Key Points
Hermansky-Pudlak syndrome exhibits impaired granule exocytosis and PDI secretion that contribute to its associated bleeding disorder.
Endothelial cells deficient in HPS6 show defective secretion of granules, including Weibel-Palade bodies.
Abstract
Protein disulfide isomerase (PDI), secreted from platelets and endothelial cells after injury, is required for thrombus formation. The effect of platelet and endothelial cell granule contents on PDI-mediated thrombus formation was studied by intravital microscopy using a mouse model of Hermansky-Pudlak syndrome in which platelet dense granules are absent. Platelet deposition and fibrin generation were nearly absent, and extracellular PDI was significantly reduced in HPS6−/− mice after vascular injury. HPS6−/− platelets displayed impaired PDI secretion and impaired exocytosis of α granules, lysosomes, and T granules due to decreased sensitivity to thrombin, but these defects could be corrected by addition of subthreshold amounts of adenosine 5′-diphosphate (ADP). Human Hermansky-Pudlak syndrome platelets demonstrated similar characteristics. Infusion of wild-type platelets rescued thrombus formation in HPS6−/− mice. Human umbilical vein endothelial cells in which the HPS6 gene was silenced displayed impaired PDI secretion and exocytosis of Weibel-Palade bodies. Defective thrombus formation in Hermansky-Pudlak syndrome, associated with impaired exocytosis of residual granules in endothelial cells and platelets, the latter due to deficiency of ADP, is characterized by a defect in T granule secretion, a deficiency in extracellular PDI secretion, and impaired fibrin generation and platelet aggregation. Hermansky-Pudlak syndrome is an example of a hereditary disease whereby impaired PDI secretion contributes to a bleeding phenotype.
Introduction
Hermansky-Pudlak syndrome is an autosomal recessive disorder characterized by oculocutaneous albinism, platelet dysfunction associated with bleeding, and lysosomal storage defects.1,2 In mice, 16 loci are associated with Hermansky-Pudlak syndrome, including HPS6. The HPS6 gene encodes a novel protein (HPS6) in the biogenesis of lysosome-related organelles complex (BLOC)-2 of HPS3, HPS5, and HPS6, which regulates the synthesis of lysosome-related organelles, including melanosomes and platelet dense granules.3-5 HPS6−/− platelets, deficient in dense granules in mice,3 have not been fully characterized in humans, although all HPS6 patients studied have lacked dense bodies.6,7 These mice offer an opportunity to explore the contribution of granules during thrombus formation. Among the contents of platelet dense granules, polyphosphates have been proposed as activators through factor XII or factor V on platelet stimulation and polyphosphate secretion.8-10 The absence of dense granules has been hypothesized as a cause of bleeding associated with Hermansky-Pudlak syndrome.9
Protein disulfide isomerase (PDI) in the endoplasmic reticulum plays a critical role in protein synthesis. However, PDI has an extracellular role in thrombus formation.11-14 Stimulated platelets and endothelial cells both secrete PDI11,15 from storage granules: T granules in platelets16 and small Gro-α-containing granules in endothelial cells.15 Extracellular PDI is captured in flowing blood by activated αIIbβ3 on platelets and activated αVβ3 on the endothelium.17 PDI is required for thrombus formation, and inhibition of PDI activity blocks both platelet accumulation and fibrin generation at the site of injury.11,18
We evaluated the role of platelet and endothelial cell granules and their contents in fibrin generation and platelet thrombus formation in Hermansky-Pudlak syndrome characterized by platelets lacking dense granules. Using a mouse model of Hermansky-Pudlak syndrome, we determined that these mice demonstrate a defect in thrombus formation. Although Hermansky-Pudlak syndrome platelets and wild-type (WT) endothelial cells in which the HPS6 gene has been silenced contain PDI, their remaining granules demonstrate decreased sensitivity to thrombin as an agonist and show impaired release of PDI and other granule constituents in vitro and in vivo; the addition of subthreshold amounts of ADP rescued this defect in platelets in vitro. Human Hermansky-Pudlak syndrome platelets also showed impaired α granule exocytosis, thiol isomerase activity secretion, and PDI antigen release. Defective thrombus formation in Hermansky-Pudlak syndrome, associated with impaired exocytosis of the residual granules in platelets due to a deficiency of ADP, is characterized by a defect in T granule secretion, a deficiency in extracellular PDI, and impaired fibrin generation and platelet aggregation. Hermansky-Pudlak syndrome is a hereditary disease whereby impaired PDI secretion contributes to a bleeding phenotype.
Materials and methods
Mice
C57BL/6J mice and B6.Cg-Hps6ru/J mice were obtained from The Jackson Laboratory (Bar Harbor, ME). The Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee approved all animal care and procedures.
Preparation of mouse platelets
Sodium citrate–treated mouse blood was obtained from WT or HPS6−/− mice, and the platelet-rich plasma was collected and centrifuged in the presence of 0.5 μM prostaglandin E1. The pellet was washed in N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES)–buffered Tyrode (HTG) solution containing 0.25 μM prostaglandin E1.
Preparation of human platelets
Human platelets were prepared from whole blood obtained by venipuncture from volunteers and a Hermansky-Pudlak syndrome patient (type 1)11 with Institutional Review Board approval.
PCR genotyping
Polymerase chain reaction (PCR) was performed with TaKaRa Premix Taq Hot-Start DNA Polymerase (Clontech Laboratories) with HPS6-specific forward (5′-GTTAAGGACCAACCAGAGCAGCTTTCAACG-3′) and reverse (5′-CAAGTGTCCCCTTGTTTGGGA-3′) primers. PCR amplification (35 cycles) was carried out at 94°C for 2 minutes, 94°C for 0.5 minutes, 60°C for 0.5 minutes, and 72°C for 1 minute, followed by final extension at 72°C for 10 minutes. PCR-purified DNA was digested with BsrD1 (New England Biolabs) and analyzed by agarose gel electrophoresis. Genotypes were distinguished by their banding patterns: HPS6+/+, bands at 182 bp and 123 bp; HPS6+/−, bands at 305 bp, 182 bp, and 123 bp; HPS6−/−, band at 305 bp.
Intravital microscopy in a mouse model of laser-induced arteriolar thrombosis
Intravital videomicroscopy of the cremaster muscle microcirculation was performed as described previously.18,19 Antibodies labeled with Alexa Fluor or DyLight were infused into mice prior to arteriolar wall injury. Image analysis was performed using SlideBook v5.0 (Intelligent Imaging Innovations). Representative images are presented, but the median curves include the full data. The kinetics of PDI accumulation, fibrin generation, and platelet thrombus formation were analyzed by determining median fluorescence values over time in ∼19 to 30 thrombi.
Antibodies and reagents
Anti-platelet CD42b monoclonal antibody conjugated to DyLight 649 was purchased from Emfret Analytics (Eibelstadt, Germany); anti-PDI antibody RL90 from Abcam (Cambridge, MA); polyclonal rabbit anti-PDI antibody DL-11 from Sigma-Aldrich (St Louis, MO); fluorescein isothiocyanate–labeled anti-mouse CD62P from BD Biosciences; Alexa Fluor 488–labeled anti-mouse lysosome-associated membrane protein (LAMP)-1 from BioLegend; anti- Toll-like receptor 9 (TLR9) from Imgenex; and mouse thrombin from Haematologic Technologies (Essex Junction, VT). A mouse monoclonal anti-fibrin-specific antibody was derived from hybridoma cell line 59D8.20 Immunoaffinity-purified rabbit anti-PDI antibody, mouse anti-fibrin antibody, and nonimmune control immunoglobulin G were labeled with Alexa Fluor 488.
Di-E-GSSG assay for thiol isomerase activity
Reductase activity was assayed by measuring the reduction of a di-eosin glutathione disulfide (di-E-GSSG) probe in the presence of dithiothreitol. The probe was synthesized by reacting eosin isothiocyanate with oxidized glutathione.21
P-selectin, TLR9, LAMP1, and PF-4 exocytosis
P-selectin, TLR9, and LAMP1 expression of the activated platelet surface was monitored by flow cytometry using specific antibodies. Platelet factor 4 (PF-4) was quantitated using a sandwich enzyme-linked immunosorbent assay kit (Abcam). For PDI quantitation, releasate from stimulated platelets was resolved using sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with anti-PDI antibodies.
Immunogold electron microscopy
Washed WT and HPS6−/− platelets were examined for PDI localization by electron microscopy.16 Electron microscopy was performed in a JEOL 1200 EX transmission electron microscope, and images were recorded with an AMT 2k CCD camera.
RNA silencing of HPS6 in endothelial cells
Human umbilical vein endothelial cells (HUVECs; Lonza) were cultured in endothelial growth medium (EGM-2; Lonza) supplemented with 2% fetal bovine serum, 0.4% human fibroblast growth factor, 0.1% vascular endothelial growth factor, 0.1% R3 insulin-like growth factor 1, 0.1% human epidermal growth factor, 0.04% hydrocortisone, 0.1% ascorbic acid, 0.1% heparin, and 0.1% gentamicin-ampicillin. Cells from passages 2 to 4 were used for transfection. Cells were seeded and grown to 80% confluence, washed with endothelial growth basal medium (EBM-2; Lonza) and incubated with transfection media containing 50 pmol of pooled HPS6 small interfering (si)RNA (Sigma-Aldrich) or control siRNA for 6 hours. Cells were used for exocytosis assays 72 hours posttransfection.
Regulated secretion of PDI and VWF from endothelial cells
The cells were incubated with human α-thrombin (1 NIH U/mL) in basal media, and aliquots of releasate were collected at indicated times in buffer containing β-mercaptoethanol. The cells were lysed with radioimmunoprecipitation assay buffer, and lysates were collected. The releasates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by western blot analysis for PDI (DL-11) and von Willebrand factor (VWF; rabbit polyclonal; Dako). The band intensities were quantitated using the GE ImageQuant LAS 4000 imaging system. The transfection efficiency was confirmed using western blot analysis of the cell lysates using rabbit polyclonal anti-human HPS antibody (HPS6d), a gift from Dr Esteban Dell’Angelica (University of California, Los Angeles).5
Results
Thrombus formation is defective in HPS6−/− mice
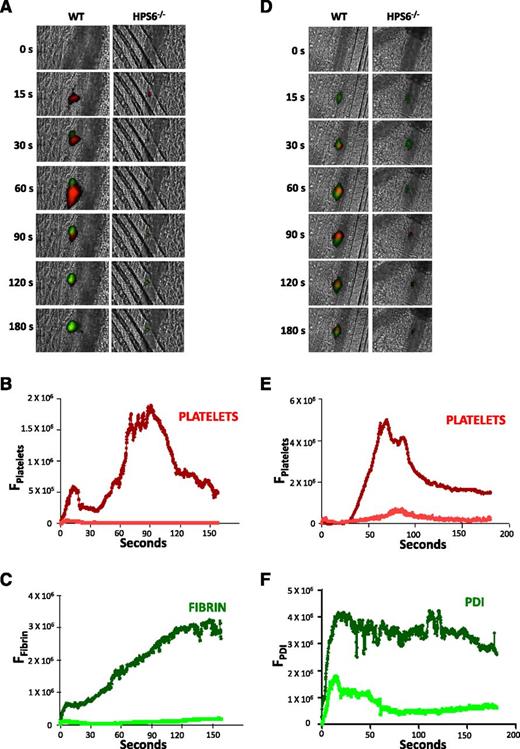
HPS6−/− platelets lack dense granules; however, α granules and lysosomes are present in normal abundance.6 To characterize thrombus formation in HPS6−/− mice, we compared platelet accumulation and fibrin generation after laser injury of mouse cremaster arterioles using intravital microscopy. Platelet accumulation and fibrin generation at the site of vascular injury, visualized using fluorescently labeled antibodies, were nearly absent in HPS6−/− mice when compared with WT mice (Figure 1A). Quantitative results are presented for the median fluorescence associated with platelets and fibrin from multiple thrombi in each group (Figure 1B-C). Minimal platelet accumulation and fibrin generation were observed in HPS6−/− mice compared with WT mice. These results demonstrate defective platelet thrombus formation and fibrin generation after injury in mice lacking platelet dense granules.
Platelet thrombus formation, fibrin generation, and PDI secretion after laser-induced arteriolar wall injury in HPS6−/− and WT mice. Platelet-specific anti-CD42b antibody and fibrin-specific mouse anti-human fibrin monoclonal antibody were infused into mice, and the cremaster arteriole subjected to laser injury and the induction of thrombus formation. (A) Representative images of the fluorescence associated with fibrin (green) and platelets (red) over 180 seconds after laser-induced vessel wall injury. (B) Median integrated platelet fluorescence intensity vs time in WT mice (dark red) and HPS6−/− mice (red). (C) Median integrated fibrin fluorescence intensity vs time in WT mice (dark green) and HPS6−/− mice (green). Data are from 30 thrombi in 3 mice for HPS6−/− and WT mice. In panels A-C, platelet-specific anti-CD42b antibody and nonblocking polyclonal anti-PDI antibody were infused into mice to detect platelets and PDI during thrombus formation. (D) Representative images of fluorescence associated with PDI (green) and platelets (red) over 180 seconds of thrombus formation after laser-induced vessel wall injury in WT and HPS6−/− mice. (E) Median integrated platelet fluorescence intensity during thrombus formation in WT mice (dark red) and HPS6−/− mice (red). (F) Median integrated PDI fluorescence intensity during thrombus formation in WT mice (dark green) and HPS6−/− mice (green). F, fluorescence intensity.
Platelet thrombus formation, fibrin generation, and PDI secretion after laser-induced arteriolar wall injury in HPS6−/− and WT mice. Platelet-specific anti-CD42b antibody and fibrin-specific mouse anti-human fibrin monoclonal antibody were infused into mice, and the cremaster arteriole subjected to laser injury and the induction of thrombus formation. (A) Representative images of the fluorescence associated with fibrin (green) and platelets (red) over 180 seconds after laser-induced vessel wall injury. (B) Median integrated platelet fluorescence intensity vs time in WT mice (dark red) and HPS6−/− mice (red). (C) Median integrated fibrin fluorescence intensity vs time in WT mice (dark green) and HPS6−/− mice (green). Data are from 30 thrombi in 3 mice for HPS6−/− and WT mice. In panels A-C, platelet-specific anti-CD42b antibody and nonblocking polyclonal anti-PDI antibody were infused into mice to detect platelets and PDI during thrombus formation. (D) Representative images of fluorescence associated with PDI (green) and platelets (red) over 180 seconds of thrombus formation after laser-induced vessel wall injury in WT and HPS6−/− mice. (E) Median integrated platelet fluorescence intensity during thrombus formation in WT mice (dark red) and HPS6−/− mice (red). (F) Median integrated PDI fluorescence intensity during thrombus formation in WT mice (dark green) and HPS6−/− mice (green). F, fluorescence intensity.
PDI accumulation on the injured vessel wall is reduced in HPS6−/− mice
Although the defect in platelet accumulation was expected on the basis of the absence of platelet dense granules, thus confirming previous studies demonstrating diminished platelet thrombi in ruby-eye mice in response to vessel wall injury,22 the defect in fibrin generation was unanticipated. The absence of fibrin generation cannot be explained by the absence of platelet accumulation because we have previously shown normal fibrin formation in Par4−/− mice in whom platelets cannot be thrombin activated23 ; in WT mice treated with eptifibatide, an inhibitor that blocks platelet accumulation15,24 ; and in chimeric mice lacking platelet β3 integrins.17 In contrast, there is no fibrin generation in β3−/− mice lacking both platelet αIIbβ3 and endothelial αVβ3.17 Given the importance of PDI in fibrin generation,11,18 we examined whether the absence of fibrin generation in the HPS6−/− mice was related to diminished platelet and endothelial cell secretion of PDI.
PDI accumulation in thrombi was evaluated in vivo in WT and HPS6−/− mice in parallel with platelet thrombus formation. PDI was detected using a noninhibitory anti-PDI antibody,11 and platelets were detected using an antibody directed against GPIbβ (Figure 1D). Platelet accumulation in WT mice and in HPS6−/− mice is shown in Figure 1A,D. WT mice showed the characteristic kinetics of platelet and PDI accumulation,11,15 with platelets peaking at ∼80 to 90 seconds (Figure 1E) and PDI appearing immediately after vessel wall injury and remaining elevated at the site of injury over the 180 seconds of observation (Figure 1F). In contrast, HPS6−/− mice showed minimal platelet accumulation (Figure 1E) and an initial significantly smaller accumulation of extracellular PDI at the site of vessel injury (Figure 1F); this accumulation dissipated rapidly.
HPS6−/− platelets store and secrete PDI
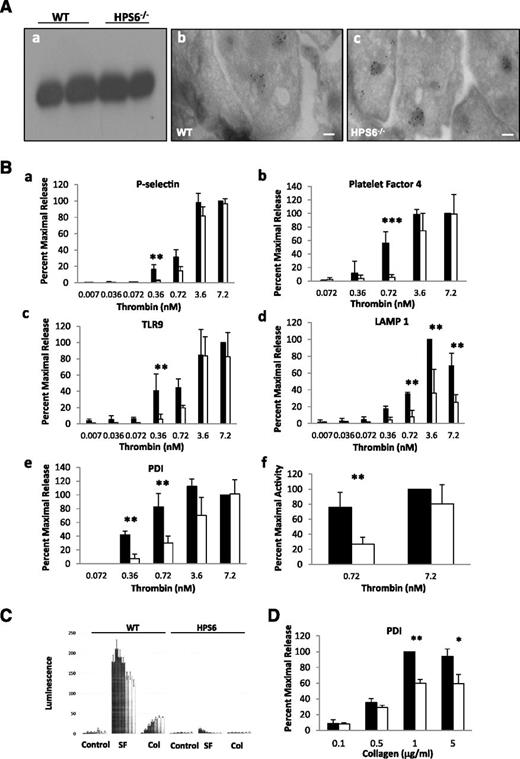
HPS6−/− platelets were examined for their ability to store PDI. PDI is stored in unstimulated WT platelets and released with the addition of agonists.11 We investigated whether deficiency in platelet dense granules influences the storage of PDI. As determined by immunoblotting, the lysates of unstimulated WT and HPS6−/− mouse platelets contained approximately equivalent amounts of PDI (Figure 2Aa). The results indicate that the diminished amount of extracellular PDI during thrombus formation in vivo in the HPS6−/− mice is not due to diminished PDI storage.
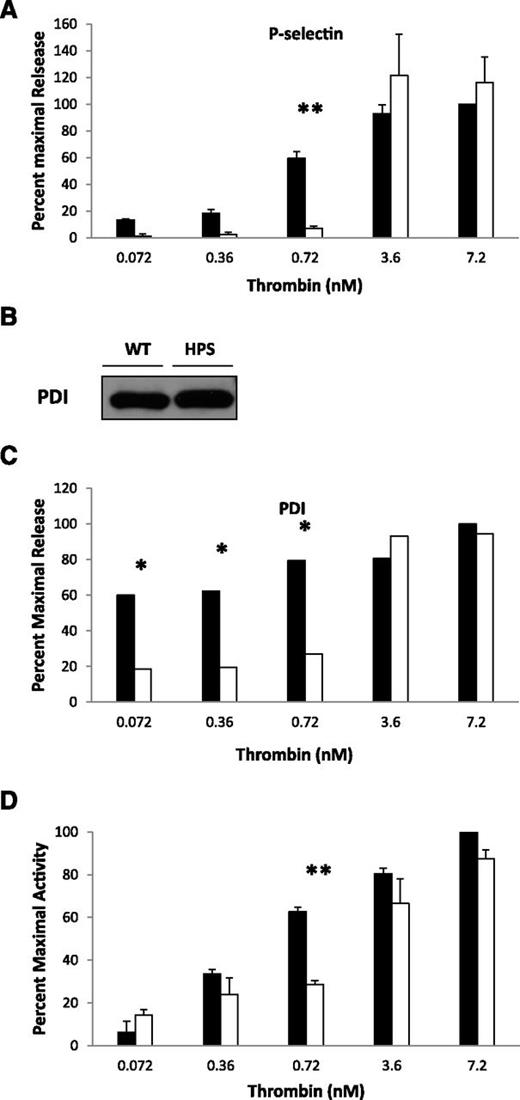
Localization of PDI and granule release in WT and HPS6−/− platelets. (A) Localization of PDI in platelet granules. (a) Unstimulated platelets isolated from WT and HPS6−/− mice were sedimented by centrifugation. The pellet was solubilized with SDS lysis buffer to generate the lysate. Lysate of intact resting platelets from WT and HPS6−/− mice was subjected to SDS-PAGE and blotted with anti-PDI antibodies DL-11. (b) Washed WT mouse resting platelets were fixed, frozen, and sectioned before mounting on Formvar carbon-coated copper grids. Ultrathin platelet sections were probed for PDI, and bound antibody labeled with Protein A-gold. Samples were examined by transmission electron microscopy and reveal distribution of PDI in platelet T granules. Bar represents 100 nm. (c) As per panel Ab, but HPS6−/− mouse platelets were examined. (B) Decreased thrombin sensitivity of granule exocytosis in HPS6−/− platelets. Comparing WT platelets and HPS6−/− platelets, thrombin-induced granule exocytosis was studied in vitro to characterize α granule, T granule, and lysosomes. Mouse platelets in HTG buffer (250 × 105 platelets per 100 μL of 5 mM d-glucose, 134 mM sodium chloride, 0.34 mM disodium phosphate, 2.9 mM potassium chloride, 12 mM sodium bicarbonate, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and 1 mM magnesium chloride, pH 7.3) were incubated with mouse α-thrombin for 10 minutes at room temperature. Twenty microliters of thrombin-stimulated or resting platelets were incubated with fluorescein isothiocyanate–conjugated P-selectin, TLR9, or Alexa Fluor 488–labeled LAMP1 antibodies for 15 minutes. Surface expression of platelet granule markers was measured using CellQuest on a FACSCalibur flow cytometer (Becton Dickinson). Data are expressed as percent maximal release. Immunoflow cytometry of P-selectin monitored α granule exocytosis, whereas α granule content release, monitored by PF-4 secretion, was measured by enzyme-linked immunosorbent assay. T granule exocytosis was monitored by surface exposure of TLR9 using flow cytometry and lysosome exocytosis by the release of LAMP1. For PF-4 and PDI, hirudin (1 U/mL) was added to quench thrombin activity, then platelets were sedimented by centrifugation, the supernatant was collected and ultracentrifuged at 71 000g for 30 minutes, and the releasate was assayed. Platelets from WT or HPS6−/− mice were activated with varying amounts of thrombin, and the markers for granule exocytosis were measured. Thrombin agonist concentrations were 0.007, 0.036, 0.072, 0.36, 0.72, 3.6, and 7.2 nM; the average of 5 measurements defines each point ± SD. (a) P-selectin (α granules); (b) PF-4 (α granules); (c) TLR9 (T granules); (d) LAMP 1 (lysosomes); **P < .01, ***P < .001. (e) Band densities of PDI antigen in releasates of thrombin-stimulated WT and HPS6−/− platelets detected by SDS-PAGE, followed by immunoblotting with anti-PDI antibodies (DL-11; 1 μg/mL). Data represent mean ± SD (n = 2; **P < .01). (f) Thiol isomerase secretion after platelet activation with 0.72 or 7.2 nM thrombin. Thiol isomerase activity was monitored by the reduction of a di-E-GSSG as a substrate. The increase in fluorescence was measured at excitation/emission of 525/540 nm for 20 minutes at 25°C. **P < .01. WT platelets, closed bars; HPS6−/− platelets, open bars. (C) Agonist-induced adenosine triphosphate release as a marker of dense granule release in WT and HPS6−/− platelets as measured by luminometry. WT and HPS6−/− platelets were activated with SFLLRN (SF; 252 μM), collagen (Col; 19 μg/mL), or buffer control (Control), and the kinetics of release of adenosine triphosphate was monitored as a function of time (0, 5, 10, 15, 20, 25, and 30 seconds, left to right) by luminometry. (D) Decreased collagen sensitivity of granule exocytosis from HPS6−/− platelets. Band densities of PDI antigen in releasates of collagen-stimulated (0.1-5 μg/mL of type 1 equine collagen; 10 minutes) WT and HPS6−/− platelets detected by SDS-PAGE, followed by immunoblotting with anti-PDI antibodies (DL-11; 1 μg/mL) (n = 3; mean ± SD; **P = .001, *P = .01). WT platelets, closed bars; HPS6−/− platelets, open bars. SD, standard deviation; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Localization of PDI and granule release in WT and HPS6−/− platelets. (A) Localization of PDI in platelet granules. (a) Unstimulated platelets isolated from WT and HPS6−/− mice were sedimented by centrifugation. The pellet was solubilized with SDS lysis buffer to generate the lysate. Lysate of intact resting platelets from WT and HPS6−/− mice was subjected to SDS-PAGE and blotted with anti-PDI antibodies DL-11. (b) Washed WT mouse resting platelets were fixed, frozen, and sectioned before mounting on Formvar carbon-coated copper grids. Ultrathin platelet sections were probed for PDI, and bound antibody labeled with Protein A-gold. Samples were examined by transmission electron microscopy and reveal distribution of PDI in platelet T granules. Bar represents 100 nm. (c) As per panel Ab, but HPS6−/− mouse platelets were examined. (B) Decreased thrombin sensitivity of granule exocytosis in HPS6−/− platelets. Comparing WT platelets and HPS6−/− platelets, thrombin-induced granule exocytosis was studied in vitro to characterize α granule, T granule, and lysosomes. Mouse platelets in HTG buffer (250 × 105 platelets per 100 μL of 5 mM d-glucose, 134 mM sodium chloride, 0.34 mM disodium phosphate, 2.9 mM potassium chloride, 12 mM sodium bicarbonate, 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, and 1 mM magnesium chloride, pH 7.3) were incubated with mouse α-thrombin for 10 minutes at room temperature. Twenty microliters of thrombin-stimulated or resting platelets were incubated with fluorescein isothiocyanate–conjugated P-selectin, TLR9, or Alexa Fluor 488–labeled LAMP1 antibodies for 15 minutes. Surface expression of platelet granule markers was measured using CellQuest on a FACSCalibur flow cytometer (Becton Dickinson). Data are expressed as percent maximal release. Immunoflow cytometry of P-selectin monitored α granule exocytosis, whereas α granule content release, monitored by PF-4 secretion, was measured by enzyme-linked immunosorbent assay. T granule exocytosis was monitored by surface exposure of TLR9 using flow cytometry and lysosome exocytosis by the release of LAMP1. For PF-4 and PDI, hirudin (1 U/mL) was added to quench thrombin activity, then platelets were sedimented by centrifugation, the supernatant was collected and ultracentrifuged at 71 000g for 30 minutes, and the releasate was assayed. Platelets from WT or HPS6−/− mice were activated with varying amounts of thrombin, and the markers for granule exocytosis were measured. Thrombin agonist concentrations were 0.007, 0.036, 0.072, 0.36, 0.72, 3.6, and 7.2 nM; the average of 5 measurements defines each point ± SD. (a) P-selectin (α granules); (b) PF-4 (α granules); (c) TLR9 (T granules); (d) LAMP 1 (lysosomes); **P < .01, ***P < .001. (e) Band densities of PDI antigen in releasates of thrombin-stimulated WT and HPS6−/− platelets detected by SDS-PAGE, followed by immunoblotting with anti-PDI antibodies (DL-11; 1 μg/mL). Data represent mean ± SD (n = 2; **P < .01). (f) Thiol isomerase secretion after platelet activation with 0.72 or 7.2 nM thrombin. Thiol isomerase activity was monitored by the reduction of a di-E-GSSG as a substrate. The increase in fluorescence was measured at excitation/emission of 525/540 nm for 20 minutes at 25°C. **P < .01. WT platelets, closed bars; HPS6−/− platelets, open bars. (C) Agonist-induced adenosine triphosphate release as a marker of dense granule release in WT and HPS6−/− platelets as measured by luminometry. WT and HPS6−/− platelets were activated with SFLLRN (SF; 252 μM), collagen (Col; 19 μg/mL), or buffer control (Control), and the kinetics of release of adenosine triphosphate was monitored as a function of time (0, 5, 10, 15, 20, 25, and 30 seconds, left to right) by luminometry. (D) Decreased collagen sensitivity of granule exocytosis from HPS6−/− platelets. Band densities of PDI antigen in releasates of collagen-stimulated (0.1-5 μg/mL of type 1 equine collagen; 10 minutes) WT and HPS6−/− platelets detected by SDS-PAGE, followed by immunoblotting with anti-PDI antibodies (DL-11; 1 μg/mL) (n = 3; mean ± SD; **P = .001, *P = .01). WT platelets, closed bars; HPS6−/− platelets, open bars. SD, standard deviation; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
PDI is stored in T granules in HPS6−/− platelets
PDI localization was examined by immunogold electron microscopy in HPS6−/− and WT mouse platelets. PDI was observed in electron dense granular compartments in WT (Figure 2Ab), as described previously,16 and in HPS6−/− (Figure 2Ac) mouse platelets.
Differential sensitivity of platelet granule content release as a function of thrombin concentration in WT and HPS6−/− platelets
We examined whether impaired granule content release at physiological agonist concentrations might explain the low levels of PDI secreted in vivo in the HPS6−/− mice. Exocytosis of α granules, lysosomes, and T granules from HPS6−/− platelets showed decreased sensitivity to thrombin agonists compared to WT platelets (Figure 2B). Using P-selectin as a marker of α granule membrane translocation into the plasma membrane and PF-4 as a marker of α granule content release, higher concentrations of thrombin were necessary to obtain comparable release from HPS6−/− platelets as from WT platelets (Figure 2Ba,b). Similarly, using TLR9 as a marker of T granule content release and LAMP1 as a marker of lysosome content release, higher concentrations of thrombin were necessary to obtain comparable release from HPS6−/− platelets as from WT platelets (Figure 2Bc,d). At thrombin agonist concentrations of 0.36 and 0.72 nM, there was a significant difference in sensitivity to thrombin (approximately 20-fold and threefold, respectively) in T granule release. At higher concentrations of thrombin (eg, 3.6 nM), no differences were observed.
To investigate whether HPS6−/− platelets showed impaired PDI release, WT and HPS6−/− platelets were stimulated with thrombin (0.072-7.2 nM) and PDI antigen was quantitated in the releasates. HPS6−/− platelets demonstrated impairment in PDI antigen release at thrombin concentrations of 0.36 and 0.72 nM compared to WT platelets (Figure 2Be). Additionally, thiol isomerase activity of WT and HPS6−/− platelets was examined by measuring the reductase activity of thiol isomerases secreted from activated HPS6−/− mouse platelets using the di-E-GSSG assay.19 Platelets from HPS6−/− and WT mice were stimulated with 0.72 or 7.2 nM thrombin, and the reductase activity of thiol isomerases released from activated WT platelets and HPS6−/− mouse platelets were compared (Figure 2Bf). At 0.72 nM (but not 7.2 nM) thrombin, HPS6−/− platelets demonstrated impaired release of thiol isomerase activity. Using quercetin-3-rutinoside, an inhibitor of PDI that does not inhibit ERp5 and ERp57 associated with the platelet releasate,16 we demonstrated that PDI is responsible for most of the thiol isomerase reductase activity observed (data not shown).
In parallel, we confirmed the absence of dense granule release from HPS6−/− platelets as compared to WT platelets (Figure 2C) using both collagen and the thrombin receptor–activating peptide SFLLRN as agonists. Impaired release of PDI was also observed when collagen was employed as agonist in HPS6−/− platelets (Figure 2D).
ADP rescues impaired release of PDI in HPS6−/− platelets, and apyrase attenuates PDI release in WT platelets
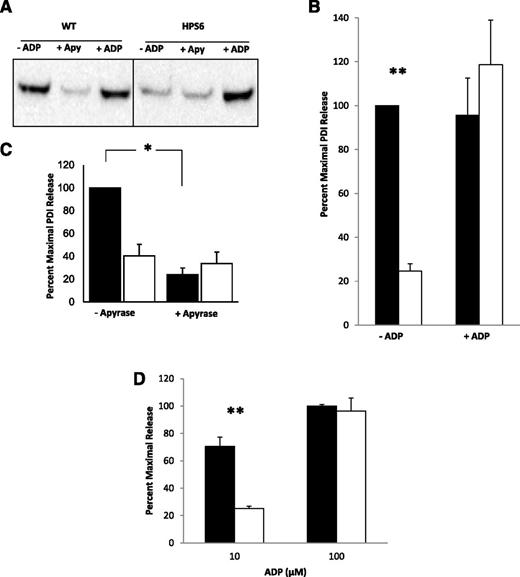
Adenosine 5′-diphosphate (ADP), lacking in HPS6−/− mice due to the absence of dense granules, is known to enhance agonist sensitivity of Hermansky-Pudlak syndrome platelets.25 WT and HPS6−/− platelets were treated with 1 μM ADP, a concentration insufficient to activate platelets in the absence of thrombin. Subsequently, the platelets were activated with low levels of thrombin that were associated with impaired granule secretion. As shown in Figure 3A-B, the addition of ADP corrected the level of PDI secreted in the HPS6−/− platelet releasate to levels similar to those in platelets from WT mice when treated with low levels of thrombin. These results suggest that the absence of dense granules, and thus ADP, is responsible for impaired granule secretion in Hermansky-Pudlak syndrome.
Rescue of thrombin-induced PDI release from HPS6−/− platelets using substimulatory ADP, and reduction of PDI release from WT platelets with apyrase. PDI antigen in releasate of thrombin-stimulated (0.36 nM) WT and HPS6−/− platelets with and without substimulatory concentrations of ADP (1 μM) or with and without apyrase (Apy; 0.1 U/mL). Samples were obtained 10 minutes after thrombin activation. (A) PDI antigen in releasates of thrombin-stimulated WT and HPS6−/− platelets were detected by SDS-PAGE, followed by immunoblotting with anti-PDI antibodies (DL-11; 1 mg/mL). (B) Bar graph comparing PDI release in WT and HPS6−/− platelets in the presence (+) or absence (-) of ADP. Data represent mean ± SD; n = 2; **P < .01. (C) Bar graph comparing PDI release in WT and HPS6−/− platelets in the presence (+) or absence (-) of apyrase. Data represent mean ± SD (n = 2; *P < .01). (D) Effect of high levels of ADP on HPS6−/− release of PDI. WT and HPS6−/− platelets were incubated with 10 μM (-) or 100 μM (+) of ADP, and releasate was collected and resolved on SDS-PAGE. PDI was quantitated by immunoblotting with rabbit polyclonal anti-PDI antibody (n = 2; mean ± SD; **P = .02). WT, closed bars; HPS6−/−, open bars.
Rescue of thrombin-induced PDI release from HPS6−/− platelets using substimulatory ADP, and reduction of PDI release from WT platelets with apyrase. PDI antigen in releasate of thrombin-stimulated (0.36 nM) WT and HPS6−/− platelets with and without substimulatory concentrations of ADP (1 μM) or with and without apyrase (Apy; 0.1 U/mL). Samples were obtained 10 minutes after thrombin activation. (A) PDI antigen in releasates of thrombin-stimulated WT and HPS6−/− platelets were detected by SDS-PAGE, followed by immunoblotting with anti-PDI antibodies (DL-11; 1 mg/mL). (B) Bar graph comparing PDI release in WT and HPS6−/− platelets in the presence (+) or absence (-) of ADP. Data represent mean ± SD; n = 2; **P < .01. (C) Bar graph comparing PDI release in WT and HPS6−/− platelets in the presence (+) or absence (-) of apyrase. Data represent mean ± SD (n = 2; *P < .01). (D) Effect of high levels of ADP on HPS6−/− release of PDI. WT and HPS6−/− platelets were incubated with 10 μM (-) or 100 μM (+) of ADP, and releasate was collected and resolved on SDS-PAGE. PDI was quantitated by immunoblotting with rabbit polyclonal anti-PDI antibody (n = 2; mean ± SD; **P = .02). WT, closed bars; HPS6−/−, open bars.
If ADP is required for thrombin-induced platelet PDI release at low concentrations of agonist, then treatment of WT platelets with apyrase, which converts ADP released from activated platelets to inactive adenosine 5′-monophosphate (AMP), should decrease the release of PDI from WT platelets. This effect is shown in Figure 3A-C. However, apyrase had no effect on the level of PDI release from HPS6−/− platelets. These results indicate that it is ADP, not another constituent of dense granules, whose secretion is lacking in HPS6−/− platelets. Thus, ADP is responsible for sensitization for thrombin activation.
Using higher levels of ADP to activate HPS6−/− platelets, we demonstrate that PDI release from these platelets is similar to WT platelets (Figure 3D). This finding argues further for the effect of low ADP potentiating the effect of thrombin in rescuing PDI release in HPS6−/− platelets, whereas higher concentrations directly activate platelets.
Infusion of WT platelets rescues thrombus formation
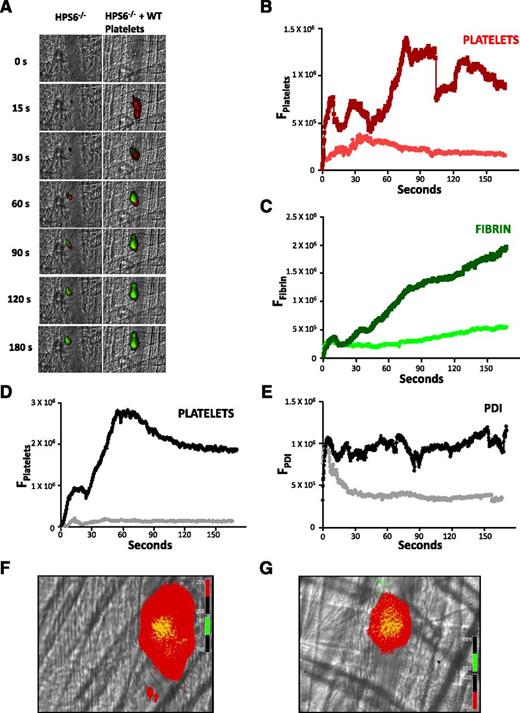
To determine whether the defect in thrombus formation in HPS6−/− mice could be corrected by the infusion of WT platelets into the mouse circulation, we infused platelets from WT mice into HPS6−/− mice. The infusion of washed WT mouse platelets, in which the final concentration of WT platelets in the circulation represented ∼10% to 20% of the total mouse platelets in the HPS6−/− mice, resulted in complete rescue of the platelet thrombus and fibrin generation (Figure 4A-C). Infusion of WT platelets into WT mice had no additional effect on thrombus formation (data not shown).
Rescue of thrombus formation in HPS6−/− mice by infusion of WT platelets. Washed platelets isolated from WT mice were infused into HPS6−/− mice to a final approximate concentration of 10% to 20% of the total mouse platelet count. Platelet-specific anti-CD42b antibody and fibrin-specific mouse anti-human fibrin monoclonal antibody were infused to detect platelets and fibrin during in vivo thrombus formation initiated by laser-induced injury. (A) Representative images of the fluorescence associated with fibrin (green) and platelets (red) over 180 seconds after laser-induced vessel wall injury in an HPS6−/− mouse (left) and an HPS6−/− mouse treated with WT mouse platelets (right). (B) Median integrated platelet fluorescence intensity as a function of time in the absence (red) and presence (dark red) of WT platelets. (C) Median integrated fibrin fluorescence intensity as a function of time in the absence (green) and presence (dark green) of WT platelets. Under identical experimental conditions, a nonblocking anti-PDI antibody was employed instead of the fibrin-specific monoclonal antibody to visualize PDI. (D) Median integrated platelet fluorescence intensity as a function of time in the absence (gray) and presence (black) of infused WT platelets. (E) Median integrated PDI fluorescence intensity as a function of time in the absence (gray) and presence (black) of infused WT platelets. Data in panels B-E are from 30 thrombi in 3 mice for each condition. (F) Distribution of calcein-labeled WT donor platelets in a developing thrombus following their infusion into an HPS6−/− mouse. (G) Distribution of calcein-labeled WT donor platelets in a developing thrombus following their infusion into a WT mouse. Donor WT platelets, green; platelets, red; merge, yellow.
Rescue of thrombus formation in HPS6−/− mice by infusion of WT platelets. Washed platelets isolated from WT mice were infused into HPS6−/− mice to a final approximate concentration of 10% to 20% of the total mouse platelet count. Platelet-specific anti-CD42b antibody and fibrin-specific mouse anti-human fibrin monoclonal antibody were infused to detect platelets and fibrin during in vivo thrombus formation initiated by laser-induced injury. (A) Representative images of the fluorescence associated with fibrin (green) and platelets (red) over 180 seconds after laser-induced vessel wall injury in an HPS6−/− mouse (left) and an HPS6−/− mouse treated with WT mouse platelets (right). (B) Median integrated platelet fluorescence intensity as a function of time in the absence (red) and presence (dark red) of WT platelets. (C) Median integrated fibrin fluorescence intensity as a function of time in the absence (green) and presence (dark green) of WT platelets. Under identical experimental conditions, a nonblocking anti-PDI antibody was employed instead of the fibrin-specific monoclonal antibody to visualize PDI. (D) Median integrated platelet fluorescence intensity as a function of time in the absence (gray) and presence (black) of infused WT platelets. (E) Median integrated PDI fluorescence intensity as a function of time in the absence (gray) and presence (black) of infused WT platelets. Data in panels B-E are from 30 thrombi in 3 mice for each condition. (F) Distribution of calcein-labeled WT donor platelets in a developing thrombus following their infusion into an HPS6−/− mouse. (G) Distribution of calcein-labeled WT donor platelets in a developing thrombus following their infusion into a WT mouse. Donor WT platelets, green; platelets, red; merge, yellow.
Similar experiments monitoring extracellular PDI accumulation and platelet thrombus formation revealed both the absence of platelets and the early appearance (and rapid disappearance) of PDI in HPS6−/− mice (Figure 4D-E). When WT platelets were infused, platelet thrombus formation was rescued, as was the association of extracellular PDI with the thrombus.
Given that a relatively small number of WT donor platelets were infused into a recipient HPS6−/− mouse, but that the thrombus size/formation was comparable to that observed in WT mice, the platelets that composed the thrombus were likely dominated by HPS6−/− platelets. To prove this, donor platelets were isolated from a WT mouse and labeled with calcein-AM, and these fluorescent platelets were infused separately into an HPS6−/− mouse and a WT mouse. The platelet thrombus in both mice contained some donor platelets, but most of the platelets were not labeled with calcein, thus indicating that the endogenous platelets dominated the platelet thrombus (Figure 4F-G). These results suggest that the infused WT platelets play a catalytic role, that of releasing ADP, which in turn promotes thrombus formation.
Is Hermansky-Pudlak syndrome in humans characterized by impaired PDI secretion?
Examination of platelet secretion in a patient with Hermansky-Pudlak syndrome type 1 allowed us to determine whether impaired α granule and T granule secretion is present in Hermansky-Pudlak syndrome in humans. Platelet-rich plasma was isolated from a female patient with known Hermansky-Pudlak syndrome type I, characterized by a phenotype that includes a significant history of bleeding. P-selectin expression as a marker of α granule release was impaired in the Hermansky-Pudlak syndrome platelets compared to normal platelets when thrombin was in the range of 0.36 to 0.72 nM (Figure 5A). The lysates of the Hermansky-Pudlak syndrome and normal platelets revealed equivalent amounts of stored PDI (Figure 5B). However, PDI antigen release (Figure 5C) and the release of thiol isomerase activity (Figure 5D) are impaired in the range of 0.36 to 0.72 nM thrombin. Although these analyses are limited to a single patient, the results comparing human and mouse α granule release, PDI secretion, and thiol isomerase release are parallel.
Hermansky-Pudlak syndrome in humans: impaired α granules and PDI secretion in a patient with Hermansky-Pudlak syndrome. Comparing normal human platelets and human Hermansky-Pudlak syndrome platelets, thrombin-induced granule exocytosis was studied in vitro to characterize α granule release, thiol isomerase secretion, and PDI antigen secretion. Platelet activation was performed with indicated amounts of thrombin. (A) P-selectin (α granules) expression by flow cytometry in platelets from a Hermansky-Pudlak syndrome patient and from a normal subject. (B) PDI antigen in lysate of resting normal and Hermansky-Pudlak syndrome platelets. (C) PDI antigen secretion monitored by western blot analysis of the releasate of thrombin-activated normal and Hermansky-Pudlak syndrome platelets. (D) Thiol isomerase activity monitored by the reduction of a di-E-GSSG as a substrate. Normal platelets, closed bars; Hermansky-Pudlak syndrome platelets, open bars. N = 2 experiments; mean ± SD; *P = .02, **P < .01.
Hermansky-Pudlak syndrome in humans: impaired α granules and PDI secretion in a patient with Hermansky-Pudlak syndrome. Comparing normal human platelets and human Hermansky-Pudlak syndrome platelets, thrombin-induced granule exocytosis was studied in vitro to characterize α granule release, thiol isomerase secretion, and PDI antigen secretion. Platelet activation was performed with indicated amounts of thrombin. (A) P-selectin (α granules) expression by flow cytometry in platelets from a Hermansky-Pudlak syndrome patient and from a normal subject. (B) PDI antigen in lysate of resting normal and Hermansky-Pudlak syndrome platelets. (C) PDI antigen secretion monitored by western blot analysis of the releasate of thrombin-activated normal and Hermansky-Pudlak syndrome platelets. (D) Thiol isomerase activity monitored by the reduction of a di-E-GSSG as a substrate. Normal platelets, closed bars; Hermansky-Pudlak syndrome platelets, open bars. N = 2 experiments; mean ± SD; *P = .02, **P < .01.
Silencing of the HPS6 gene in endothelial cells impairs PDI release and granule exocytosis
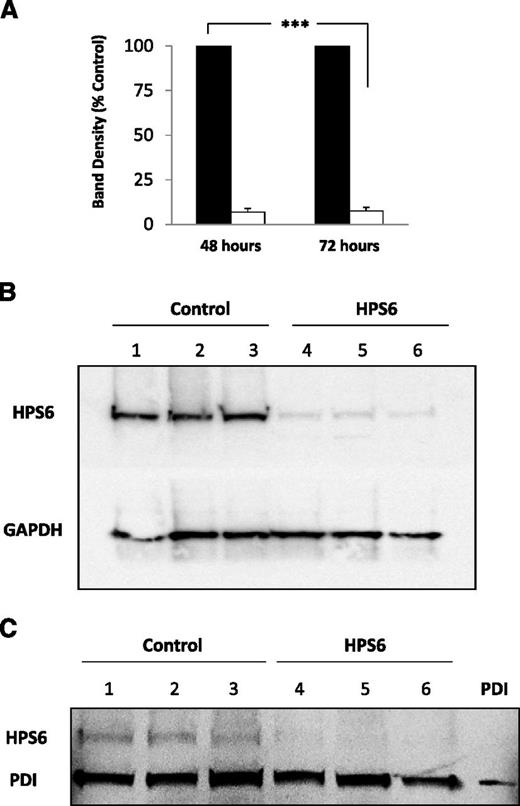
The HPS6 gene encodes the HPS6 protein, a component of the BLOC-2 complex that plays a role in organelle biogenesis associated with melanosomes, platelet dense granules, and lysosomes. Forty-eight and 72 hours after the introduction of HPS6 siRNA to HUVECs, cells were analyzed for HPS6 in cell lysates (Figure 6A). Cells treated with the HPS6 siRNA showed levels of HPS6 protein that were reduced by about 90% compared to the cells treated with control siRNA. Furthermore, HPS6 expression was equivalent from cells treated 48 or 72 hours earlier with siRNA. These results were confirmed using a glyceraldehyde-3-phosphate dehydrogenase loading sample control (Figure 6B). To assure that the knockdown of HPS6 did not impact the levels of PDI in unstimulated endothelial cells, the lysates of cells treated with the HPS6 siRNA and control siRNA were compared for HPS6 protein and PDI. The levels of PDI in HUVECs treated with HPS6 siRNA and cells treated with control siRNA were similar, whereas HPS6 protein was suppressed in the HUVECs treated with HPS6 siRNA (Figure 6C).
HPS6 knockdown in HUVECs treated with HPS6 siRNA. HPS6 in HUVECs 48 and 72 hours posttransfection with HPS6 or control siRNA were detected by SDS-PAGE of cell lysates followed by immunoblotting with polyclonal anti-HPS6 antibodies (HPS6d; 2.5 μg/mL). (A) Band densities of HPS6 at 48 and 72 hours posttransfection with control siRNA (closed bars) or HPS6 siRNA (open bars). Control is shown as 100%. Data represent mean ± SD (n = 3; ***P < .001). (B) Immunoblot of HPS6 (50 μL of lysate from confluent monolayer, ∼0.25 × 106 cells) 72 hours posttransfection with control siRNA (lanes 1, 2, and 3) or HPS6 siRNA (lanes 4, 5, and 6). The membranes were immunoblotted for GAPDH (polyclonal anti-GAPDH, 1 μg/mL) as a loading control. (C) HPS6 knockdown does not affect PDI storage in HUVECs. PDI and HPS6 in HUVECs 72 hours posttransfection with HPS6 or control siRNA were detected by SDS-PAGE of cell lysates, followed by immunoblotting with polyclonal anti-PDI antibody (DL-11; 1 μg/mL) and polyclonal anti-HPS6 antibody (HPS6d; 2.5 μg/mL), respectively. Immunoblot of PDI and HPS6 (25 μL of lysate from confluent monolayer, ∼0.25 × 106 cells) 72 hours posttransfection. HUVEC lysate from cells treated with control siRNA (lanes 1, 2, and 3) or HPS6 siRNA (lanes 4, 5, and 6). Recombinant PDI (15 ng) was run as positive control. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
HPS6 knockdown in HUVECs treated with HPS6 siRNA. HPS6 in HUVECs 48 and 72 hours posttransfection with HPS6 or control siRNA were detected by SDS-PAGE of cell lysates followed by immunoblotting with polyclonal anti-HPS6 antibodies (HPS6d; 2.5 μg/mL). (A) Band densities of HPS6 at 48 and 72 hours posttransfection with control siRNA (closed bars) or HPS6 siRNA (open bars). Control is shown as 100%. Data represent mean ± SD (n = 3; ***P < .001). (B) Immunoblot of HPS6 (50 μL of lysate from confluent monolayer, ∼0.25 × 106 cells) 72 hours posttransfection with control siRNA (lanes 1, 2, and 3) or HPS6 siRNA (lanes 4, 5, and 6). The membranes were immunoblotted for GAPDH (polyclonal anti-GAPDH, 1 μg/mL) as a loading control. (C) HPS6 knockdown does not affect PDI storage in HUVECs. PDI and HPS6 in HUVECs 72 hours posttransfection with HPS6 or control siRNA were detected by SDS-PAGE of cell lysates, followed by immunoblotting with polyclonal anti-PDI antibody (DL-11; 1 μg/mL) and polyclonal anti-HPS6 antibody (HPS6d; 2.5 μg/mL), respectively. Immunoblot of PDI and HPS6 (25 μL of lysate from confluent monolayer, ∼0.25 × 106 cells) 72 hours posttransfection. HUVEC lysate from cells treated with control siRNA (lanes 1, 2, and 3) or HPS6 siRNA (lanes 4, 5, and 6). Recombinant PDI (15 ng) was run as positive control. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
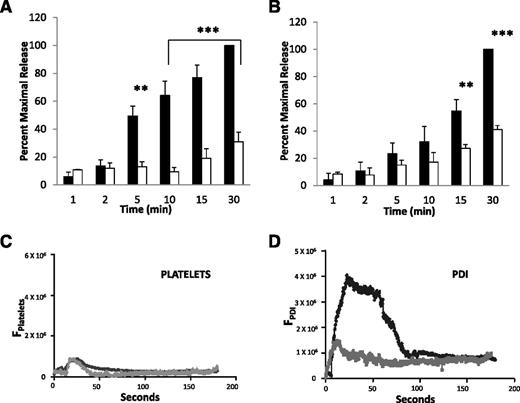
The kinetics of the release of PDI from HUVECs treated with HPS6 siRNA and HUVECs treated with control siRNA after thrombin stimulation were compared to determine whether decreased levels of HPS6 in HUVECs impaired granule release. HPS6 siRNA–treated HUVECs and control siRNA–treated HUVECs were activated with thrombin, and PDI antigen was measured in the releasate over 30 minutes. These band densities were quantitated to allow direct comparison of the release of PDI from control siRNA–treated HUVECs and the HPS6 siRNA–treated HUVECs. PDI released from the HPS6 siRNA–treated HUVECs was reduced by ∼70% at 30 minutes compared to PDI released from control siRNA–treated HUVECs (Figure 7A).
Thrombin-induced PDI and VWF release from HUVECs treated with HPS6 siRNA. Thrombin-induced PDI release and VWF release from HUVECs 72 hours posttransfection with HPS6 or control siRNA were detected by SDS-PAGE, followed by immunoblotting with anti-PDI antibodies (DL-11; 1 μg/mL) and anti-VWF antibodies (rabbit polyclonal; 1 μg/mL), respectively. Band densities of released PDI from HUVECs (A) or VWF released from HUVECs (B) over 30 minutes after thrombin stimulation (1 NIH U/mL) 72 hours posttransfection with control siRNA (closed bars) or HPS6 siRNA (open bars). Control is shown as 100%. Data represent mean ± SD (n = 3; ***P < .001, **P < .01). (C) When eptifibatide (10 μg/g mouse) was infused, platelet accumulation was prevented in WT mice (black) and in HPS6−/− mice (gray). (D) Eptifibatide did not blunt the median integrated PDI fluorescence intensity during thrombus formation in WT mice (black) but did so in HPS6−/− mice (gray). These results are consistent with defective PDI release from the endothelium in HPS6−/− mice. Data in panels C and D are from 30 thrombi in 3 mice for HPS6−/− and for WT mice.
Thrombin-induced PDI and VWF release from HUVECs treated with HPS6 siRNA. Thrombin-induced PDI release and VWF release from HUVECs 72 hours posttransfection with HPS6 or control siRNA were detected by SDS-PAGE, followed by immunoblotting with anti-PDI antibodies (DL-11; 1 μg/mL) and anti-VWF antibodies (rabbit polyclonal; 1 μg/mL), respectively. Band densities of released PDI from HUVECs (A) or VWF released from HUVECs (B) over 30 minutes after thrombin stimulation (1 NIH U/mL) 72 hours posttransfection with control siRNA (closed bars) or HPS6 siRNA (open bars). Control is shown as 100%. Data represent mean ± SD (n = 3; ***P < .001, **P < .01). (C) When eptifibatide (10 μg/g mouse) was infused, platelet accumulation was prevented in WT mice (black) and in HPS6−/− mice (gray). (D) Eptifibatide did not blunt the median integrated PDI fluorescence intensity during thrombus formation in WT mice (black) but did so in HPS6−/− mice (gray). These results are consistent with defective PDI release from the endothelium in HPS6−/− mice. Data in panels C and D are from 30 thrombi in 3 mice for HPS6−/− and for WT mice.
The kinetics of the release of VWF antigen was quantitated in the releasate of thrombin-activated control siRNA–treated HUVECs and HPS6 siRNA–treated HUVECs. These results show impairment of Weibel-Palade body exocytosis in HPS6 siRNA–treated HUVECs (Figure 7B). VWF antigen released from the HPS6 siRNA–treated HUVECs was reduced by 58% at 30 minutes compared to VWF antigen released from control siRNA–treated HUVECs.
These results predict defective release of PDI from the endothelium during thrombus formation in vivo. To test this hypothesis in the absence of platelets, eptifibatide, an inhibitor of αIIbβ3, was infused into WT mice and HPS6−/− mice. Minimal platelet accumulation was observed in both mouse strains (Figure 7C). However, release of PDI in the absence of platelet accumulation was markedly reduced in HPS6−/− mice (Figure 7D). These results are consistent with a defect in delivery of endothelial cell–derived PDI to the injury site in HPS6−/− mice.
Discussion
Hermansky-Pudlak syndrome is a genetic disorder in which platelet dense granules are absent or nearly absent. In humans, Hermansky-Pudlak syndrome type 6 is associated with clinically significant bleeding, presumably due to the loss of components in the dense granules, including ADP.3,7 Here, we demonstrate that HPS6−/− mice have defective thrombus formation, including platelet accumulation and fibrin formation. Furthermore, both endothelial cells and platelets have impaired release of the contents of their remaining granules at low thrombin concentrations comparable to those in vivo after vessel wall injury.
Endothelial cells have not been known to contribute to the bleeding syndrome associated with Hermansky-Pudlak syndrome. In 2 separate series of experiments, we demonstrated an endothelial defect. First, when platelet accumulation was blocked with eptifibatide, PDI accumulation contributed by the endothelium was significantly less in HPS6−/− mice than in WT mice after vessel injury. Second, HUVECs in which the HPS6 protein was knocked down demonstrated decreased sensitivity to thrombin in secretion of both PDI and VWF.
The cause of bleeding in patients with Hermansky-Pudlak syndrome has been thought to be related to the absence of ADP due to platelet dense granule deficiency. Indeed, this might serve to explain the observed in vivo defect in platelet thrombus formation. However, fibrin generation in our model was absent as well. Yet, fibrin generation is not dependent on platelet accumulation in this model because we have previously shown normal fibrin formation in Par4−/− mice in whom platelets cannot be thrombin-activated23 ; in WT mice treated with eptifibatide, an inhibitor that blocks platelet accumulation15 ; and in chimeric mice lacking platelet β3 integrins.17 Given the critical importance of PDI in fibrin generation,11,18 we explored impaired PDI release from platelets and endothelial cells in Hermansky-Pudlak syndrome, and now suggest that this is the cause of defective fibrin generation in the HPS6−/− mice and possibly platelet accumulation after vessel wall injury.
In the current study, we posit that defective release of granules secreting PDI in platelets and endothelial cells explains the decreased sensitivity of HPS6−/− mice to thrombin activation. ADP deficiency, in the absence of dense granules in Hermansky-Pudlak syndrome, contributes directly to impaired exocytosis of T granules and, thus, impaired PDI release. Decreased sensitivity of HPS6−/− platelets to various agonists has been previously described. In Hermansky-Pudlak syndrome, only a combination of ADP plus thrombin could restore normal platelet aggregation (with normal α granule and lysosome release),25 and impaired thrombin-induced α granule exocytosis, as measured by aggregation, was reversed by subthreshold concentrations of ADP.22 Among several dense granule components tested, ADP was the only one capable of correcting impaired release of acid hydrolase in storage pool–deficient platelets.26 In the in vivo setting, ADP release may be required to increase the sensitivity of platelets to thrombin and enable the release of endogenous platelet PDI during thrombus formation.
The mechanism by which ADP alters the activation threshold of thrombin-induced activation and allows rescue of PDI from T granules remains speculative. Subthreshold ADP sensitizes platelets to activation by thrombin and subsequent degranulation.22,25 ADP interacts with P2Y1 and P2Y12. P2Y1 couples to Gq,27 whereas P2Y12 is coupled to Gi-type G proteins, in particular Gi2.28 Studies using receptor agonists suggest that activation of both receptors is required for a full response of platelets to ADP.29 These ADP receptors, stimulating Gαq and Gαi, lead to a decrease in cyclic AMP.30 With lower levels of cyclic AMP, thrombin action through the Par-1 leads to increased granule release.
Hermansky-Pudlak syndrome is a rare autosomal recessive disorder in humans. Among its clinical characteristics are defects in hemostasis. Previously, the absence of dense granules and attendant ADP was thought to be the cause of the bleeding phenotype; however, although this may explain the absence of platelet thrombus formation, it does not explain the absence of fibrin generation in our mouse model. Based on the results obtained in this model of thrombus formation in HPS6−/− mice, we suggest that the sequelae leading to a bleeding disorder in Hermansky-Pudlak syndrome are initiated by the absence of secreted ADP, which is required for priming the physiological release of the remaining granules in platelets. In the absence or near absence of ADP, PDI release is impaired, and PDI is required for fibrin generation and platelet accumulation during thrombus formation. The variability of the magnitude of bleeding in patients with Hermansky-Pudlak syndrome may relate to the variability of the level of ADP deficiency. Hermansky-Pudlak syndrome is a hereditary disease whereby impaired PDI secretion related to ADP deficiency contributes to a phenotype characterized by bleeding.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Esteban Dell’Angelica for generously providing antibodies to HPS6.
This work was supported by National Institutes of Health National Heart, Lung, and Blood Institute grants PO1 HL087203, R01 HL092125, R01 HL112809, R01 HL125275, and U54 HL112302.
Authorship
Contribution: A.S., S.H.K., S.G., and R.J. designed and performed the experiments, analyzed the results, and edited the manuscript; R.F. and B.C.F. designed the experiments, analyzed the results, and edited the manuscript; and B.F. designed the experiments, analyzed the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce Furie, Center for Life Science, Room 903, 3 Blackfan Circle, Boston MA 02215; e-mail: bfurie@bidmc.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal