Key Points
Removal of αβ+ T and CD19+ B cells is an effective strategy for successful HLA-haploidentical hematopoietic stem cell transplantation.
The high probability of disease-free survival renders this transplant option attractive for any child with a nonmalignant disorder.
Twenty-three children with nonmalignant disorders received HLA-haploidentical hematopoietic stem cell transplantation (haplo-HSCT) after ex vivo elimination of αβ+ T cells and CD19+ B cells. The median number of CD34+, αβ+CD3+, and B cells infused was 16.8 × 106, 40 × 103, and 40 × 103 cells/kg, respectively. No patient received any posttransplantation pharmacologic prophylaxis for graft-versus-host disease (GVHD). All but 4 patients engrafted, these latter being rescued by a second allograft. Three patients experienced skin-only grade 1 to 2 acute GVHD. No patient developed visceral acute or chronic GVHD. Cumulative incidence of transplantation-related mortality was 9.3%. With a median follow-up of 18 months, 21 of 23 children are alive and disease-free, the 2-year probability of disease-free survival being 91.1%. Recovery of γδ+ T cells was prompt, but αβ+ T cells progressively ensued over time. Our data suggest that this novel graft manipulation strategy is safe and effective for haplo-HSCT. This trial was registered at www.clinicaltrials.gov as #NCT01810120.
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) still remains either the only or the best curative option for many children with life-threatening, hematologic or immune nonmalignant disorders.1 However, only 25% of patients have an HLA-identical sibling, and a suitable HLA-compatible unrelated volunteer can be promptly located for fewer than 70% of the remaining patients.2 In the absence of an HLA-matched donor, alternative donors such as HLA-haploidentical relatives are being increasingly used. A major breakthrough for successful haploidentical HSCT (haplo-HSCT) was the demonstration that an efficient T-cell depletion of the graft prevented both acute and chronic graft-versus-host disease (GVHD), even when the donor/recipient pair differed for an entire HLA haplotype.3,,-6 So far, haplo-HSCT has been performed mainly by using positively selected CD34+ cells, and many studies showed that, through this approach, sustained engraftment of donor hematopoiesis without the occurrence of GVHD can be obtained in most patients. Delayed recovery of adaptive immunity and increased risk of transplantation-related mortality (TRM) compared with HLA-matched unmanipulated allografts are still unsolved obstacles to the success of the procedure.7,8 To overcome these hurdles, we recently implemented a novel method of ex vivo T- and B-cell depletion based on the selective elimination of αβ+ T cells (the lymphocyte subset responsible for GVHD occurrence) through labeling with biotinylated anti-T-cell receptor-αβ (anti-TCR-αβ) and anti-CD19 antibodies, followed by incubation with anti-biotin antibodies conjugated to paramagnetic beads (CliniMACS; Miltenyi Biotec, Bergisch Gladbach, Germany).9,-11 We herein report outcome and immune recovery of 23 children with nonmalignant disorders who lacked a compatible donor or were in urgent need of HSCT who were given this type of allograft.
Study design
Patients received a transplantation from February 2011 to November 2013 at a single center and were enrolled in a trial approved by the local ethical committee. Written informed consent was obtained from all parents and/or legal guardians in accordance with the Declaration of Helsinki. Details on patient’s and donor’s characteristics are reported in Table1. Eight patients had severe combined immunodeficiency (SCID), 4 had Fanconi anemia, 4 had severe aplastic anemia (SAA), and 1 each had osteopetrosis, immunodeficiency with polyendocrinopathy and enteropathy X-linked, DOCK8-mutated hyperimmunoglobulin E syndrome, Shwachman-Diamond syndrome, congenital amegakaryocytic thrombocytopenia, primary hemophagocytic lymphohistiocytosis, and thalassemia major with autoimmune hemolytic anemia.
Patient, donor, and transplantation characteristics
| Characteristic . | No. . | % . | Median . | Range . |
|---|---|---|---|---|
| No. of patients | 23 | 100 | ||
| Gender | ||||
| Male | 15 | 65 | ||
| Female | 8 | 35 | ||
| Age at HSCT, y | 3.3 | 0.4-12 | ||
| Original disorders | ||||
| SCID | 8 | 35 | ||
| SAA | 4 | 17 | ||
| Fanconi anemia | 4 | 17 | ||
| Immunodeficiency with polyendocrinopathy and enteropathy X-linked | 1 | 4.3 | ||
| Congenital amegakaryocytic thrombocytopenia | 1 | 4.3 | ||
| Shwachmann-Diamond syndrome | 1 | 4.3 | ||
| UNC13D-mutated hemophagocytic lymphohistiocytosis | 1 | 4.3 | ||
| DOCK-8–mutated hyper-IgE syndrome | 1 | 4.3 | ||
| Osteopetrosis | 1 | 4.3 | ||
| Thalassemia | 1 | 4.3 | ||
| Type of donor | ||||
| Father | 8 | 35 | ||
| Mother | 15 | 65 | ||
| Donor age, y | 35 | 28-52 | ||
| Donor/recipient gender combinations | ||||
| Female donor/male recipient | 7 | 30 | ||
| Other combinations | 16 | 70 | ||
| Donor KIR genotype† | ||||
| A/A | 5 | 24 | ||
| B/x | 16 | 76 | ||
| Donor B-content value† | ||||
| 0-1 | 10 | 48 | ||
| >2 | 11 | 52 | ||
| Donor-recipient NK alloreactivity‡ | ||||
| Yes | 4 | 17 | ||
| No | 19 | 83 | ||
| Human CMV serology | ||||
| Negative donor/negative recipient | 2 | 9 | ||
| Negative donor/positive recipient | 1 | 4.3 | ||
| Positive donor/negative recipient | 4 | 17 | ||
| Positive donor/positive recipient | 16 | 70 | ||
| Conditioning regimen§ | ||||
| Busulfan-thiotepa-fludarabine | 3 | 13 | ||
| Treosulfan-thiotepa-fludarabine | 4 | 17 | ||
| Treosulfan-fludarabine|| | 8 | 35 | ||
| Fludarabine-cyclophosphamide ± single-dose TBI¶ | 8 | 35 | ||
| No. of cells infused | ||||
| CD34+ × 106 per kg | 15.8 | 10.2-40.0 | ||
| TCR-αβ+CD3+ × 104 per kg | 4 | 1-9.5 | ||
| TCR-γδ+CD3+ × 106 per kg | 9.4 | 1.6-95.4 | ||
| CD3–CD16+CD56+ × 106 per kg | 38.2 | 15.7-176.8 | ||
| CD20+ × 104 per kg | 4 | 0.5-15 | ||
| No. of days to polymorphonuclear neutrophil recovery# | 13 | 10-20 | ||
| No. of days to platelet recovery** | 10 | 7-40 | ||
| Graft failure†† | 4 | 20 | ||
| Chimerism at time of last follow-up‡‡ | 100a | 80-100 |
| Characteristic . | No. . | % . | Median . | Range . |
|---|---|---|---|---|
| No. of patients | 23 | 100 | ||
| Gender | ||||
| Male | 15 | 65 | ||
| Female | 8 | 35 | ||
| Age at HSCT, y | 3.3 | 0.4-12 | ||
| Original disorders | ||||
| SCID | 8 | 35 | ||
| SAA | 4 | 17 | ||
| Fanconi anemia | 4 | 17 | ||
| Immunodeficiency with polyendocrinopathy and enteropathy X-linked | 1 | 4.3 | ||
| Congenital amegakaryocytic thrombocytopenia | 1 | 4.3 | ||
| Shwachmann-Diamond syndrome | 1 | 4.3 | ||
| UNC13D-mutated hemophagocytic lymphohistiocytosis | 1 | 4.3 | ||
| DOCK-8–mutated hyper-IgE syndrome | 1 | 4.3 | ||
| Osteopetrosis | 1 | 4.3 | ||
| Thalassemia | 1 | 4.3 | ||
| Type of donor | ||||
| Father | 8 | 35 | ||
| Mother | 15 | 65 | ||
| Donor age, y | 35 | 28-52 | ||
| Donor/recipient gender combinations | ||||
| Female donor/male recipient | 7 | 30 | ||
| Other combinations | 16 | 70 | ||
| Donor KIR genotype† | ||||
| A/A | 5 | 24 | ||
| B/x | 16 | 76 | ||
| Donor B-content value† | ||||
| 0-1 | 10 | 48 | ||
| >2 | 11 | 52 | ||
| Donor-recipient NK alloreactivity‡ | ||||
| Yes | 4 | 17 | ||
| No | 19 | 83 | ||
| Human CMV serology | ||||
| Negative donor/negative recipient | 2 | 9 | ||
| Negative donor/positive recipient | 1 | 4.3 | ||
| Positive donor/negative recipient | 4 | 17 | ||
| Positive donor/positive recipient | 16 | 70 | ||
| Conditioning regimen§ | ||||
| Busulfan-thiotepa-fludarabine | 3 | 13 | ||
| Treosulfan-thiotepa-fludarabine | 4 | 17 | ||
| Treosulfan-fludarabine|| | 8 | 35 | ||
| Fludarabine-cyclophosphamide ± single-dose TBI¶ | 8 | 35 | ||
| No. of cells infused | ||||
| CD34+ × 106 per kg | 15.8 | 10.2-40.0 | ||
| TCR-αβ+CD3+ × 104 per kg | 4 | 1-9.5 | ||
| TCR-γδ+CD3+ × 106 per kg | 9.4 | 1.6-95.4 | ||
| CD3–CD16+CD56+ × 106 per kg | 38.2 | 15.7-176.8 | ||
| CD20+ × 104 per kg | 4 | 0.5-15 | ||
| No. of days to polymorphonuclear neutrophil recovery# | 13 | 10-20 | ||
| No. of days to platelet recovery** | 10 | 7-40 | ||
| Graft failure†† | 4 | 20 | ||
| Chimerism at time of last follow-up‡‡ | 100a | 80-100 |
IgE, immunoglobulin E; TBI, total body irradiation.
One patient each had RAG2, JAK3, CD3ε, and ARTEMIS deficiency, and 4 had RAG1 deficiency.
Two donors were not investigated for KIR haplotype and B content.
NK alloreactivity was evaluated according to the KIR/KIR-ligand mismatch model in GVHD direction.7
Busulfan 16 mg/kg over 4 days; thiotepa 10 mg/kg divided into 2 doses; fludarabine 40 mg/m2 per day for 4 consecutive days or 30 mg/m2 per day for 4 consecutive days in patients with Fanconi anemia and SAA; treosulfan 14 g/m2 per day for 3 consecutive days; cyclophosphamide 300 mg/m2 per day for 4 consecutive days.
This regimen was used in patients with SCID.
This regimen was used in patients with either SAA or Fanconi anemia, the former being those given single-dose TBI at 200 cGy.
Defined as the time needed to reach an absolute neutrophil count ≥0.5 × 109 per liter.
Defined as the time needed to reach an unsupported platelet count ≥50 × 109 per liter.
Defined as either the absence of hematopoietic recovery of donor origin on day +35 after the allograft (primary graft rejection) or as loss of donor cells after transient engraftment of donor-origin hematopoiesis (secondary graft rejection).
Hematopoietic chimerism was evaluated by using DNA obtained from peripheral blood by microsatellite analysis.
Two patients had 80% donor chimerism.
Donor peripheral blood stem cells were mobilized after subcutaneous administration of 10 to 12 µg/kg per day granulocyte colony-stimulating factor from day −5 until leukapheresis (day 0). In 2 donors with circulating CD34+ cell counts <0.04 × 109/L on day −1, single-dose plerixafor was added as an adjunct to the mobilization regimen at 240 µg/kg.
The donor was chosen according to immunogenetic criteria, mainly privileging natural killer (NK) cell B haplotype, higher B content, and NK alloreactivity evaluated according to the killer immunoglobulin-like receptor (KIR) KIR ligand model.7,12,13 In particular, we gave priority to B-haplotype, which includes more NK cell–activating receptors than AA-haplotype and, among B-haplotype donors, we privileged those with a higher B-content score.13 The donor was the mother in 15 patients and the father in 8. All patients were given a pretransplant conditioning regimen that differed according to the original disorder (Table1). Antithymocyte globulin-Fresenius 4 mg/kg per day from day −5 to day −3 was administered for preventing both graft rejection and GVHD through in vivo depletion and/or modulation of bidirectional alloreactivity. On day −1, children also received rituximab 200 mg/m2 for in vivo donor and recipient B-cell depletion to reduce as much as possible the risk of Epstein-Barr virus (EBV)-related posttransplantation lymphoproliferative disease (PTLD).We also reasoned that recipient B-cell depletion could help lower the risk of GVHD.14 No patient was given any posttransplantation pharmacologic GVHD prophylaxis.
Chimerism analysis was performed weekly for the first 3 months and monthly thereafter. Immune recovery (count of TCRαβ+, TCRγδ+, CD4+ [CD45RA+,CD45RO+], CD8+ [CD45RA+, CD45RO+], NK [CD3–CD56+], and CD19+ cells) was investigated at 1, 3, 6, 9, and 12 months after transplantation.
Patients surviving more than 14 and 100 days after transplantation were evaluated for acute and chronic GVHD, respectively, which were diagnosed and graded according to the Seattle criteria.15,16
Data were analyzed as of May 1, 2014. Patients were censored at time of death or last follow-up. Probability of overall survival, disease-free survival, and event-free survival (EFS) were estimated by the Kaplan-Meier product limit and were expressed as percentage ± standard error (SE). For calculation of EFS probability, death, disease recurrence/persistence, and graft failure were considered as events. Probabilities of acute and chronic GVHD, graft failure, and TRM were calculated as cumulative incidence curves ± SE to adjust the analysis for competing risks.17,18
Results and discussion
Graft composition is detailed in Table 1; all children received >10 × 106 CD34+ cells per kg and <1 × 105 αβ+ T cells per kg. Three of the 8 patients with SCID had active viral infections at time of transplantation and 1 of them, as well as the child with immunodeficiency with polyendocrinopathy and enteropathy X-linked, was transplanted while receiving mechanical ventilation. Two cycles of immune-suppressive therapy had failed for all children with SAA.
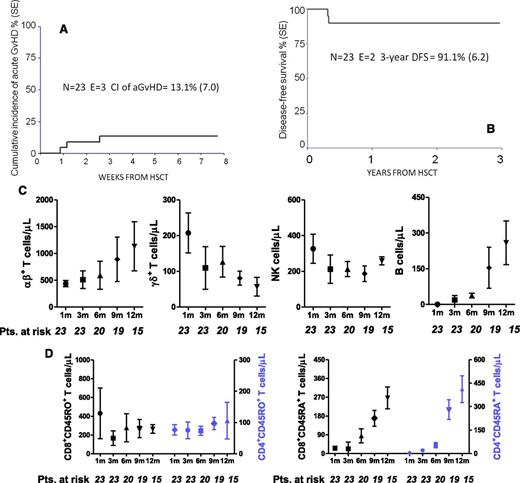
Twenty-one of the 23 patients engrafted; in addition to the 2 children who had primary rejection, associated with fever, recipient lymphocytosis, and increased levels of lactate dehydrogenase, 2 more children experienced secondary graft failure, both 1 month after HSCT, the cumulative incidence of graft failure being 16.2% (SE, 8.1). All of the 4 patients experiencing either primary or secondary graft failure were successfully re-transplanted, 2 from the same donor and 2 from the other parent, and are alive and disease-free. Neither donor age nor median number of CD34+ cells infused differed between patients who did or did not experience graft failure (data not shown). Hematologic recovery was fast; details on neutrophil and platelet recovery are shown in Table 1. Three children developed skin-only grade 1 to 2 acute GVHD (aGVHD); no patient developed grade 3 to 4 or visceral aGVHD. The cumulative incidence of grade 1 to 2 aGVHD was 13.1% (SE, 7.0; Figure 1A). None of the 21 patients at risk developed chronic GVHD.
Outcome and immune reconstitution of patients enrolled in the study. (A) Cumulative incidence (CI) of aGVHD in the study population. (B) Probability of disease-free survival in the study population. (C) Reconstitution kinetics of αβ+ and γδ+ T cells, as well as NK cells and B lymphocytes, in children receiving T-cell receptor (TCR)–αβ/CD19-depleted haploidentical HSCT. Peripheral blood samples were obtained 1, 3, 6, 9, and 12 months after HSCT. NK cells were identified and counted on the basis of their CD3–CD56+ phenotype. The graph depicts the absolute number of each cell subset, which is shown in terms of mean and standard error of the mean (SEM). Absolute numbers were calculated by multiplying the frequency of each individual cell subset by the white blood cell count. (D) Reconstitution kinetics of naïve and memory CD4+ and CD8+ T cells in children receiving TCR-αβ/CD19–depleted haploidentical HSCT. Peripheral blood samples were obtained 1, 3, 6, 9, and 12 months after HSCT. Naïve and memory T cells were identified and counted on the basis of their reciprocal expression of the two CD45 isoforms (CD45RA and CD45RO, respectively). The graph depicts the absolute number of each cell subset, which is shown in terms of mean and SEM. Absolute numbers were calculated by multiplying the frequency of each individual cell subset by the white blood cell count. DFS, disease-free survival; E, events; Pts., number of patients evaluable for the occurrence of immune reconstitution.
Outcome and immune reconstitution of patients enrolled in the study. (A) Cumulative incidence (CI) of aGVHD in the study population. (B) Probability of disease-free survival in the study population. (C) Reconstitution kinetics of αβ+ and γδ+ T cells, as well as NK cells and B lymphocytes, in children receiving T-cell receptor (TCR)–αβ/CD19-depleted haploidentical HSCT. Peripheral blood samples were obtained 1, 3, 6, 9, and 12 months after HSCT. NK cells were identified and counted on the basis of their CD3–CD56+ phenotype. The graph depicts the absolute number of each cell subset, which is shown in terms of mean and standard error of the mean (SEM). Absolute numbers were calculated by multiplying the frequency of each individual cell subset by the white blood cell count. (D) Reconstitution kinetics of naïve and memory CD4+ and CD8+ T cells in children receiving TCR-αβ/CD19–depleted haploidentical HSCT. Peripheral blood samples were obtained 1, 3, 6, 9, and 12 months after HSCT. Naïve and memory T cells were identified and counted on the basis of their reciprocal expression of the two CD45 isoforms (CD45RA and CD45RO, respectively). The graph depicts the absolute number of each cell subset, which is shown in terms of mean and SEM. Absolute numbers were calculated by multiplying the frequency of each individual cell subset by the white blood cell count. DFS, disease-free survival; E, events; Pts., number of patients evaluable for the occurrence of immune reconstitution.
Two patients, 1 each affected by SAA and congenital amegakaryocytic thrombocytopenia, died of infectious complications (cytomegalovirus [CMV]-related pneumonia and disseminated adenovirus infection) 120 and 116 days after HSCT, respectively. The child who succumbed to CMV-related pneumonia had previously rejected an unrelated-donor cord blood transplant performed in another center. Both of these patients had an αβ+T-cell count lower than 400 cells per μL at 3 months after transplantation, although the median αβ+T-cell count of the surviving patients was 570 cells per μL. No patient developed EBV-related PTLD. The cumulative incidence of TRM was 9.3% (SE, 6.1).
With a median follow-up of 18 months (range, 5 to 40 months), the 2-year probability of both overall survival and disease-free survival was 91.1% (SE, 6.2; Figure 1B). Considering both primary and secondary graft failure as events, the probability of EFS was 74% (SE, 8.6).
Nine children experienced viral infections and/or reactivations, the cumulative incidence of CMV and adenovirus infection being 38% (SE, 5.0). Recovery of lymphoid subsets investigated is detailed in Figure 1C-D, showing prompt recovery of γδ+ cells in the early posttransplant period and progressive emergence of αβ+ T lymphocytes. Remarkably, all children with SCID had donor B-cell engraftment and are free of immunoglobulin replacement therapy.
Although the results of this study pertain to patients with heterogeneous disorders, the results of this study suggest that the infusion of αβ+ T-cell and B-cell–depleted hematopoietic progenitors from an HLA-haploidentical parent is an effective option for children with life-threatening nonmalignant disorders, either congenital or acquired. aGVHD involved only the skin and was confined to 3 patients, even in the absence of any posttransplantation GVHD prophylaxis. The absence of chronic GVHD in these children is particularly noteworthy, considering the detrimental impact that this complication can have on quality of life of patients who, being affected by nonmalignant disorders, would not benefit from this immune reaction.19 We cannot exclude that elimination of rituximab, and lower dosage of antithymocyte globulin may result in comparable efficacy in terms of GVHD and EBV-related PTLD prevention.
We can interpret the rapid hematopoietic recovery as being the result of a large infusion of stem cells and committed progenitors. The 4 patients who experienced graft failure had SAA (n = 2), thalassemia with allo-/autosensitization (n = 1), and osteopetrosis (n = 1), diseases known to be associated with increased risk of rejection.20,,-23 Because 2 graft failures occurred in patients with SAA, it is reasonable to envision for the future the use of more immune-suppressive preparation, including higher dosages of cyclophosphamide or of total body irradiation. However, the lack of or loss of donor cell engraftment did not jeopardize the successful rescue of these children with a second allograft. The number of fatal events afflicting these children is comparable or even lower than that expected to occur after HSCT from HLA-matched unrelated volunteer or cord blood transplantation.20,,,,,-26 If these results are further confirmed in a larger cohort of patients and with longer follow-up, they indicate that this transplant option might be promptly offered to any child with a nonmalignant disorder who is in need of an allograft and is lacking an HLA-identical sibling. Infusion of titrated numbers of donor-derived αβ+ T cells transduced with a suicide gene for controlling possible alloreactive reactions could further optimize this type of allograft, by accelerating recovery of adaptive immunity.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
There is an Inside Blood Commentary on this article in this issue.
Acknowledgments
This work was supported by research grants from Associazione Italiana per la Ricerca sul Cancro (5 × 1000 Special Grant No. 9962) (A.M., L.M., and F.L.) and Investigator Grant Project No. 10643 (A.M.), by Progetti di Rilevante Interesse Nazionale 2010 (F.L.) and 2012 (S.R.), by Ospedale Bambino Gesù, Roma, Progetto di Ricerca Corrente 2012-2013 (A.B. and F.L.), and Finanziaria Laziale di Sviluppo Adult Stem Cells (S.R.).
Authorship
Contribution: A.B. designed the study, performed transplantation, and wrote the article; P.M., D. Pagliara, and M.E.B. designed the study and performed transplantation; S.R. designed the study, coordinated graft manipulation, and wrote the article; R.M. analyzed data; D. Pende. and M.F. performed natural killer cell characterization studies; R.H. contributed to study design; F.M., B.L., and L.P.B. performed transplantation and followed patients; G.L.P. performed graft manipulation; M.T. performed donor-recipient pair HLA typing; C.C., N.K., and A.F. diagnosed children with primary immune deficiencies and followed patients after the allograft; R.C. performed immunologic reconstitution studies; A.M. and L.M. contributed to study design and supervised NK cell characterization studies; and F.L. designed the study, interpreted data, performed transplantation, and wrote the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alice Bertaina, Department of Pediatric Hematology and Oncology, Istituto di Ricovero e Cura a Carattere Scientifico Ospedale Pediatrico Bambino Gesù, Piazza S.Onofrio 4, 00165, Rome, Italy; e-mail: alice.bertaina@opbg.net.