, phenotye group, PEG-ADA treatment withdrawn for 2 weeks to allow adenosine accumulation; and group 4, rescued group, PEG-ADA treatment withdrawn for 11 days to allow adenosine accumulation and followed with an additional dose of PEG-ADA treatment to lower adenosine. At the end of experiments, mice were euthanized, plasma and erythrocytes were collected, and circulating adenosine and erythrocyte SphK1 activity was measured. As expected, we found that after stopping PEG-ADA treatment, plasma adenosine was significantly increased in the Ada−/− mice compared with the control Ada+/− (Figure 3B). The elevated plasma adenosine led to significantly increased erythrocyte SphK1 activity in the Ada−/− mice (Figure 3C). In contrast, elevation of plasma adenosine was successfully prevented in the Ada−/− mice (prevention group) when continuously treated with PEG-ADA (Figure 3B). As such, SphK1 activity was not induced in the erythrocytes in the prevention group with continuous treatment of PEG-ADA (Figure 3C). Moreover, when we decreased the plasma adenosine level by readministering PEG-ADA in the rescued group (Figure 3B), the erythrocyte SphK1 activity was also reduced (Figure 3C). Therefore, our results provided solid genetic evidence that increased plasma adenosine levels induce erythrocyte SphK1 activity in vivo, and our preclinical studies revealed that PEG-ADA is a safe and effective drug to regulate adenosine levels and subsequently control erythrocyte SphK1 activity.
Elevated plasma adenosine induces erythrocyte SphK1 activity increase in vivo. (A) Schematic representation of mouse treatment strategy. (B) Plasma adenosine and (C) erythrocyte SphK1 activity in Ada+/−, Ada−/− with PEG-ADA treatment, Ada−/− without PEG-ADA treatment, and Ada−/− rescue. Values shown represent the mean ± SEM (n = 6 for each group). *P < .05 Ada−/− vs Ada−/−+ PEGADA;**P < .05 Ada−/− vs Ada−/− rescue.
Elevated plasma adenosine induces erythrocyte SphK1 activity increase in vivo. (A) Schematic representation of mouse treatment strategy. (B) Plasma adenosine and (C) erythrocyte SphK1 activity in Ada+/−, Ada−/− with PEG-ADA treatment, Ada−/− without PEG-ADA treatment, and Ada−/− rescue. Values shown represent the mean ± SEM (n = 6 for each group). *P < .05 Ada−/− vs Ada−/−+ PEGADA;**P < .05 Ada−/− vs Ada−/− rescue.
Genetic deletion of ADORA2B or antagonism of ADORA2B attenuates adenosine-induced SphK1 activity in cultured primary normal and sickle erythrocytes from mice and humans
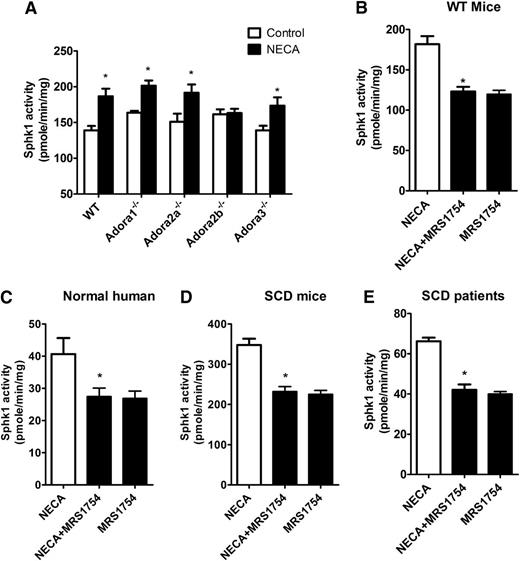
Adenosine is a potent signaling molecule that functions primarily by activating G protein–coupled receptors on target cells.34 There are 4 adenosine receptors: ADORA1, ADORA2A, ADORA2B, and ADORA3, each with a distinct affinity for adenosine and a distinct cellular and tissue distribution.35,36 To dissect which of the 4 adenosine receptors regulates erythrocyte SphK1 activity, we first took a genetic approach by using 4 adenosine receptor-deficient mice. First, we isolated erythrocytes from WT and 4 adenosine receptor-deficient mice and treated each with NECA. We found that NECA induced SphK1 activity in erythrocytes isolated from Adora1−/−, Adora2a−/−, and Adora3−/− mice similar to WT mice (Figure 4A). However, NECA-induced SphK1 activity was completely abolished in erythrocytes isolated from Adora2b−/− mice (Figure 4A), indicating that ADORA2B is essential for adenosine-induced SphK1 activity in normal mouse erythrocytes.
Adenosine signals through ADORA2B to induce erythrocyte SphK1 activity increase. (A) SphK1 activity in cultured primary erythrocytes from 4 adenosine receptor-deficient mice after NECA (10 μM) treatment for 30 min. (B-E) SphK1 activity in cultures of primary erythrocytes from (B) WT mice, (C) normal human subjects, (D) SCD transgenic mice, and (E) SCD patients after NECA (10 μM) treatment with and without AODRA2B antagonist MRS1754 (10 μM). Values shown represent the mean ± SEM (n = 5 for SCD patients and normal human subjects; n = 6 for SCD transgenic mice and WT mice). *P < .05 NECA vs NECA + MRS 1754 or NECA vs control.
Adenosine signals through ADORA2B to induce erythrocyte SphK1 activity increase. (A) SphK1 activity in cultured primary erythrocytes from 4 adenosine receptor-deficient mice after NECA (10 μM) treatment for 30 min. (B-E) SphK1 activity in cultures of primary erythrocytes from (B) WT mice, (C) normal human subjects, (D) SCD transgenic mice, and (E) SCD patients after NECA (10 μM) treatment with and without AODRA2B antagonist MRS1754 (10 μM). Values shown represent the mean ± SEM (n = 5 for SCD patients and normal human subjects; n = 6 for SCD transgenic mice and WT mice). *P < .05 NECA vs NECA + MRS 1754 or NECA vs control.
Next, to extend our mouse genetic studies, we conducted pharmacological studies to test the effects of blocking ADORA2B signaling with its specific antagonist in normal erythrocytes isolated from both mice and humans. We treated normal mouse and human erythrocytes with NECA in the presence or absence of the potent ADORA2B antagonist MRS1754 and found that MRS1754 completely blunted the induction of erythrocyte SphK1 activity by NECA (Figure 4B-C). Thus, our genetic and pharmacological studies provide strong evidence that ADORA2B signaling underlies adenosine-induced erythrocyte SphK1 activity in normal mouse and human erythrocytes. Finally, we further conducted pharmacological study to determine whether a specific ADORA2B antagonist has an effect on adenosine-induced SphK1 activity in sickle erythrocytes isolated from patients and mice. Similarly, we found that MRS1754 significantly attenuated NECA-induced SphK1 activities in cultured human and mouse sickle erythrocytes (Figure 4D-E). Altogether, we provided both human and mouse evidence that ADORA2B is required for adenosine-induced SphK1 activity in normal and sickle erythrocytes and that blocking ADORA2B signaling effectively inhibits adenosine-induced erythrocyte SphK1 activity.
Genetic deletion of ADORA2B abolishes excess plasma adenosine-induced erythrocyte SphK1 activity in vivo
Our in vitro studies demonstrated ADORA2B is required for adenosine-induced erythrocyte SphK1 activity. Next, to investigate the importance of ADORA2B in excess circulating adenosine-induced erythrocyte SphK1 activity in Ada−/− mice, we used ADA and ADORA2B double-deficient mice (Ada−/−/Adora2b−/−).20 Similarly, we took advantage of PEG-ADA enzyme therapy to regulate adenosine levels in these mice. As shown in Figure 5A, we treated both Ada−/−/Adora2b−/− and Ada−/−/Adora2b+/+ mice with PEG-ADA until they were 8 weeks old. Some of the mice were continuously treated with PEG-ADA, and for others, PEG-ADA treatment was terminated for 2 weeks. Without PEG-ADA treatment for 2 weeks, we found that plasma adenosine significantly increased in both Ada−/−/Adora2b−/− and Ada−/−/Adora2b+/+ mice compared with the treated groups (Figure 5B,D). When both Ada−/−/Adora2b+/+ and Ada−/−/Adora2b−/− were maintained with PEG-ADA enzyme therapy, they have a similar level of erythrocyte SphK1 activity (Figure 5C,E). However, 2 weeks after the enzyme therapy was withdrawn, although adenosine levels increased in both mice (Figure 5B and D), only Ada−/−/Adora2b+/+ mice showed significantly increased erythrocyte SphK1 activity (Figure 5C), whereas the SphK1 activity in Ada−/−/Adora2b−/− mice erythrocytes remained unchanged (Figure 5E). These results validated our in vitro studies and provided strong in vivo genetic evidence that ADORA2B is required for adenosine-mediated induction of erythrocyte SphK1 activity in Ada−/− mice.
Elevated plasma adenosine increases erythrocyte SphK1 activity through ADORA2B. (A) Schematic representation of mouse treatment strategy. (B) Plasma adenosine levels and (C) erythrocyte SphK1 activity in Ada−/−Adora2b+/+ mice before (+PEG-ADA) and after PEG-ADA treatment withdrawn (−PEG-ADA). (D) Plasma adenosine levels and (E) erythrocyte SphK1 activity in Ada−/−Adora2b−/− mice before (+PEG-ADA) and after PEG-ADA treatment withdraw (−PEG-ADA). Values shown represent the mean ± SEM (n = 6 for each group). *P < .05 −PEG-ADA vs +PEG-ADA.
Elevated plasma adenosine increases erythrocyte SphK1 activity through ADORA2B. (A) Schematic representation of mouse treatment strategy. (B) Plasma adenosine levels and (C) erythrocyte SphK1 activity in Ada−/−Adora2b+/+ mice before (+PEG-ADA) and after PEG-ADA treatment withdrawn (−PEG-ADA). (D) Plasma adenosine levels and (E) erythrocyte SphK1 activity in Ada−/−Adora2b−/− mice before (+PEG-ADA) and after PEG-ADA treatment withdraw (−PEG-ADA). Values shown represent the mean ± SEM (n = 6 for each group). *P < .05 −PEG-ADA vs +PEG-ADA.
PKA-mediated ERK1/2 activation functions downstream of ADORA2B and underlies adenosine-induced SphK1 activity in normal and sickle erythrocytes from both human and mice
The Gs-coupled ADORA2B signaling involves many downstream components including PKA37 and ERK1/2.38 Previous study showed the involvement of PKA in adenosine-ADORA2B-mediated induction of 2,3-bisphosphoglycerate in erythrocytes.25 Also, an early study showed that ERK1/2 can directly phosphorylate and activate SphK1.39 Therefore, we sought to test whether PKA and ERK1/2 are important intracellular signaling molecules functioning downstream of ADORA2B responsible for adenosine-induced SphK1 activity in erythrocytes. To directly test this possibility, we took advantage of using primary erythrocyte cultures coupled with pharmacological tools. First, we treated erythrocytes isolated from normal human and mice with or without NECA in the presence or absence of a specific PKA inhibitor, H89, or ERK1/2 inhibitor, PD98059. We found that NECA-induced SphK1 activity was significantly reduced by either H89 or PD98059 as MRS1754, a specific ADORA2B antagonist (Figure 6A-B). PKA and ERK inhibitors each prevented NECA-mediated induction of erythrocyte SphK1 activity, suggesting that PKA and ERK1/2 work in an upstream and downstream manner. To test this intriguing possibility, we treated normal human and mouse erythrocytes with forskolin, a potent and specific PKA agonist, in the presence or absence of PD98059. We found that forskolin treatment directly induced SphK1 activity and PD98059 significantly attenuated forskolin-induced SphK1 activity in primary cultured normal human and mouse erythrocytes (Figure 6A-B).
PKA-mediated activation of ERK1/2 underlies adenosine-ADORA2B-mediated erythrocyte SphK1 activation in WT mice and normal human individuals. SphK1 activity, membrane bound total, and phosphorylated SphK1 in primary erythrocytes from WT mice (A and C) and normal human subjects (B and D) after treatment with N (10 μM NECA), N+M (10 μM NECA + 10 μM MRS1754), N+H (10 μM NECA + 10 μM H89), N+P (10 μM NECA + 20 μM PD98059), F (10 μM Forskolin), and F+P (10 μM Forskolin+ 20 μM PD98059) for 30 minutes. Values shown represent the mean ± SEM (n = 5 for normal human subjects and n = 4 for WT mice). *P < .05 N or F vs control; **P < .05 N+M, N+H, N+P vs N; ***P < .05 F+P vs F.
PKA-mediated activation of ERK1/2 underlies adenosine-ADORA2B-mediated erythrocyte SphK1 activation in WT mice and normal human individuals. SphK1 activity, membrane bound total, and phosphorylated SphK1 in primary erythrocytes from WT mice (A and C) and normal human subjects (B and D) after treatment with N (10 μM NECA), N+M (10 μM NECA + 10 μM MRS1754), N+H (10 μM NECA + 10 μM H89), N+P (10 μM NECA + 20 μM PD98059), F (10 μM Forskolin), and F+P (10 μM Forskolin+ 20 μM PD98059) for 30 minutes. Values shown represent the mean ± SEM (n = 5 for normal human subjects and n = 4 for WT mice). *P < .05 N or F vs control; **P < .05 N+M, N+H, N+P vs N; ***P < .05 F+P vs F.
Because an early study showed that ERK1/2 directly induced serine225 phosphorylation and trans-localization of SphK1 to the cell membrane,39 it is possible that NECA treatment induces SphK1 phosphorylation at serine 225 and subsequent trans-localization to the membrane in an ADORA2B-PKA-ERK-dependent manner. To test this idea, we isolated membrane proteins from normal mouse and human erythrocytes treated with or without NECA in the presence or absence of an ADORA2B antagonist (MRS1754), PKA inhibitor (H-89), or ERK1/2 inhibitor. Some of those cells were treated with the PKA agonist forskolin. First, western blot analysis coupled with image quantification demonstrated that NECA and forskolin treatment induced specific phosphorylation of SphK1 at the serine 225 residue and increased SphK1 trans-localization to the membrane of normal human and mouse erythrocytes (Figure 6C-D). Additionally, we found that NECA-mediated induction of phosphorylated SphK1 at Ser225 and membrane bound SphK1 levels were significantly reduced by ADORA2B antagonist (M), PKA inhibitor (H), and ERK1/2 (P) inhibitor, respectively (Figure 6C-D). Similar to Sphk1 activity, we found that the ERK1/2 inhibitor (P) significantly reduced forskolin-induced phosphorylation of SphK1 at Ser225 and membrane-bound SphK1 levels (Figure 6C-D). Thus, both SphK1 activity and western blot analysis provide strong evidence that adenosine signaling via ADORA2B activation coupled with PKA-mediated ERK1/2 activation directly phosphorylates SphK1, increases its trans-localization to the membrane, and thereby induces its activity in normal human and mouse erythrocytes.
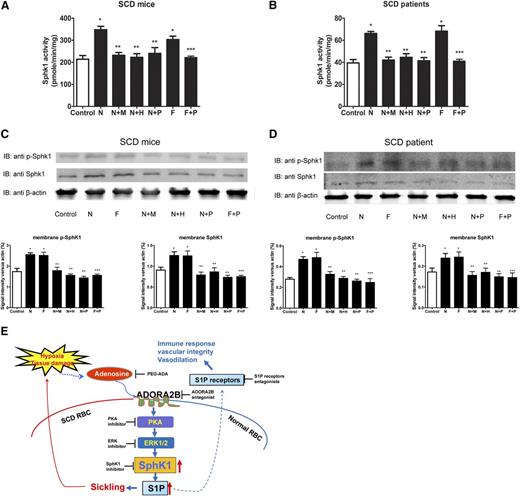
Next, we extended normal erythrocytes to sickle erythrocytes to assess whether PKA-ERK1/2 is the key intracellular signaling cascade functioning downstream of ADORA2B responsible for adenosine-induced SphK1 activity in cultured primary sickle erythrocytes isolated from both patients and mice. Similarly, we found that NECA-induced SphK1 activities, Ser225 phosphorylated SphK1, and membrane-bound SphK1 in sickle mouse and human cells (Figure 7A-D). Moreover, the NECA-mediated increase of SphK1 activity, SphK1 phosphorylation, and membrane trans-localization was significantly reduced by H89 or PD98059 in sickle erythrocytes from both patients and mice (Figure 7A-D). Finally, we found that forskolin directly induced erythrocyte SphK1 activation, phosphorylation, and membrane trans-localization, whereas PD98059 blocked forskolin-induced SphK1 activity, phosphorylation, and membrane trans-localization (Figure 7A-D). Thus, our studies revealed that ADORA2B-mediated activation of PKA is responsible for adenosine-induced SphK1 activity by ERK1/2-dependent phosphorylation of SphK1 in both normal and sickle erythrocytes.
PKA-mediated activation of ERK1/2 underlies adenosine-ADORA2B-mediated erythrocyte SphK1 activation in SCD Tg mice and SCD patients. SphK1 activity, membrane bound total, and phosphorylated SphK1 in primary erythrocytes from (A,C) SCD transgenic mice and (B,D) SCD patients after treatment with N (10 μM NECA), N+M (10 μM NECA + 10 μM MRS1754), N+H (10 μM NECA + 10 μM H89), N+P (10 μM NECA + 20 μM PD98059), F (10 μM Forskolin), and F+P (10 μM Forskolin+ 20 μM PD98059) for 30 minutes. Values shown represent the mean ± SEM (n = 3 for SCD patients and n = 5 for normal human subjects; n = 3 for SCD transgenic mice and n = 4 for WT mice). *P < .05 N or F vs control; **P < .05 N+M, N+H, N+P vs N; ***P < .05 F+P vs F. (E) Working model: hypoxia or tissue damage leads to increased plasma adenosine which signals through ADORA2B and subsequent PKA and ERK1/2 pathways to activate SphK1 and produce more S1P in erythrocyte.
PKA-mediated activation of ERK1/2 underlies adenosine-ADORA2B-mediated erythrocyte SphK1 activation in SCD Tg mice and SCD patients. SphK1 activity, membrane bound total, and phosphorylated SphK1 in primary erythrocytes from (A,C) SCD transgenic mice and (B,D) SCD patients after treatment with N (10 μM NECA), N+M (10 μM NECA + 10 μM MRS1754), N+H (10 μM NECA + 10 μM H89), N+P (10 μM NECA + 20 μM PD98059), F (10 μM Forskolin), and F+P (10 μM Forskolin+ 20 μM PD98059) for 30 minutes. Values shown represent the mean ± SEM (n = 3 for SCD patients and n = 5 for normal human subjects; n = 3 for SCD transgenic mice and n = 4 for WT mice). *P < .05 N or F vs control; **P < .05 N+M, N+H, N+P vs N; ***P < .05 F+P vs F. (E) Working model: hypoxia or tissue damage leads to increased plasma adenosine which signals through ADORA2B and subsequent PKA and ERK1/2 pathways to activate SphK1 and produce more S1P in erythrocyte.
Discussion
Erythrocyte SphK1 plays an important role in supplying plasma S1P, a bioactive signaling lipid involved in multiple cellular and systemic functions.40 Recent studies revealed that SphK1 activity is elevated in sickle erythrocytes of human and mice with SCD and that elevated SphK1 activity contributes to disease pathophysiology.17 Additional evidence showed that SphK1 activity is further induced under hypoxic conditions.41 However, specific molecules and signaling pathways responsible for regulating SphK1 activity in sickle erythrocytes remain unidentified. Testing a series of hypoxia-related molecules and molecules known to induce SphK1 activity in other cell types, we identified that adenosine signaling via ADORA2B is a previously unrecognized signaling pathway that stimulates SphK1 activity in cultured primary normal and sickle erythrocytes isolated from both humans and mice. Intriguingly, using a genetic approach, we provided in vivo evidence that excess plasma adenosine induces erythrocyte SphK1 activity in ADA-deficient mice. ADA enzyme therapy or genetic deletion of ADORA2B completely abolishes excess adenosine-induced erythrocyte SphK1 activity in ADA-deficient mice. Finally, we provided both human and mouse evidence that ADORA2B activation-mediated PKA signaling is responsible for adenosine-induced SphK1 activity by ERK1/2-mediated phosphorylation of SphK1 in both normal and sickle erythrocytes. Taken together, we revealed a novel role of adenosine signaling in erythrocyte physiology and pathology by regulating SphK1 activity and thereby identified a new means to regulate SphK1 activity in normal individuals and SCD patients.
Erythrocytes contain high SphK1 activity and lack S1P degrading enzymes.13 Thus, erythrocytes are considered to be one of the major cell types producing circulating S1P.11 We showed that SphK1 activity in human normal and sickle erythrocytes is only one third of normal and sickle mouse erythrocytes (Figures 1 and 2). Consistently, human plasma S1P level is only one third of that in mice.3,4 However, this does not compromise the importance of elevated SphK1 in human physiology and pathology, specifically in SCD. For example, we and other group have independently reported that circulating and erythrocyte S1P levels are significantly elevated in SCD patients.17,42 Moreover, we showed that inhibiting SphK1 activation directly decreases hypoxia-induced sickling by reducing S1P production in human erythrocyte in vitro.17 Thus, lower basal level of SphK1 activity in human erythrocyte relative to mouse erythrocyte does not exclude the important role of elevated SphK1-mediated S1P production in human sickle erythrocytes. Here, we found adenosine, a signaling molecule well known to be induced by hypoxia, induces erythrocyte SphK1 activity by activating ADORA2B in a PKA/ERK1/2-dependent manner. Moreover, we report for the first time that ERK1/2 functions downstream of PKA in a linear sequence rather than parallel pathways underlying ADORA2B-mediated activation of SphK1 by phosphorylation of SphK1 specifically on Ser225 and increasing membrane-bound SphK1 levels in both normal and sickle human and mouse erythrocytes. Thus, we revealed a previously unrecognized signaling cascade regulating erythrocyte SphK1 in normal and sickle erythrocytes from human and mice. These findings also point out that the difference of SphK1 activity between human and mouse erythrocytes is likely due to the difference of basal protein levels of our newly identified molecules involved regulating erythrocyte SphK1 activity including ADORA2B, PKA, ERK, and SphK1 itself in those 2 species. The comparison of the basal expression levels of all of those identified candidates involved in regulating SphK1 activity in human and mouse erythrocyte is interesting for future investigation.
More importantly, previous studies demonstrated that circulating adenosine level is significantly increased in patients and mice with SCD and that excess circulating adenosine functions via ADORA2B, contributing to sickling by inducing 2,3-bisphosphoglycerate (2,3-BPG), a potent allosteric modulator, triggering O2 release and thereby inducing polymerization of deoxy-hemoglobin S and sickling.25 Thus, our recent studies have added significant new insights by revealing dual mechanisms underlying elevated adenosine signaling via ADORA2B in sickling: (1) inducing 2,3-bisphosphoglycerate (BPG) and deoxygenation and of sickle hemoglobin leading to polymerization and sickling25 ; and (2) inducing SphK1 activity and elevation of erythrocyte and plasma S1P (Figure 7E). Without interference, increased sickling leads to more severe hypoxia and additional adenosine production. As such, elevated adenosine signaling via ADOAR2B functions as a malicious cycle to induce more 2,3-BPG and further elevated SphK1 and eventually disease progression. Thus, lowering circulating adenosine and interfering with erythrocyte ADORA2B are likely promising therapies to lower SphK1 activity and 2,3-BPG and therefore reduce sickling and disease progression. Notably, previous studies also indicated potential benefits incurred by adenosine via ADORA2A to inhibit invariant nature killer cells and nature killer cells to reduce pulmonary inflammation and injury in SCD.43,44 Taken together, these studies indicate that adenosine signaling exerts multiple functions in SCD depending on the different tissue and cell types and adenosine receptor expression profile. Targeting specific adenosine receptors will probably provide better treatment strategies for SCD patients.
Our innovative finding that adenosine signaling via ADORA2B induces SphK1 activity in a PKA/ERK1/2-dependent manner in sickle erythrocytes led us to further discover that this novel signaling network also plays an important role in stimulating SphK1 activity in both human and mouse normal erythrocytes. It is well known that the concentration of adenosine in circulation increases under energy depletion and ischemic or hypoxic conditions.45-47 It has been speculated that increased adenosine levels under acute hypoxic conditions may be beneficial by increasing blood flow to ischemic or hypoxic tissue owing to its potent vasodilatory effect. More recent studies showed that increased adenosine signaling via ADORA2B promotes 2,3-BPG induction in normal erythrocytes, and thus it may prevent hypoxia-mediated acute tissue injury.25 Unfortunately, there are situations in which persistent chronically elevated adenosine signaling functions to exacerbate tissue injury and fibrosis. For example, the uncontrolled accumulation of adenosine in ADA-deficient mice leads to widespread activation of adenosine receptors with detrimental effects in many areas of pathology including pulmonary inflammatory damage,20,48,49 priapism,50,51 and chronic kidney injury.37 Our study here using ADA-deficient mice and PEG-ADA enzyme therapy provides genetic evidence indicating the important and previously unknown function of excess adenosine signaling in regulating erythrocyte SphK1 activity. PEG-ADA enzyme therapy has been used to treat ADA-deficient humans for >30 years. We showed here that PEG-ADA can successfully prevent or reverse excess adenosine-induced erythrocyte SphK1 activity. Moreover, genetic deletion of ADORA2B in ADA-deficient mice prevented excess adenosine-induced stimulatory effects on erythrocyte SphK1 activity in vivo. Also, using ADA-deficient mice and ADA/ADORA2B double-deficient mice coupled with PEG-ADA enzyme therapy to regulate adenosine levels, we provided solid in vivo genetic evidence that adenosine signaling via ADORA2B contributes to increased SphK1 activity in normal mouse erythrocytes. Thus, our findings support a new working model: increased circulating adenosine due to hypoxia or tissue injury causes increased erythrocyte SphK1 activity. Increased SphK1 leads to elevated S1P production in the erythrocytes and subsequent increased circulating S1P. Elevated S1P in the circulation likely exerts multiple important effects in endothelial integrity, inflammation, and tissue damage in response to ischemic injuries52 (Figure 7E).
In conclusion, nothing was known about the role of adenosine signaling in activation of erythrocyte SphK1 in normal and SCD prior to our studies. Therefore, this discovery sheds new light on how adenosine, a fast-responsive signaling molecule to energy depletion and tissue injury, activates ADORA2B to induce erythrocyte SphK1 activity, resulting in the elevation of S1P in erythrocytes and circulation. Moreover, besides erythrocytes, endothelial cells and platelets also contribute a significant part of circulating S1P.53,54 Our finding on adenosine signaling via ADORA2B-inducing erythrocyte SphK1 activity is likely not limited to erythrocytes and may also regulate SphK1 activity and S1P generation in other cell types, including endothelial cells, platelets, and cancer cells. Finally, our findings are highly significant because the novel role of adenosine signaling via ADORA2B in erythrocyte SphK1 activity provides a new means to regulate SphK1 activity in normal individuals and SCD patients.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by National Institute of Health National Institute of Diabetes and Digestive and Kidney Diseases grant DK083559 (to Y.X.), and National Heart, Lung, and Blood Institute grants HL119549 and HL113574 (to Y.X.) and American Heart Association grant 12IRG9150001 (to Y.X.).
Authorship
Contribution: K.S. carried out the generation and maintenance of Ada−/− and Ada−/−/Adora2b−/− mice, collection and withdrawal of mouse blood, culture and treatment of primary erythrocytes, measurement of plasma adenosine and SphK1 activity, erythrocyte membrane isolation and western blot, statistical analysis, and contributed to the generation of figures; Y.Z. and A.S. provided assistance in adenosine measurement and primary erythrocyte culture; M.V.B. helped to set up SphK1 activity assay system; Y.Z. and H.W. generated and maintained SCD transgenic mice; J.L. contributed to the breeding of 4 adenosine receptor-deficient mice; W.D. provided expertise in lipid kinase activity analysis; M.I. and H.S.J. were the hematologists that identified and collected normal and SCD patient blood; J.G.M. provided expertise in generating and maintaining Ada−/− and Ada−/−/Adora2b−/− mice; M.R.B. provided Ada−/−/Adora2b−/− mice; R.E.K. helped in analyzing adenosine signaling and manuscript editing; and Y.X. was the principal investigator, oversaw the design of experiments and interpretation of results, wrote and organized the manuscript including the text and figures, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Yang Xia, Department of Biochemistry and Molecular Biology, University of Texas-Houston Medical School, 6431 Fannin, MSB 6.202 Houston, TX 77030; e-mail: yang.xia@uth.tmc.edu.