Key Points
Obesity is associated with increased risk for persistent minimal residual disease after induction therapy for pediatric BP-ALL.
Abstract
Obesity is associated with poorer event-free survival (EFS) in pediatric acute lymphoblastic leukemia (ALL). Persistent minimal residual disease (MRD) in the bone marrow as measured by multidimensional flow cytometry (MDF) is a key early prognostic indicator and is strongly associated with EFS. We therefore hypothesized that obesity during induction would be associated with positive end-of-induction MRD (≥0.01%). We analyzed MDF of end-induction bone marrow samples from a historical cohort of 198 children newly diagnosed with B-precursor ALL (BP-ALL) and treated with Children’s Oncology Group induction regimens. We assessed the influence of body mass index on risk for positive end-induction MRD in the bone marrow. In our cohort of BP-ALL, 30 children (15.2%) were overweight and 41 (20.7%) were obese at diagnosis. Independent of established predictors of treatment response, obesity during induction was associated with significantly greater risk for persistent MRD (odds ratio, 2.57; 95% confidence interval, 1.19 to 5.54; P = .016). Obesity and overweight were associated with poorer EFS irrespective of end-induction MRD (P = .012). Obese children with newly diagnosed BP-ALL are at increased risk for positive end-induction MRD and poorer EFS.
Introduction
Obesity is associated with increased mortality from a variety of cancers.1 In pediatric acute lymphoblastic leukemia (ALL), obesity at the time of diagnosis has similarly been associated with an increased risk of relapse and poorer event-free survival (EFS).2-5 As opposed to traditional host or biologic predictors of survival, however, weight and body composition fluctuate over the multiple years of ALL-directed therapy.6,7 We have recently shown that duration of obesity during the initial intensive months of chemotherapy independently predicts EFS.3 Whether obesity poses a uniform risk during this period or whether it contributes greater influence during certain phases of treatment has yet to be investigated. On modern treatment regimens for ALL, disease response to initial chemotherapy as measured by end-induction minimal residual disease (MRD) in the bone marrow is among the strongest predictors of EFS.8,9 Because of the association between obesity and EFS and the prognostic significance of end-induction MRD, we hypothesized that obesity during induction is associated with persistent leukemia as evidenced by end-induction MRD positivity in the bone marrow. Recent studies from our group have described the active nature of adipocytes in the regulation of chemotherapy response in ALL cell lines and provided the rationale for such an effect.10-12 To the best of our knowledge, this is the first study examining the association of obesity and end-induction MRD.
Methods
Study cohort
Children between the ages of 1 and 21 years diagnosed with ALL between January 2008 and January 2013 at Children’s Hospital Los Angeles were included in this analysis of a historical cohort. Infant leukemia, acute leukemia of ambiguous lineage, and Philadelphia chromosome (BCR-ABL) –positive B-precursor leukemia were excluded from analysis because of their unique leukemia biology and associated risk characteristics. T-cell ALL was similarly considered a distinct disease13 and was excluded because of a limited sample size. Patients with B-precursor ALL (BP-ALL) were classified per National Cancer Institute/Rome criteria (NCI/Rome)14 as standard-risk ALL (SR-ALL) or high-risk ALL (HR-ALL) and treated according to Children’s Oncology Group risk-stratified ALL regimens (SR-ALL: CCG-1991, AALL0331, AALL0932; HR-ALL: CCG-1961, AALL08P1, AALL0232, AALL1131). Per protocol, they received either a 3-drug (vincristine, dexamethasone or prednisone, and pegylated E.colil-asparaginase) or 4-drug induction regimen (adding daunorubicin). Per institutional standard of care and national guidelines,15 chemotherapy was dosed by actual body weight except for vincristine, which was capped per current recommendations at 2 mg. The database included age at diagnosis, gender, race, ethnicity, presence of trisomy 21 (Down syndrome), initial white blood cell count (WBC), central nervous system disease, translocations via fluorescence in situ hybridization, and leukemia cytogenetics. Race and ethnicity were recorded separately, but ethnicity alone was analyzed because of a high correlation between the two. Cytogenetics were classified into favorable (presence of trisomy 4 and trisomy 10 and/or an ETV-RUNX1 fusion), unfavorable (hypodiploid leukemia with <44 chromosomes or DNA index <0.81, and/or presence of intrachromosomal amplification of chromosome 21, and/or mixed-lineage leukemia gene rearrangement), or neutral (none of the above).16 The study was reviewed and approved by the hospital’s Institutional Review Board, and was conducted in accordance with the Declaration of Helsinki.
Weight classification
Height and weight at diagnosis and at the end of induction were used to calculate each child’s body mass index (BMI). Expected variations in height between time points were found within the margin of error for clinical measurement (mean difference of <3% for the cohort) and were accounted for by using the greater value to conservatively determine BMI. The BMI score was then converted to a percentile through population norms for age and gender published by the Centers for Disease Control and Prevention (CDC).17 Each child’s percentile was then classified into a weight category according to the CDC thresholds for overweight (85% to 94% inclusive), and obese (≥95th percentile). Children below the 85th percentile were classified for this study as “lean.”
Analysis of MRD
MRD was obtained during the study period from end-of-induction bone marrow specimens and was determined by using 4-color multidimensional flow cytometry. Bone marrow specimens were analyzed with a dual-laser FACSCalibur flow cytometer with CellQuest and CellQuestPro software (BD Biosciences, San Jose, CA) in the institutional clinical flow cytometry laboratory, which tests routinely for MRD for research and clinical requirements. MRD was determined by an abnormal cluster of cells that deviated from normal patterns of antigen expression for cell lineage–specific stages of maturation compared with normal or regenerating bone marrow cells and, where present, a cluster of cells that expressed leukemia-specific immunophenotypes identified at diagnosis.18-21 MRD was calculated as the proportion of residual leukemic blasts compared with total mononuclear cells.22 Because quantity of detectable MRD (ie, the depth of remission) confers additional risk for relapse,8 MRD was analyzed as a continuous variable. MRD was also analyzed as positive or negative (defined by using a threshold of 0.01% residual leukemia blasts) as established from previous large cohorts in pediatric ALL8 and as incorporated into current first-line Children’s Oncology Group studies and therapy.16
Statistical analysis
Distribution of MRD positivity was evaluated by Pearson’s χ2 test across end-of-induction weight categories by weight category at diagnosis. Logistic regression analysis23 was used to analyze the effect of weight-category predictors on the presence or absence of positive MRD at the end of induction. Covariates that may influence response to treatment (Table 1) were screened by univariate analyses. Two multivariate models were then constructed by using reverse stepwise selection with retention of significant variables at P < .05 (additional details are provided in the supplemental Data available at the Blood Web site). Obese status (obese vs not obese) and weight category (lean, overweight, obese) were analyzed in separate multivariate models. As a sensitivity analysis to investigate whether the imbalance in obesity as a result of ethnicity affected the results, the significance of obesity or weight category was tested in the context of a multivariate model inclusive of ethnicity. In addition, because weight is not static, we assessed the effect of change in weight during induction. BMI percentile was also analyzed as a continuous variable to illustrate the prevalence of positive MRD without regard to the CDC-defined thresholds. Finally, to explore the contributions of weight status and MRD at the end of induction to EFS, we examined postinduction EFS, defined as the time until disease progression, recurrence, secondary malignancy, or death as a result of any cause. Patients without an observed event were censored at the time of last contact. A Cox regression model24 using reverse stepwise selection was constructed for the logistic regression starting with predictors present at diagnosis and incorporating end-induction MRD as an established predictor of EFS. Unless otherwise stated, P values refer to 2-sided tests. Statistical computations were performed with Stata 1125 and R software.26
Demographic and disease characteristics
| Characteristic . | All patients . | Weight category at diagnosis . | P* . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Lean . | Overweight . | Obese . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | ||
| Total | 198 | 100.0 | 127 | 64.1 | 30 | 15.2 | 41 | 20.7 | |
| BMI percentile at diagnosis | |||||||||
| Mean ± SD | 60.3% ± 33.3% | ||||||||
| Range | <1%-99.9% | ||||||||
| Gender | .042 | ||||||||
| Male | 101 | 51.0 | 58 | 45.7 | 15 | 50.0 | 28 | 68.3 | |
| Female | 97 | 49.0 | 69 | 54.3 | 15 | 50.0 | 13 | 31.7 | |
| Age, years† | .002 | ||||||||
| Mean ± SD | 8.2 ± 5.4 | ||||||||
| Range | 1.2-18.9 | ||||||||
| <13 | 144 | 72.7 | 99 | 78.0 | 24 | 80.0 | 21 | 51.2 | |
| ≥13 | 54 | 27.3 | 28 | 22.0 | 6 | 20.0 | 20 | 48.8 | |
| Ethnicity | .001 | ||||||||
| Hispanic | 157 | 79.3 | 94 | 74.0 | 22 | 73.3 | 41 | 100.0 | |
| Non-Hispanic | 41 | 20.7 | 33 | 26.0 | 8 | 26.7 | 0 | 0.0 | |
| Trisomy 21 | .009 | ||||||||
| Absent | 179 | 90.4 | 118 | 92.9 | 29 | 96.7 | 32 | 78.0 | |
| Present | 19 | 9.6 | 9 | 7.1 | 1 | 3.3 | 9 | 22.0 | |
| NCI/Rome risk | .098 | ||||||||
| Standard | 111 | 56.1 | 75 | 59.1 | 19 | 63.3 | 17 | 41.5 | |
| High | 87 | 43.9 | 52 | 40.9 | 11 | 36.7 | 24 | 58.5 | |
| Initial WBC count (per μL) | .536 | ||||||||
| Median ± SD | 9.0 ± 67.86 | ||||||||
| Range | 600-562 000 | ||||||||
| <50 000 | 162 | 81.8 | 102 | 80.3 | 24 | 80.0 | 36 | 87.8 | |
| ≥50 000 | 36 | 18.2 | 25 | 19.7 | 6 | 20.0 | 5 | 12.2 | |
| CNS disease‡ | .029 | ||||||||
| CNS1 | 175 | 88.4 | 114 | 89.8 | 30 | 100.0 | 31 | 75.6 | |
| CNS2 | 21 | 10.6 | 12 | 9.4 | 0 | 0.0 | 9 | 22.0 | |
| CNS3 | 2 | 1.0 | 1 | 0.8 | 0 | 0.0 | 1 | 2.4 | |
| Leukemia cytogenetics | .432 | ||||||||
| Neutral | 133 | 67.2 | 80 | 63.0 | 20 | 66.7 | 33 | 80.5 | |
| Favorable | 58 | 29.3 | 41 | 32.3 | 10 | 33.3 | 7 | 17.1 | |
| Unfavorable | 6 | 3.0 | 5 | 3.9 | 0 | 0.0 | 1 | 2.4 | |
| Unavailable | 1 | 0.5 | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | |
| End of induction morphology§ | NA | ||||||||
| M1 | 194 | 98.0 | 196 | 99.0 | 0 | 0.0 | 196 | 99.0 | |
| M2 | 3 | 1.5 | 2 | 1.0 | 0 | 0.0 | 1 | 0.5 | |
| M3 | 1* | 0.5 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | |
| MRD, % | .032 | ||||||||
| Range | 0-30|| | ||||||||
| <0.01 | 143 | 72.2 | 98 | 77.2 | 22 | 73.3 | 23 | 56.1 | |
| ≥0.01 | 55 | 27.8 | 29 | 22.8 | 8 | 26.7 | 18 | 43.9 | |
| Characteristic . | All patients . | Weight category at diagnosis . | P* . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Lean . | Overweight . | Obese . | |||||||
| No. . | % . | No. . | % . | No. . | % . | No. . | % . | ||
| Total | 198 | 100.0 | 127 | 64.1 | 30 | 15.2 | 41 | 20.7 | |
| BMI percentile at diagnosis | |||||||||
| Mean ± SD | 60.3% ± 33.3% | ||||||||
| Range | <1%-99.9% | ||||||||
| Gender | .042 | ||||||||
| Male | 101 | 51.0 | 58 | 45.7 | 15 | 50.0 | 28 | 68.3 | |
| Female | 97 | 49.0 | 69 | 54.3 | 15 | 50.0 | 13 | 31.7 | |
| Age, years† | .002 | ||||||||
| Mean ± SD | 8.2 ± 5.4 | ||||||||
| Range | 1.2-18.9 | ||||||||
| <13 | 144 | 72.7 | 99 | 78.0 | 24 | 80.0 | 21 | 51.2 | |
| ≥13 | 54 | 27.3 | 28 | 22.0 | 6 | 20.0 | 20 | 48.8 | |
| Ethnicity | .001 | ||||||||
| Hispanic | 157 | 79.3 | 94 | 74.0 | 22 | 73.3 | 41 | 100.0 | |
| Non-Hispanic | 41 | 20.7 | 33 | 26.0 | 8 | 26.7 | 0 | 0.0 | |
| Trisomy 21 | .009 | ||||||||
| Absent | 179 | 90.4 | 118 | 92.9 | 29 | 96.7 | 32 | 78.0 | |
| Present | 19 | 9.6 | 9 | 7.1 | 1 | 3.3 | 9 | 22.0 | |
| NCI/Rome risk | .098 | ||||||||
| Standard | 111 | 56.1 | 75 | 59.1 | 19 | 63.3 | 17 | 41.5 | |
| High | 87 | 43.9 | 52 | 40.9 | 11 | 36.7 | 24 | 58.5 | |
| Initial WBC count (per μL) | .536 | ||||||||
| Median ± SD | 9.0 ± 67.86 | ||||||||
| Range | 600-562 000 | ||||||||
| <50 000 | 162 | 81.8 | 102 | 80.3 | 24 | 80.0 | 36 | 87.8 | |
| ≥50 000 | 36 | 18.2 | 25 | 19.7 | 6 | 20.0 | 5 | 12.2 | |
| CNS disease‡ | .029 | ||||||||
| CNS1 | 175 | 88.4 | 114 | 89.8 | 30 | 100.0 | 31 | 75.6 | |
| CNS2 | 21 | 10.6 | 12 | 9.4 | 0 | 0.0 | 9 | 22.0 | |
| CNS3 | 2 | 1.0 | 1 | 0.8 | 0 | 0.0 | 1 | 2.4 | |
| Leukemia cytogenetics | .432 | ||||||||
| Neutral | 133 | 67.2 | 80 | 63.0 | 20 | 66.7 | 33 | 80.5 | |
| Favorable | 58 | 29.3 | 41 | 32.3 | 10 | 33.3 | 7 | 17.1 | |
| Unfavorable | 6 | 3.0 | 5 | 3.9 | 0 | 0.0 | 1 | 2.4 | |
| Unavailable | 1 | 0.5 | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | |
| End of induction morphology§ | NA | ||||||||
| M1 | 194 | 98.0 | 196 | 99.0 | 0 | 0.0 | 196 | 99.0 | |
| M2 | 3 | 1.5 | 2 | 1.0 | 0 | 0.0 | 1 | 0.5 | |
| M3 | 1* | 0.5 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | |
| MRD, % | .032 | ||||||||
| Range | 0-30|| | ||||||||
| <0.01 | 143 | 72.2 | 98 | 77.2 | 22 | 73.3 | 23 | 56.1 | |
| ≥0.01 | 55 | 27.8 | 29 | 22.8 | 8 | 26.7 | 18 | 43.9 | |
Pearson’s χ2 test for distribution of predictors across BMI weight category.
Additionally analyzed per Children’s Oncology Group “very-high-risk” age threshold.
CNS1, no WBCs or blasts; CNS2, <5 WBCs and blasts; CNS3, ≥5 WBCs and blasts.
M1, <5% blasts; M2, 5% to 25% blasts; M3, >25% blasts.
Same patient with M3 marrow and 30% residual leukemia by flow cytometry.
CNS, central nervous system; NA, not applicable; SD, standard deviation.
Results
Of 237 patients diagnosed with ALL during the study period, the final cohort consisted of 198 patients with BP-ALL after excluding ineligible (n = 26) or not evaluable (n = 13) patients (supplemental Figure 1). Information for the cohort on demographics, disease, and treatments is presented in Table 1. Approximately one-third of the cohort was either overweight (n = 30 [15.2%]) or obese (n = 41 [20.7%]) at time of diagnosis. More than half the cohort (n = 110 [55.5%]) gained weight during induction. Of those who were lean at time of diagnosis (n = 127), 45 (35.4%) gained sufficient weight to be classified as overweight (n = 23/127 [18.1%]) or obese (n = 22/127 [17.3%]) by the end of induction (supplemental Table 1). Of note, the study included a predominantly Hispanic population on the basis of institutional demographics (n = 157/198 [79.3%]). As previously reported nationally27 and in a recent ALL population,28 obesity was more prevalent in our cohort in the Hispanic population. Consistent with national averages, obesity was more prevalent in our cohort in males,27 adolescents (particularly those age 13 years or older),27 and in children with Down syndrome.29 No significant differences were found in NCI/Rome risk category and cytogenetic classification.
Risk for persistent MRD by weight status at diagnosis
Obese children with BP-ALL had significantly increased prevalence of MRD positivity on univariate analysis (Table 1). On multivariate analysis (Table 2), obesity at diagnosis remained significantly associated with a more than twofold increase in risk for MRD positivity at the end of induction compared with those who were not obese at diagnosis (odds ratio [OR], 2.57; 95% confidence interval [CI], 1.19 to 5.54; P = .016). Similarly, when all 3 weight categories were considered, children who were overweight or obese at diagnosis continued to demonstrate increased risk for MRD positivity (OR, 1.37 [95% CI, 0.53 to 3.55] and OR, 2.74 [95% CI, 1.24 to 6.10], respectively; P = .046). When ethnicity was also included in each final model, the effect of Hispanic ethnicity on MRD was not significant (P > .70 for both models; multivariate model 1 [MV1]: OR, 1.13 [95% CI, 0.46 to 2.76]; MV2: OR, 1.15 [95% CI, 0.47 to 2.80]) and significance levels and ORs for other variables in the models changed only negligibly. Analysis of BMI percentile as a continuous variable further illustrates the increased risk for persistent MRD in the highest BMI percentiles (P = .02; Figure 1 and supplemental Data). Previously established predictors of early response to treatment, such as starting WBC and cytogenetics, were independently associated with MRD positivity in both multivariate models (Table 2).
Multivariate logistic regression for persistent end-of-induction MRD ≥0.01%
| Variable* . | Univariate analyses . | Multivariate model 1 . | Multivariate model 2 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR . | 95% CI . | P . | OR . | 95% CI . | P . | OR . | 95% CI . | P . | |
| Age (y), continuous | 1.07 | 1.01-1.14 | .014 | ||||||
| Age at diagnosis (y) | .030 | ||||||||
| <13 | 1 | ||||||||
| ≥13 | 2.12 | 1.08-4.15 | |||||||
| NCI/Rome risk | .011 | ||||||||
| Standard | 1 | ||||||||
| High | 2.27 | 1.20-4.27 | |||||||
| WBC (per microliter), continuous | 1.01 | 1.00-1.01 | .052 | 1.91 | 1.10-3.33 | .021 | 1.91 | 1.10-3.34 | .021 |
| WBC, per microliter | .035 | ||||||||
| <50 000 | 1 | ||||||||
| ≥50 000 | 2.28 | 1.07-4.88 | |||||||
| Cytogenetics† | <.001 | .004 | .003 | ||||||
| Neutral | 1 | 1 | 1 | ||||||
| Favorable | 0.28 | 0.12-0.66 | 0.34 | 0.14-0.83 | 0.34 | 0.14-0.83 | |||
| Unfavorable | 4.05 | 0.71-22.94 | 5.25 | 0.89-31.84 | 5.59 | 0.91-34.32 | |||
| Obese | .013 | .016 | |||||||
| No | 1 | 1 | |||||||
| Yes | 2.51 | 1.23-5.16 | 2.57 | 1.19-5.54 | |||||
| Weight category | .042 | .046 | |||||||
| Lean | 1 | 1 | |||||||
| Overweight | 1.22 | 0.49-3.02 | 1.37 | 0.53-3.55 | |||||
| Obese | 2.62 | 1.24-5.50 | 2.74 | 1.24-6.10 | |||||
| Weight change during induction‡ | .016 | ||||||||
| Gain | 1 | ||||||||
| Loss | 2.17 | 1.15-4.08 | |||||||
| Variable* . | Univariate analyses . | Multivariate model 1 . | Multivariate model 2 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR . | 95% CI . | P . | OR . | 95% CI . | P . | OR . | 95% CI . | P . | |
| Age (y), continuous | 1.07 | 1.01-1.14 | .014 | ||||||
| Age at diagnosis (y) | .030 | ||||||||
| <13 | 1 | ||||||||
| ≥13 | 2.12 | 1.08-4.15 | |||||||
| NCI/Rome risk | .011 | ||||||||
| Standard | 1 | ||||||||
| High | 2.27 | 1.20-4.27 | |||||||
| WBC (per microliter), continuous | 1.01 | 1.00-1.01 | .052 | 1.91 | 1.10-3.33 | .021 | 1.91 | 1.10-3.34 | .021 |
| WBC, per microliter | .035 | ||||||||
| <50 000 | 1 | ||||||||
| ≥50 000 | 2.28 | 1.07-4.88 | |||||||
| Cytogenetics† | <.001 | .004 | .003 | ||||||
| Neutral | 1 | 1 | 1 | ||||||
| Favorable | 0.28 | 0.12-0.66 | 0.34 | 0.14-0.83 | 0.34 | 0.14-0.83 | |||
| Unfavorable | 4.05 | 0.71-22.94 | 5.25 | 0.89-31.84 | 5.59 | 0.91-34.32 | |||
| Obese | .013 | .016 | |||||||
| No | 1 | 1 | |||||||
| Yes | 2.51 | 1.23-5.16 | 2.57 | 1.19-5.54 | |||||
| Weight category | .042 | .046 | |||||||
| Lean | 1 | 1 | |||||||
| Overweight | 1.22 | 0.49-3.02 | 1.37 | 0.53-3.55 | |||||
| Obese | 2.62 | 1.24-5.50 | 2.74 | 1.24-6.10 | |||||
| Weight change during induction‡ | .016 | ||||||||
| Gain | 1 | ||||||||
| Loss | 2.17 | 1.15-4.08 | |||||||
Only significant variables are depicted in the table. Gender, ethnicity, presence of trisomy 21, and presence of CNS disease were not significantly associated with persistent MRD in the bone marrow. See “Methods” for a description of multivariate model construction and retention of predictors.
A single patient with unknown cytogenetics was excluded from this analysis.
Weight change was also tested for an interaction with weight category and was not significant (P = .101 in model 1, and P = .157 in model 2).
Prevalence of positive MRD by BMI percentile. To illustrate the relationship between MRD status and BMI percentile as a continuous variable without regard to CDC-defined thresholds, a monotone nondecreasing logistic model was fit to the data (curved line; error bars correspond to 95% confidence intervals for each of the 6 equal groups [n = 33 per group]). This showed an increased prevalence of MRD positivity at the highest BMI percentiles as depicted in the figure. The significance of this finding was confirmed via permutation test (P = .02).
Prevalence of positive MRD by BMI percentile. To illustrate the relationship between MRD status and BMI percentile as a continuous variable without regard to CDC-defined thresholds, a monotone nondecreasing logistic model was fit to the data (curved line; error bars correspond to 95% confidence intervals for each of the 6 equal groups [n = 33 per group]). This showed an increased prevalence of MRD positivity at the highest BMI percentiles as depicted in the figure. The significance of this finding was confirmed via permutation test (P = .02).
Risk for persistent MRD by weight change during induction
Weight change during induction was not found to be a significant predictor of MRD when controlling for starting weight (any weight loss compared with no change and/or weight gain, MV1: OR, 1.28 [95% CI, 0.63 to 2.60; P = .49]; MV2: OR, 1.27 [95% CI, 0.62 to 2.57; P = .51]). Analysis of the interaction between weight change and starting weight category also was not significant in either multivariate model (MV1: P = .101; MV2: P = .157). Examination of children whose BMI changed over induction showed that children who started lean but gained sufficient weight to be classified as overweight or obese did not show different rates of MRD than those who stayed lean (P = .98; supplemental Table 1); a similar lack of an effect on MRD from changes in weight was found in those who started as overweight or obese (P = .50 and P = .26, respectively; supplemental Table 1).
Obesity and EFS
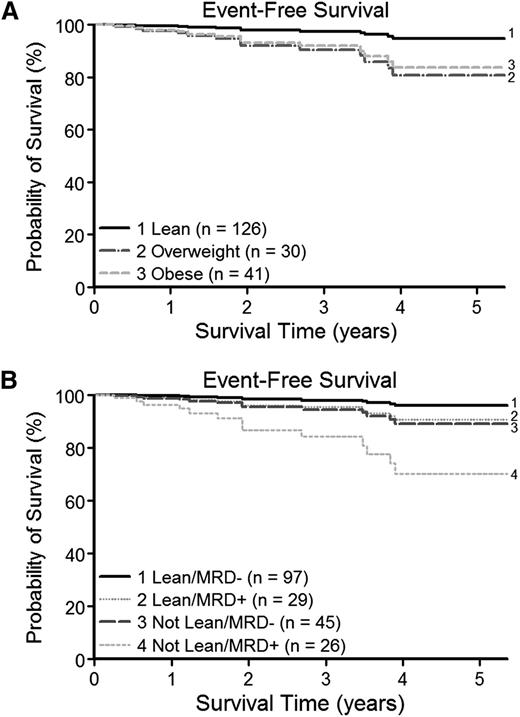
The BP-ALL cohort who were alive and event free had a median follow-up of 1.9 years with a maximum follow-up of 5.4 years from the time of diagnosis. Despite this relatively short follow-up, overweight and obese patients had poorer EFS than the lean group (Figure 2A). On multivariate analysis controlling for NCI/Rome risk category and end-induction MRD as a continuous variable (MV1; Table 3 and supplemental Data), those overweight or obese at diagnosis had a significantly increased risk for an event (hazard ratio [HR], 7.70 [95% CI, 1.37 to 43.12] and HR, 6.65 [95% CI, 1.40 to 31.63], respectively; P = .012). When EFS was analyzed instead in a model inclusive of the clinically used threshold for MRD positivity (MV2; Table 3), the same trend toward an increased risk for an event in the obese and overweight group was present (HR, 5.65 [95% CI, 1.17 to 27.30] and HR, 2.57 [95% CI, 0.70 to 9.36], respectively; P = .081). In comparing the relative contributions to EFS of BMI (lean vs not lean) and MRD positivity (MRD positive vs MRD negative), the survival curves show a clear pattern of differences in EFS by weight category, even within MRD groups (Figure 2B). Children who were lean and MRD negative had the best EFS, and MRD positivity and being overweight or obese were associated with poorer EFS. Children who were overweight or obese and also MRD positive had the lowest EFS. As expected, NCI/Rome high risk and positive end-induction MRD were also both significantly associated with poorer EFS on the multivariate analysis (NCI/Rome high risk: HR, 5.96 [95% CI, 1.25 to 28.32; P = .010] and positive end-induction MRD: HR, 3.48 [95% CI, 1.00 to 12.12; P = .045]; Table 3). Similar to the preceding analysis for MRD, there was no evidence that weight change during induction affected EFS after controlling for weight category at diagnosis.
Weight status and EFS. Cox regression analysis was used to evaluate the effect of weight category at diagnosis and MRD on postinduction EFS. At early ∼2-year median follow-up, NCI/Rome risk category–adjusted survival curves revealed poorer survival for (A) children overweight or obese at time of diagnosis and (B) an additional contribution of BMI category (lean or not lean) to EFS even within MRD groups (end-induction MRD positive or negative). Survival curves plotted from time of diagnosis for clarity.
Weight status and EFS. Cox regression analysis was used to evaluate the effect of weight category at diagnosis and MRD on postinduction EFS. At early ∼2-year median follow-up, NCI/Rome risk category–adjusted survival curves revealed poorer survival for (A) children overweight or obese at time of diagnosis and (B) an additional contribution of BMI category (lean or not lean) to EFS even within MRD groups (end-induction MRD positive or negative). Survival curves plotted from time of diagnosis for clarity.
Multivariate Cox regression model of EFS
| Variable* . | Univariate analyses . | Multivariate model 1 . | Multivariate model 2 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P† . | HR . | 95% CI . | P† . | |
| Age at diagnosis (continuous, years) | 1.24 | 1.10-1.39 | .001 | ||||||
| At diagnosis | .001 | ||||||||
| <13 | 1 | ||||||||
| ≥13 | 7.12 | 2.19-23.17 | |||||||
| Gender | .349 | ||||||||
| Female | 1 | ||||||||
| Male | 1.69 | ||||||||
| Ethnicity | .160 | ||||||||
| Non-Hispanic | 1 | ||||||||
| Hispanic | 3.42 | 0.44-26.31 | |||||||
| Trisomy 21 | .470 | ||||||||
| Absent | 1 | ||||||||
| Present | 1.82 | 0.40-8.20 | |||||||
| NCI/Rome risk | .001 | .016 | .010 | ||||||
| Standard | 1 | 1 | 1 | ||||||
| High | 7.77 | 1.72-35.07 | 5.60 | 1.14-27.34 | 5.96 | 1.25-28.32 | |||
| WBC (per μL), continuous | 4.83 | 2.14-10.90 | <.001 | ||||||
| WBC (per μL) | .008 | ||||||||
| <50 000 | 1 | ||||||||
| ≥50 000 | 4.87 | 1.63-14.51 | |||||||
| Cytogenetics | .044 | ||||||||
| Neutral | 1 | ||||||||
| Favorable | 0.20 | 0.02-1.53 | |||||||
| Unfavorable | 6.90 | 0.80-59.75 | |||||||
| Obese | .061 | ||||||||
| No | 1 | ||||||||
| Yes | 2.95 | 0.99-8.80 | |||||||
| Weight category | .045 | .012 | .081 | ||||||
| Lean | 1 | 1 | 1 | ||||||
| Overweight | 3.78 | 0.84-16.91 | 7.70 | 1.37-43.12 | 5.65 | 1.17-27.30 | |||
| Obese | 4.31 | 1.22-15.29 | 6.65 | 1.40-31.63 | 2.57 | 0.70-9.36 | |||
| Weight change during induction | .011 | ||||||||
| Gain | 1 | ||||||||
| Loss | 4.57 | 1.26-16.63 | |||||||
| End-induction MRD (%), continuous | —‡ | <.001 | —‡ | <.001 | |||||
| End-induction MRD | .006 | .045 | |||||||
| Negative | 1 | 1 | |||||||
| Positive | 4.72 | 1.54-14.45 | 3.48 | 1.00-12.12 | |||||
| Variable* . | Univariate analyses . | Multivariate model 1 . | Multivariate model 2 . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR . | 95% CI . | P . | HR . | 95% CI . | P† . | HR . | 95% CI . | P† . | |
| Age at diagnosis (continuous, years) | 1.24 | 1.10-1.39 | .001 | ||||||
| At diagnosis | .001 | ||||||||
| <13 | 1 | ||||||||
| ≥13 | 7.12 | 2.19-23.17 | |||||||
| Gender | .349 | ||||||||
| Female | 1 | ||||||||
| Male | 1.69 | ||||||||
| Ethnicity | .160 | ||||||||
| Non-Hispanic | 1 | ||||||||
| Hispanic | 3.42 | 0.44-26.31 | |||||||
| Trisomy 21 | .470 | ||||||||
| Absent | 1 | ||||||||
| Present | 1.82 | 0.40-8.20 | |||||||
| NCI/Rome risk | .001 | .016 | .010 | ||||||
| Standard | 1 | 1 | 1 | ||||||
| High | 7.77 | 1.72-35.07 | 5.60 | 1.14-27.34 | 5.96 | 1.25-28.32 | |||
| WBC (per μL), continuous | 4.83 | 2.14-10.90 | <.001 | ||||||
| WBC (per μL) | .008 | ||||||||
| <50 000 | 1 | ||||||||
| ≥50 000 | 4.87 | 1.63-14.51 | |||||||
| Cytogenetics | .044 | ||||||||
| Neutral | 1 | ||||||||
| Favorable | 0.20 | 0.02-1.53 | |||||||
| Unfavorable | 6.90 | 0.80-59.75 | |||||||
| Obese | .061 | ||||||||
| No | 1 | ||||||||
| Yes | 2.95 | 0.99-8.80 | |||||||
| Weight category | .045 | .012 | .081 | ||||||
| Lean | 1 | 1 | 1 | ||||||
| Overweight | 3.78 | 0.84-16.91 | 7.70 | 1.37-43.12 | 5.65 | 1.17-27.30 | |||
| Obese | 4.31 | 1.22-15.29 | 6.65 | 1.40-31.63 | 2.57 | 0.70-9.36 | |||
| Weight change during induction | .011 | ||||||||
| Gain | 1 | ||||||||
| Loss | 4.57 | 1.26-16.63 | |||||||
| End-induction MRD (%), continuous | —‡ | <.001 | —‡ | <.001 | |||||
| End-induction MRD | .006 | .045 | |||||||
| Negative | 1 | 1 | |||||||
| Positive | 4.72 | 1.54-14.45 | 3.48 | 1.00-12.12 | |||||
Only significant variables are depicted in the table.
Likelihood ratio P values.
Regression coefficient for MRD as a log10(MRD% + 1) transformed continuous variable included in multivariate model 1.
Discussion
To the best of our knowledge, this is the first report describing the association between obesity (via BMI) and the key prognostic marker of end-induction MRD in pediatric ALL. Children with BP-ALL who were obese at diagnosis had a poorer response to induction therapy as evidenced by a more than twofold greater risk for persistent MRD in the bone marrow. Irrespective of weight category, children with the highest BMI percentiles at diagnosis were more likely to remain MRD positive following induction therapy. Those who remained MRD positive at the end of induction had inferior EFS as would be predicted,8,22 but BMI contributed significant additional risk for an event even after accounting for end-induction MRD. Overweight or obesity may thus continue to exert an adverse impact on EFS even after the induction phase. The association reported here between obesity and end-induction MRD, however, indicates that the effect of adiposity on survival in pediatric ALL is already present and significant from the time of diagnosis.
The pathophysiology of this relationship between obesity and leukemia response to therapy has yet to be fully elucidated. Early studies explored the question of host-chemotherapy interactions by examining differences in chemotherapy pharmacodynamics in obese vs non-obese individuals. The preponderance of data demonstrated either no effect of BMI on pharmacokinetics30 or increased exposure to chemotherapy in the obese.31-33 The latter may contribute to postinduction treatment failure because obese children have an increased risk for treatment-associated hepatic and pancreatic organ damage, two important dose-limiting toxicities3 ; however, this would not explain our finding of decreased efficacy of induction chemotherapy in the obese. Recent studies have therefore moved the focus from the physiology of the obese to the active nature of adipose tissue itself, exploring adipokines and adipocyte-leukemia interactions. Adipocytes represent a major component of the bone marrow microenvironment, becoming particularly prominent following induction chemotherapy.11 Leukemia cells have been shown to migrate toward and integrate within adipose tissue,10 thus increasing their immediate exposure to adipokines. Furthermore, the presence of adipocytes markedly reduced the efficacy of chemotherapy agents that form the backbone of induction therapy (vincristine, dexamethasone, asparaginase, and daunorubicin) in in vitro coculture and in vivo mouse models.10-12 In addition, adipocytes and increased fat mass have been implicated in leukemogenic pathways34 through the production of cytokines such as leptin, adiponectin, and interleukins.35,36 Because bone marrow adiposity has been shown to correlate with total body fat in some,37 but not all studies,38 investigation is ongoing to determine whether leukemia evasion from therapy is a result of the local adipose microenvironment or a global effect of obesity, for example, via circulating adipokines or altered immune function. Adipocyte-leukemia interactions represent a largely unexplored microenvironment that may mediate the adverse impact of obesity noted in pediatric ALL.
In this context, our findings regarding the influence of obesity on early disease response has significant implications for any potential intervention. The adverse impact of obesity beginning at time of diagnosis on EFS and disease-related mortality has been demonstrated across multiple studies in pediatric ALL,2-5,28 adult ALL,39 and other hematologic malignancies.40-42 The newly identified effect of obesity to increase risk for persistent MRD raises the question of whether obese patients should be considered as a higher risk group from time of diagnosis, analogous to adolescents, who could potentially benefit from more intensive therapy during induction. Alternatively, and in contrast to immutable host and leukemia risk factors, the added risk from obesity may be modifiable and amenable to intervention as suggested in CCG1961 in which normalization of BMI mitigated the adverse impact on EFS.3 Over the past decade, treatment regimens for pediatric ALL have adopted a risk-stratified approach inclusive of MRD to provide effective treatment for those at highest risk for relapse and spare toxicity in others.8,22,43-45 Approximately 1 in 5 children with SR-ALL remain MRD positive following induction therapy22 and are routinely escalated to receive augmented and toxic intensive chemotherapy to reduce risk of relapse.16,45 Successful reduction of postinduction MRD through targeting the obese state may not only improve EFS but may also spare a significant number of children with SR-ALL the need for augmented therapy associated with serious acute and late effects.46,47
Of note, our cohort consisted of a majority of Hispanic children, a population especially prone to obesity.27,48 Although self-identified Hispanic populations have been associated with poorer EFS,43,49 much of this difference was thought to be a result of reduced adherence to treatment regimens,50 a factor not typically a concern during the closely monitored induction phase. Earlier population studies of Hispanic children with leukemia, however, provided hints of a difference in disease response in this group.49 Emerging data on leukemia biology is lending increased support to this hypothesis through identification of an increased prevalence of new, higher-risk mutations in Hispanic children with ALL.51-54 When we included ethnicity in the analysis, we found a negligible effect on the study’s primary outcome of end-induction MRD. Although this supports the independent association of obesity and persistent MRD, it also highlights certain populations wherein obesity is prevalent (such as Hispanic and adolescents and young adults) and who thus constitute particular at-risk groups.
This study reports a novel association between obesity at time of diagnosis and persistence of end-induction MRD, an important predictor of leukemia relapse. Analyses of the influence of BMI on EFS or relapse risk in ALL are complicated by the continuous changes to BMI over the years of ALL therapy. The strength of this analysis therefore lies in the use of the proximal end point of MRD. Although we could not exclude all heterogeneity (specifically in the use of either dexamethasone or prednisone in the most recent HR-ALL protocols), the relative uniformity of 3- or 4-drug induction therapy within NCI/Rome risk categories and the brief time period for the primary end point minimized the risk for confounding treatment-related variables that occur over prolonged therapy. These findings improve our understanding of the connection between obesity and ALL outcomes and should be confirmed in larger studies across different populations.
The online version of this article contains a data supplement.
Presented in part at the105th Annual Meeting of the American Association for Cancer Research, San Diego, CA, April 5-9, 2014.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by a Translational Research Program grant (LLS-6249-11) from the Leukemia and Lymphoma Society.
Authorship
Contribution: E.O., S.D.M., and D.R.F. conceived the study; E.O., H.A.-A., and S.D.M. designed the study; J.T., W.A., and E.O. collected the data; H.A. established flow cytometry protocol and analyzed all MRD; R.S. and J.M. performed the statistical analysis; and all authors helped interpret the results and write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven D. Mittelman, Division of Endocrinology, Children’s Hospital Los Angeles, 4650 Sunset Blvd, Mail Stop 93, Los Angeles, CA 90027; e-mail: smittelman@chla.usc.edu; and Hisham Abdel-Azim, Division of Hematology, Oncology, Blood and Marrow Transplantation, Children’s Hospital Los Angeles, 4650 Sunset Blvd, Mail Stop 62, Los Angeles, CA 90027; e-mail: habdelazim@chla.usc.edu.
References
Author notes
H.A.-A. and S.D.M. are co-senior authors who contributed equally to the study.

![Figure 1. Prevalence of positive MRD by BMI percentile. To illustrate the relationship between MRD status and BMI percentile as a continuous variable without regard to CDC-defined thresholds, a monotone nondecreasing logistic model was fit to the data (curved line; error bars correspond to 95% confidence intervals for each of the 6 equal groups [n = 33 per group]). This showed an increased prevalence of MRD positivity at the highest BMI percentiles as depicted in the figure. The significance of this finding was confirmed via permutation test (P = .02).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/124/26/10.1182_blood-2014-08-595389/4/m_3932f1.jpeg?Expires=1769088379&Signature=ZiHYkvwy2Y6flm9rkZxtt-KRoshA9rACMstU5tcZY5llQI7Jlks2OdhwtqvqlPdCrX7hZSzB61gusmke8oTrGzkT4gTx60hLRTbDpP6OAGzOQk9PsM63wqqVNCrR2nnORBhtMhSxfUWSseyfqudCnAOp~h0RlTkKDXoVvCDy3Qfu4ybRaSCySidoPW6mKAjvsycNrGyYMAhX28Ksr1~liieAdlfB5oB2ahg7n0YHJsRW10ZuL~pJ8WEoatx8ozyCLCRR4Ph5DuX-hUg682luXJF7POTWKIHviPpuM9N1z9K8EUw9HGDGbsyO~PsBhZ7s5vKGRPbfX3RMr-12g3Lq6g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)