Key Points
MCL-1 is critical for thymic lymphoma development mediated by loss of p53.
MCL-1 is essential for sustained growth of p53-deficient thymic lymphoma cells.
Abstract
Apoptosis plays a role in normal lymphopoiesis and lymphoid malignancies. Pro-survival MCL-1 is essential for survival of T-cell progenitors, BCL-XL for immature thymocytes, and BCL-2 for mature T cells. Conversely, little is known about the regulators that are required for the survival of T-cell lymphomas. We used constitutive and conditionally gene-targeted mice to investigate which pro-survival BCL-2 family member is required for the sustained survival of thymic lymphomas initiated by loss of p53. Constitutive loss of a single Mcl-1 allele delayed tumor onset. In contrast, lymphomas emerging in p53−/− mice in which Mcl-1 could be conditionally deleted had been selected for retention of MCL-1 expression. In contrast, complete loss of BCL-XL had no impact on lymphoma development in p53−/− mice. These results demonstrate that thymic lymphomas elicited by loss of p53 must arise from cancer-initiating cells that require MCL-1 for their survival. Acute deletion of both Mcl-1 alleles abrogated the expansion of p53−/− lymphomas in mice, whereas inducible loss of BCL-XL had little impact. This reveals that MCL-1 is essential for the sustained survival of these malignant cells and suggests that targeting MCL-1 may be an attractive strategy for the treatment of T-cell lymphoma.
Introduction
Mutations in p53 have been found in ∼50% of sporadic human cancers.1 Moreover, inherited mutations in 1 p53 allele cause Li–Fraumeni syndrome, a rare autosomal disorder characterized by development of diverse cancer types at a young age after loss of the wild-type p53 allele.2,3 p53 is a transcription factor that controls diverse cellular responses to stress, including proliferation arrest, senescence, and apoptosis, by direct regulation of ∼200 target genes.4 Mice with homozygous germline loss or mutation of p53 develop tumors (mostly thymic lymphomas) with 100% incidence within 300 days.5-8
Evasion of cell death is a prerequisite for the development of cancer. Apoptosis is a genetically programmed process for the killing of cells that are no longer needed or potentially dangerous.9,10 Mutations or epigenetic changes in regulators of apoptosis cause or contribute to the development of several diseases, particularly cancer, and render malignant cells resistant to a broad range of chemotherapeutic drugs. The BCL-2-regulated apoptotic pathway can be activated by developmental cues or a diverse range of stress stimuli (eg, cytokine withdrawal, oncogene activation, DNA damage). This pathway is controlled by the 3 subgroups of the BCL-2 protein family.10 The pro-survival members BCL-2, BCL-XL, MCL-1, BCL-W and A1 (human BFL-1) are required for cell survival. The proapoptotic BH3-only proteins (BIM, PUMA, NOXA, BID, BAD, BIK, BMF, HRK) are essential for initiation of apoptosis with different death stimuli activating distinct members.10,11 The proapoptotic multi-BH (BCL-2 homology) domain proteins BAX and BAK are required for activation of the downstream phases of apoptosis: mitochondrial outer membrane permeabilization and consequent caspase activation.12,13 The BH3-only proteins bind to the pro-survival BCL-2 family proteins to unleash primed BAX/BAK allowing them to cause mitochondrial outer membrane permeabilization. Certain BH3-only proteins (eg, BIM and PUMA) can also activate BAX/BAK through direct binding.14,15
Although it has long been known that overexpression of BCL-2 (or its pro-survival relatives) can promote tumorigenesis, researchers have only recently started to explore which pro-survival BCL-2 family member expressed under endogenous control is required for the development and sustained expansion of which tumor. BCL-XL16,17 but not BCL-218 is essential for the development of MYC-driven pre-B/B lymphoma, whereas MCL-1 is critical for the development of acute myeloid leukemia (AML).19,20 Such information provided the impetus for the development of inhibitors of pro-survival BCL-2 family members (BH3-mimetics navitoclax/ABT-263: inhibits BCL-2, BCL-XL, and BCL-W; ABT-199: inhibits only BCL-2).10
In this study, we examined the impact of loss of BCL-XL or MCL-1 on the development and sustained growth of thymic lymphoma elicited by loss of p53 and show that only MCL-1 is critical.
Materials and methods
Mice
Transplantation studies
For transplantation studies, 6 C57BL/6-Ly5.1 recipient mice were injected intraperitoneally with 3 × 106 thymic lymphoma cells (TCRβ+CD4+CD8+Ly5.2+). A total of 3 to 6 independent thymic lymphoma samples were tested. Three of the recipient mice were treated by oral gavage with 4 mg tamoxifen/day (in peanut oil25 ) on days 7 and 8 (the remaining 3 recipients were left untreated) and then monitored for lymphoma development. Mice that had developed lymphoma were killed, and the lymphomas were analyzed by fluorescence-activated cell sorter analysis and western blotting. The experiment was concluded at 90 days after lymphoma injection. Mice bearing lymphoma were examined for organ weights.
Genotyping
Genotyping of mice was performed on DNA samples obtained from tail biopsies that had been digested with tail digestion buffer (Viagen Biotech, Los Angeles, CA) supplemented with proteinase K (Sigma Aldrich, Castle Hill, NSW, Australia). Oligonucleotides for genotyping were obtained from GeneWorks (Hindmarsh, SA, Australia) at polymerase chain reaction grade quality. GoTaq Green Master Mix (Promega, Alexandria, NSW, Australia) was supplemented with 10 pmol of the appropriate oligonucleotide primer pair to perform the polymerase chain reaction. Oligonucleotide sequences for genotyping will be provided on request.
Flow cytometric analysis
Lymphoid organs were harvested, and single-cell suspensions were prepared using 100-µM sieves (BD BioSciences, San Jose, CA). Red blood cells were depleted using red blood cell lysis buffer. Cells (5 × 104) were stained for surface markers using fluorochrome (fluorescein isothiocyanate, Ag-presenting cells, R-phycoerythrin; Life technologies, Mulgrave, VIC, Australia)-conjugated monoclonal antibodies (WEHI) to mouse CD4 (YTA3.2.1), CD8 (YTS169), CD3 (145-2C11), B220 (RA3-6B2), and human CD4 (RPA-T4) for 30 minutes in balanced salt solution supplemented with 2% fetal calf serum (Life Technologies, Mulgrave, VIC, Australia) and analyzed in a FACS-Calibur (BD BioSciences).
Western blot analysis
Extracts from thymic lymphomas were prepared. Blots were probed using the following monoclonal antibodies: hamster anti-mouse BCL-2 (3F11; WEHI), mouse anti-mouse BCL-XL (2F12; BD BioSciences), rat anti-mouse MCL-1 (19C4-1526 ), mouse anti-actin (AC-40; Sigma Aldrich, Castle Hill, NSW, Australia; used as a loading control), mouse anti-HSP-70 antibody (gift from Dr Robin Anderson, Peter MacCallum Cancer Research Institute, Melbourne, Australia; used as a loading control), polyclonal rabbit anti-mouse BIM (9292; Sapphire Bioscience, Waterloo, NSW, Australia) or polyclonal rabbit anti-mouse PUMA (Ab-27669; Abcam, Melbourne, VIC, Australia), and polyclonal anti-mouse ERα (HC-20; Santa Cruz Biotechnologies, Dallas, TX). Polyclonal goat antibodies against mouse, rat, hamster, or rabbit immunoglobulin (Ig)G antibodies coupled to horseradish peroxidase (In Vitro Technologies, Noble Park North, VIC, Australia) were used as secondary reagents, and enhanced chemiluminescence (GE Healthcare Life Sciences, Pittsburgh, PA) was used for detection of protein bands.
Statistical analysis
Kaplan-Meier survival curves, dot, and bar graphs for organ weights were generated and analyzed using the GraphPad Prism software using a 2-tailed Student t test comparing 2 groups with each other. Error bars are presented as standard error of mean (GraphPad Software, La Jolla, CA). Mouse survival curves were compared by log-rank Mantel Cox test. Disease incidence was calculated using χ2 test. P < .05 was considered significant.
Results
Loss of BCL-XL does not delay thymic lymphoma development
We used the loxP-targeted Bcl-x conditional knockout mouse model to determine the role of BCL-XL in tumorigenesis caused by loss of p53. These animals were crossed to the Lck-Cre strain in which a constitutively active Cre recombinase is expressed from the early TN3 (TCRβ−CD3−CD4−CD8−CD25+CD44−) T-cell progenitor stage in the thymus.
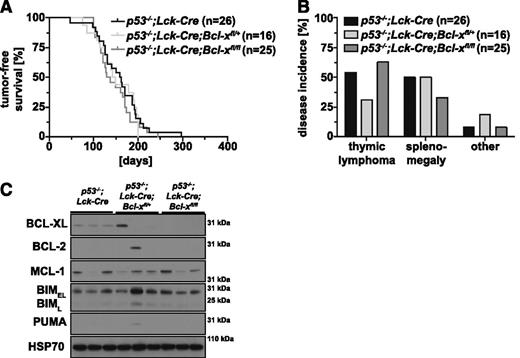
Tumor incidence and rate of tumor development were comparable between p53−/− (median survival, 156 days) and p53−/−;Lck-Cre mice (median survival, 161 days; P = .2996; supplemental Figure 1 available on the Blood Web site), demonstrating that expression of a constitutively active Cre recombinase had no adverse effect on tumor development in p53−/− mice. The survival and rate of tumor development were also comparable between p53−/−;Lck-Cre;Bcl-xfl/+ (median survival, 146 days; P = .6703), p53−/−;Lck-Cre;Bcl-xfl/fl (median survival, 134 days; P = .3236), and p53−/−;Lck-Cre mice (median survival, 161 days) (Figure 1A). Although all p53−/−;Lck-Cre;Bcl-xfl/+ mice developed cancer, the proportion of mice exhibiting thymic lymphoma was reduced compared with p53−/−;Lck-Cre mice (Figure 1B). This reduction in thymic lymphoma incidence was compensated by an increase in other tumors (eg, splenomegaly and sarcoma). The p53−/−;Lck-Cre;Bcl-xfl/fl mice displayed a minor (∼10%) increase in thymic lymphoma incidence but a ∼15% decrease in splenomegaly compared with p53−/−;Lck-Cre mice (Figure 1B). There were no significant differences in thymus or spleen weights between sick p53−/−;Lck-Cre;Bcl-xfl/+ and p53−/−;Lck-Cre;Bcl-xfl/- animals vs sick p53−/−;Lck-Cre controls (supplemental Figure 2A-B). Western blot analysis confirmed that BCL-XL expression was substantially reduced or even abrogated in thymic lymphomas from p53−/−;Lck-Cre;Bcl-xfl/+ (5/6 lymphomas) and p53−/−;Lck-Cre;Bcl-xfl/fl mice (10/10 lymphomas; representative blots shown; Figure 1C). These lymphomas did not show consistent alterations in the expression of other pro-survival (BCL-2, MCL-1) or proapoptotic (BIM and PUMA) BCL-2 family members examined (Figure 1C). These results demonstrate that BCL-XL is not required for the development of thymic lymphoma or other cancers elicited by loss of p53.
Loss of BCL-XL does not delay thymic lymphoma development in p53-deficient mice. (A) Tumor-free survival of p53−/−;Lck-Cre (control; n = 26), p53−/−;Lck-Cre;Bcl-xfl/+ (n = 16, compared with control; P = .6703), and p53−/−;Lck-Cre;Bcl-xfl/fl mice (n = 26; compared with control; P = .3236); mouse cohorts were compared using the log-rank Mantel-Cox test. (B) Incidence (as % of total mice analyzed) of thymic lymphoma, splenomegaly, and other pathologies (eg, sarcoma) in cohorts of p53−/−;Lck-Cre (n = 26), p53−/−;Lck-Cre;Bcl-xfl/+ (n = 16), and p53−/−;Lck-Cre;Bcl-xfl/fl mice (n = 24). (C) Analysis of the proteins indicated by western blotting in primary thymic lymphoma samples from sick p53−/−;Lck-Cre, p53−/−;Lck-Cre;Bcl-xfl/+, and p53−/−;Lck-Cre;Bcl-xfl/fl mice (representative of 6-10 samples for each genotype). Probing for HSP70 was used as a loading control.
Loss of BCL-XL does not delay thymic lymphoma development in p53-deficient mice. (A) Tumor-free survival of p53−/−;Lck-Cre (control; n = 26), p53−/−;Lck-Cre;Bcl-xfl/+ (n = 16, compared with control; P = .6703), and p53−/−;Lck-Cre;Bcl-xfl/fl mice (n = 26; compared with control; P = .3236); mouse cohorts were compared using the log-rank Mantel-Cox test. (B) Incidence (as % of total mice analyzed) of thymic lymphoma, splenomegaly, and other pathologies (eg, sarcoma) in cohorts of p53−/−;Lck-Cre (n = 26), p53−/−;Lck-Cre;Bcl-xfl/+ (n = 16), and p53−/−;Lck-Cre;Bcl-xfl/fl mice (n = 24). (C) Analysis of the proteins indicated by western blotting in primary thymic lymphoma samples from sick p53−/−;Lck-Cre, p53−/−;Lck-Cre;Bcl-xfl/+, and p53−/−;Lck-Cre;Bcl-xfl/fl mice (representative of 6-10 samples for each genotype). Probing for HSP70 was used as a loading control.
Constitutive loss of a single allele of Mcl-1 significantly delays thymic lymphoma development in p53−/− mice
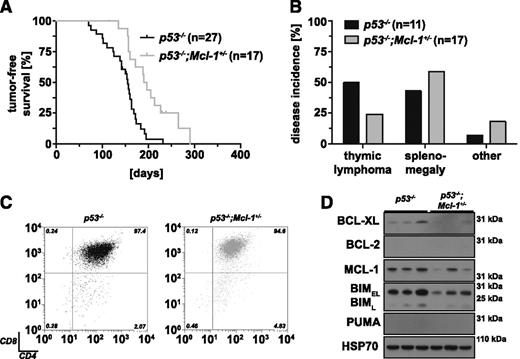
Because most thymic lymphomas in p53−/− mice express readily detectable levels of MCL-1 (Figure 1C), we generated p53-deficient mice that lack 1 allele of Mcl-1 (loss of both Mcl-1 alleles causes early embryonic lethality27 ) to examine the role of this BCL-2 pro-survival family member in T lymphomagenesis (Figure 2). In comparison with p53−/− mice (median survival, 156 days), p53−/−;Mcl-1+/− mice (median survival, 194 days) showed significantly prolonged tumor-free survival (Figure 2A; P = .0006). Compared with p53−/− controls, thymic lymphoma incidence was reduced by ∼50% in p53−/−;Mcl-1+/− mice. Instead the p53−/−;Mcl-1+/− mice were more likely to develop mature T-cell lymphoma in the spleen with liver metastasis and occasionally sarcoma (Figure 2B). Lymphoma burden (measured by the weight of the thymus and spleen) in sick p53−/−;Mcl-1+/− mice was comparable to control p53−/− animals (pthymus = .0619; pspleen = .6274; supplemental Figure 3A-B). Moreover, thymic lymphomas in both strains displayed a similar immunophenotype, mostly TCRβ+CD4+CD8+ (Figure 2C). Western blot analysis revealed reduced levels of MCL-1 (5/7 lymphomas) and also BIM (5/7 lymphomas) in the lymphomas from the p53−/−;Mcl-1+/− mice compared with tumors from control p53−/− mice (representative blots shown; Figure 2D). These results show that loss of a single allele of Mcl-1 can markedly delay the development of thymic lymphoma that is initiated by loss of p53.
Loss of 1 allele of Mcl-1 significantly delays thymic lymphoma development in p53−/− mice. (A) Tumor-free survival comparing p53−/− (control; n = 27) and p53−/−;Mcl-1+/− mice (n = 17; log-rank Mantel-Cox test, P = .0006). (B) Incidence in % of thymic lymphoma, splenomegaly, and other pathologies (eg, sarcoma) in p53−/− (n = 11) and p53−/−;Mcl-1+/− mice (n = 17). (C) Representative immunophenotyping of primary thymic lymphoma samples from p53−/− and p53−/−;Mcl-1+/− mice (gated on PI− and TCRβ+ large cells). (D) Analysis of the proteins indicated by western blotting of primary thymic lymphoma samples from mice of the indicated genotypes (representative of 7 samples each). Probing for HSP70 was used as a loading control.
Loss of 1 allele of Mcl-1 significantly delays thymic lymphoma development in p53−/− mice. (A) Tumor-free survival comparing p53−/− (control; n = 27) and p53−/−;Mcl-1+/− mice (n = 17; log-rank Mantel-Cox test, P = .0006). (B) Incidence in % of thymic lymphoma, splenomegaly, and other pathologies (eg, sarcoma) in p53−/− (n = 11) and p53−/−;Mcl-1+/− mice (n = 17). (C) Representative immunophenotyping of primary thymic lymphoma samples from p53−/− and p53−/−;Mcl-1+/− mice (gated on PI− and TCRβ+ large cells). (D) Analysis of the proteins indicated by western blotting of primary thymic lymphoma samples from mice of the indicated genotypes (representative of 7 samples each). Probing for HSP70 was used as a loading control.
Impact of conditional deletion of 1 or both alleles of Mcl-1 on T-cell lymphoma development in p53−/− mice
The ability of loss of 1 allele of Mcl-1 to significantly delay lymphoma development in p53−/− mice could be due to an impact on cells undergoing neoplastic transformation during T-lymphoid differentiation or might be a consequence of defects in the survival of more primitive hematopoietic stem/progenitor cells, known to be the cells of origin of γ radiation-induced thymic lymphoma.28,29 Furthermore, Lck-Cre;Mcl-1fl/fl mice present with a significant reduction of immature and mature T cells in the thymus, suggesting MCL-1 is critical for T-cell development (supplemental Figure 4A-B).30
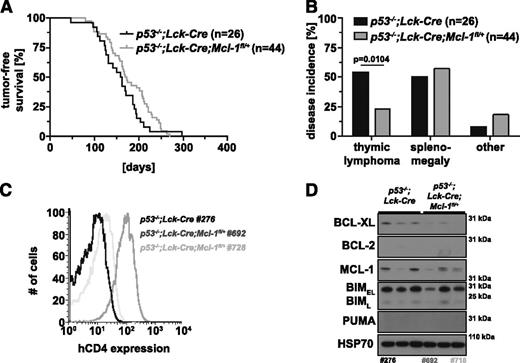
We generated p53−/−;Lck-Cre;Mcl-1fl/+ and p53−/−;Lck-Cre;Mcl-1fl/fl mice to investigate whether loss of MCL-1 specifically in T-lymphoid cells (from the TN3 stage onward) could delay thymic lymphoma development in p53−/− mice. The p53−/−;Lck-Cre;Mcl-1fl/+ mice (median survival, 171 days) did not survive significantly longer compared with the p53−/−;Lck-Cre controls (median survival, 161 days; P = .1216; Figure 3A), but we did observe a ∼50% reduction in thymic lymphoma incidence with a concomitant increase in sarcoma and certain other pathologies (Figure 3B). Thymi from tumor-bearing p53−/−;Lck-Cre;Mcl-1fl/+ mice were significantly smaller, but spleen weights were comparable to those of sick p53−/−;Lck-Cre controls (pthymus = .0045; pspleen = .6639; supplemental Figure 4A-B). To investigate whether the floxed Mcl-1 allele had been recombined in the p53−/−;Lck-Cre;Mcl-1fl/+ lymphomas, we took advantage of the human CD4 (hCD4) reporter engineered into the Mcl-1 gene targeting construct that is only expressed on Mcl-1fl recombination.20,31 Approximately 50% of p53−/−;Lck-Cre;Mcl-1fl/+ lymphomas expressed substantial levels of hCD4 and hence had recombined the floxed Mcl-1 allele (see tumor 692 in Figure 3C). The others were hCD4- (see tumor 728 in Figure 3C) and thus had been selected against loss of even a single Mcl-1 allele. Accordingly, western blotting showed that tumor 692 expressed only low levels of MCL-1 whereas tumor 728 had higher levels of MCL-1 (note that the MCL-1 protein encoded by the Mcl-1fl allele runs with slightly higher molecular weight than MCL-1 protein encoded by the wild-type allele26 ; Figure 3D). The hCD4-positive lymphomas expressing low levels of MCL-1 appeared to be selected for reduced levels of proapoptotic BIM. There were no consistent differences in the expression of BCL-XL, BCL-2, or PUMA between the different lymphomas (Figure 3D).
Impact of deletion of 1 allele of Mcl-1 selectively in T-lymphoid cells on thymic lymphoma development in p53−/− mice. (A) Tumor-free survival of p53−/−;Lck-Cre;Mcl-1fl/+ mice (n = 44) in comparison with control mice (p53−/−;Lck-Cre; n = 26; log-rank Mantel-Cox test, P = .1216). (B) Flow cytometric analysis of Mcl-1fl recombination in 2 independent primary thymic lymphoma samples (TCRβ+CD4+CD8+) from p53−/−;Lck-Cre;Mcl-1fl/+ mice compared with a p53−/−;Lck-Cre thymic lymphoma (negative control) by immunofluorescent staining for the human CD4 reporter. (C) Incidence in % of thymic lymphoma, splenomegaly, sarcoma, or other pathologies in the cohorts of p53−/−;Lck-Cre (n = 26) and p53−/−;Lck-Cre;Mcl-1fl/+ mice (n = 44). (D) Analysis of the proteins indicated by western blotting of primary thymic lymphoma samples from mice of the indicated genotypes (representative of 6-10 samples each). Probing for HSP70 was used as a loading control. The numbers below western blots indicate the tumor samples also tested in B.
Impact of deletion of 1 allele of Mcl-1 selectively in T-lymphoid cells on thymic lymphoma development in p53−/− mice. (A) Tumor-free survival of p53−/−;Lck-Cre;Mcl-1fl/+ mice (n = 44) in comparison with control mice (p53−/−;Lck-Cre; n = 26; log-rank Mantel-Cox test, P = .1216). (B) Flow cytometric analysis of Mcl-1fl recombination in 2 independent primary thymic lymphoma samples (TCRβ+CD4+CD8+) from p53−/−;Lck-Cre;Mcl-1fl/+ mice compared with a p53−/−;Lck-Cre thymic lymphoma (negative control) by immunofluorescent staining for the human CD4 reporter. (C) Incidence in % of thymic lymphoma, splenomegaly, sarcoma, or other pathologies in the cohorts of p53−/−;Lck-Cre (n = 26) and p53−/−;Lck-Cre;Mcl-1fl/+ mice (n = 44). (D) Analysis of the proteins indicated by western blotting of primary thymic lymphoma samples from mice of the indicated genotypes (representative of 6-10 samples each). Probing for HSP70 was used as a loading control. The numbers below western blots indicate the tumor samples also tested in B.
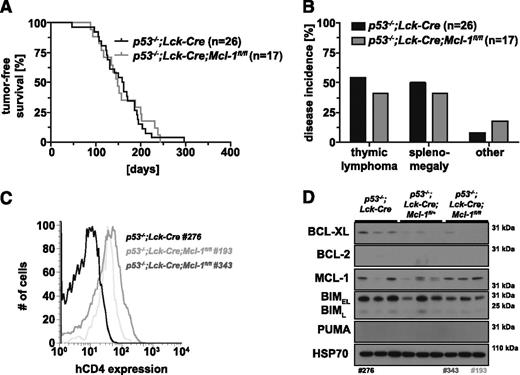
Because thymic lymphoma development in p53−/−;Lck-Cre;Mcl-1fl/+ mice was not markedly delayed compared with control p53−/−;Lck-Cre mice, we hypothesized that complete loss of MCL-1 in T-lymphoid cells might have more pronounced impact on lymphoma development in p53−/− mice. We therefore generated p53−/−;Lck-Cre;Mcl-1fl/fl mice. Remarkably, their survival (median survival, 149 days) was comparable to that of p53−/−;Lck-Cre controls (median survival, 161 days; P = .8292; Figure 4A). Compared with p53−/−;Lck-Cre controls, the p53−/−;Lck-Cre;Mcl-1fl/fl mice had a reduced incidence of thymic lymphoma and splenomegaly but presented more frequently with sarcoma or certain other pathologies (Figure 4B). Postmortem analysis of sick tumor-bearing mice did not reveal any significant differences between the 2 genotypes in terms of thymus or spleen weights (supplemental Figure 4A-B). Lymphomas from p53−/−;Lck-Cre;Mcl-1fl/fl mice had a similar immunophenotype to that of tumors from p53−/− or p53−/−;Lck-Cre mice (6/6; TCRβ+CD4+CD8+; data not shown). All p53−/−;Lck-Cre;Mcl-1fl/fl lymphomas expressed intermediate levels of the hCD4 reporter (Figure 4C), indicating that ≥1 floxed Mcl-1 allele had been recombined. Western blot analysis showed that MCL-1 was maintained at readily detectable levels in p53−/−;Lck-Cre;Mcl-1fl/fl lymphomas (5/6 lymphomas), comparable to the levels of MCL-1 in p53−/−;Lck-Cre control lymphomas (5/5 lymphomas, representative blots shown; Figure 4D). This shows that one Mcl-1fl allele had not been recombined in these lymphomas. There were no consistent differences in the levels of BCL-2 or PUMA between tumors from p53−/−;Lck-Cre and p53−/−;Lck-Cre;Mcl-1fl/fl mice, although we noted a small decrease in BCL-XL and BIM protein levels in the latter (Figure 4D).
Impact of deletion of both alleles of Mcl-1 selectively in T-lymphoid cells on thymic lymphoma development in p53−/− mice. (A) Tumor-free survival of p53−/−;Lck-Cre (n = 26) and p53−/−;Lck-Cre;Mcl-1fl/fl mice (n = 17; log-rank Mantel-Cox test, P = .8292). (B) Flow cytometric analysis of Mcl-1fl recombination in primary thymic lymphoma samples from p53−/−;Lck-Cre (negative control) and p53−/−;Lck-Cre;Mcl-1fl/fl mice by immunofluorescent staining for the human CD4 (hCD4) reporter. (C) Incidence in % of thymic lymphoma, splenomegaly, sarcoma, or other pathologies in cohorts of p53−/−;Lck-Cre (n = 26) and p53−/−;Lck-Cre;Mcl-1fl/fl mice (n = 17). (D) Analysis of the proteins indicated by western blotting of primary thymic lymphoma samples from mice of the indicated genotypes (representative of 6-10 samples each). Probing for HSP70 was used as a loading control. The numbers below western blots indicate the tumor samples also tested in B. Lanes 1 to 6 are the same image shown in Figure 3D and are presented here for comparison.
Impact of deletion of both alleles of Mcl-1 selectively in T-lymphoid cells on thymic lymphoma development in p53−/− mice. (A) Tumor-free survival of p53−/−;Lck-Cre (n = 26) and p53−/−;Lck-Cre;Mcl-1fl/fl mice (n = 17; log-rank Mantel-Cox test, P = .8292). (B) Flow cytometric analysis of Mcl-1fl recombination in primary thymic lymphoma samples from p53−/−;Lck-Cre (negative control) and p53−/−;Lck-Cre;Mcl-1fl/fl mice by immunofluorescent staining for the human CD4 (hCD4) reporter. (C) Incidence in % of thymic lymphoma, splenomegaly, sarcoma, or other pathologies in cohorts of p53−/−;Lck-Cre (n = 26) and p53−/−;Lck-Cre;Mcl-1fl/fl mice (n = 17). (D) Analysis of the proteins indicated by western blotting of primary thymic lymphoma samples from mice of the indicated genotypes (representative of 6-10 samples each). Probing for HSP70 was used as a loading control. The numbers below western blots indicate the tumor samples also tested in B. Lanes 1 to 6 are the same image shown in Figure 3D and are presented here for comparison.
These findings reveal that during thymic lymphoma development driven by loss of p53 potent selection against loss of MCL-1 operates at the TN3 differentiation stage or later.
Inducible loss of BCL-XL has no impact on the sustained expansion of p53−/− lymphomas in mice
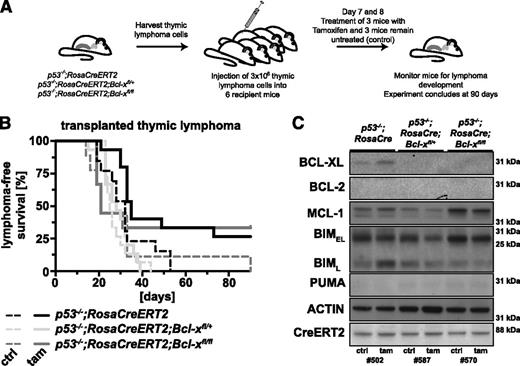
Although BCL-XL is not essential for thymic lymphoma development in p53−/− mice (Figure 1), we wondered whether it might be required for the sustained survival and expansion of these lymphomas. To examine this, we generated p53−/−;Bcl-xfl/+ and p53−/−;Bcl-xfl/fl mice that also contained the RosaCreERT2 transgene to enable acute deletion of Bcl-x in established tumors. This transgene is expressed in all cell types and encodes a conditional Cre-recombinase ER fusion protein (CreERT2) that can be activated by tamoxifen. We examined 3 to 6 p53−/−;Bcl-xfl/+ and p53−/−;Bcl-xfl/fl and (control) p53−/− lymphomas (all C57BL/6-Ly5.2). These were each injected into 6 C57BL/6-Ly5.1 recipients. On days 7 and 8, 3 of these recipients were treated with tamoxifen to activate the latent CreERT2 recombinase to delete the Bcl-xfl alleles, whereas the remainder were left untreated (Figure 5A). These recipients were monitored for lymphoma growth over a 90-day period.
Acute loss of BCL-XL has no impact on the sustained expansion of p53−/−thymic lymphomas in mice. (A) Schematic of the experimental protocol (also used for experiments shown in Figure 6). Lymphomas were harvested from p53−/−;RosaCreERT2 (control), p53−/−;RosaCreERT2;Bcl-xfl/+, p53−/−;RosaCreERT2;Bcl-xfl/fl, p53−/−;RosaCreERT2;Mcl-1fl/+, or p53−/−;RosaCreERT2;Mcl-1fl/fl mice (all Ly5.2+). Lymphoma cells (3 × 106; 3-6 independent primary lymphoma samples per genotype tested) were injected into 6 C57BL/6-Ly5.1+ recipient mice each. Three recipient mice were treated by oral gavage with 4 mg of tamoxifen on days 7 and 8 after lymphoma cell transplantation, and the other 3 recipients were left untreated and served as controls. These mice were then monitored for lymphoma development. The experiment was concluded 90 days after cell transplantation. (B) Lymphoma-free survival of the mice that had been transplanted with lymphoma cells of the genotypes indicated and treated with either tamoxifen (solid lines; to delete the floxed Bcl-x alleles) or had been left untreated (dashed lines; negative control). (C) Analysis of the proteins indicated by western blotting of transplanted thymic lymphoma samples of the indicated genotypes (representative of 3 samples each) that had grown in recipients treated with tamoxifen (tam) or in recipients that had been left untreated (ctrl). Probing for actin was used as a loading control.
Acute loss of BCL-XL has no impact on the sustained expansion of p53−/−thymic lymphomas in mice. (A) Schematic of the experimental protocol (also used for experiments shown in Figure 6). Lymphomas were harvested from p53−/−;RosaCreERT2 (control), p53−/−;RosaCreERT2;Bcl-xfl/+, p53−/−;RosaCreERT2;Bcl-xfl/fl, p53−/−;RosaCreERT2;Mcl-1fl/+, or p53−/−;RosaCreERT2;Mcl-1fl/fl mice (all Ly5.2+). Lymphoma cells (3 × 106; 3-6 independent primary lymphoma samples per genotype tested) were injected into 6 C57BL/6-Ly5.1+ recipient mice each. Three recipient mice were treated by oral gavage with 4 mg of tamoxifen on days 7 and 8 after lymphoma cell transplantation, and the other 3 recipients were left untreated and served as controls. These mice were then monitored for lymphoma development. The experiment was concluded 90 days after cell transplantation. (B) Lymphoma-free survival of the mice that had been transplanted with lymphoma cells of the genotypes indicated and treated with either tamoxifen (solid lines; to delete the floxed Bcl-x alleles) or had been left untreated (dashed lines; negative control). (C) Analysis of the proteins indicated by western blotting of transplanted thymic lymphoma samples of the indicated genotypes (representative of 3 samples each) that had grown in recipients treated with tamoxifen (tam) or in recipients that had been left untreated (ctrl). Probing for actin was used as a loading control.
Mice that were injected with p53−/−;RosaCreERT2 thymic lymphoma cells and then treated with tamoxifen (median survival, 35 days) survived slightly longer than their untreated counterparts (median survival, 32 days), demonstrating that Cre-recombinase induction has only minor cytotoxic impact on these lymphomas in vivo. Tamoxifen-treated mice bearing p53−/−;RosaCreERT2;Bcl-xfl/+ (median survival, 28 days) or p53−/−;RosaCreERT2;Bcl-xfl/fl lymphomas (median survival, 21 days) did not survive significantly longer than the tamoxifen-treated mice bearing control p53−/−;RosaCreERT2 lymphoma (median survival, 35 days; Figure 5B). Postmortem examination revealed that transplanted lymphoma cells consistently infiltrated organs in the abdominal cavity, including spleen, liver, pancreas, and ovaries, whereas only a small proportion engrafted into the thymus. There were no consistent differences in thymus, spleen, and liver weights or organ infiltration between mice bearing lymphomas of the different genotypes (also regardless of whether recipients had been left untreated or treated with tamoxifen; supplemental Figure 5A-C and histological data not shown).
Western blot analysis of thymic lymphomas that had expanded in tamoxifen-treated or control recipient mice showed similar levels of CreERT2, demonstrating no selection against expression of this latent recombinase. The reduction in BCL-XL levels in p53−/−;RosaCreERT2;Bcl-xfl/+ and p53−/−;RosaCreERT2;Bcl-xfl/fl lymphomas appeared to be compensated by an increase in MCL-1 and a decrease in BIM (Figure 5C). These results demonstrate that BCL-XL is not required for the sustained survival and expansion of p53-deficient thymic lymphoma cells.
Inducible loss of MCL-1 abrogates the growth of p53−/− lymphomas in mice
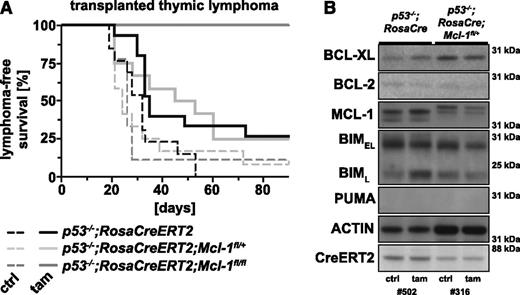
Because MCL-1 appears critical for the development of lymphoma elicited by loss of p53 (Figure 2), we examined the impact of acute loss of MCL-1 on the growth of p53−/− lymphomas in mice using the protocol outlined above and depicted in Figure 5A. Mice transplanted with p53−/−;RosaCreERT2;Mcl-1fl/+ lymphomas did not survive significantly longer (median survival, 49 days) after tamoxifen treatment (to delete 1 Mcl-1fl allele) compared with tamoxifen-treated recipients bearing p53−/−;RosaCreERT2 lymphomas (median survival, 35 days; P = .9831). In contrast, acute loss of both Mcl-1fl alleles in p53−/−;RosaCreERT2;Mcl-1fl/fl lymphomas significantly (P = .0002) prolonged the survival of mice transplanted with such tumors (Figure 6A). Remarkably, none (0/9 recipients, 3 independent thymic lymphomas) of the tamoxifen-treated recipients developed signs of lymphoma within 90 days following tumor transplantation, whereas all untreated control recipients bearing the same tumors rapidly developed lymphoma (median latency, 26 days; Figure 6A).
Acute loss of both alleles of Mcl-1 significantly prolongs survival of mice bearing p53−/− lymphomas. This experiment was conducted as described in Figure 5A. (A) Lymphoma-free survival of the mice that had been transplanted with lymphoma cells of the genotypes indicated and treated with either tamoxifen (solid line to delete the floxed Mcl-1 alleles) or that had been left untreated (dashed line; negative control). (B) Analysis of the proteins indicated by western blotting of transplanted thymic lymphoma samples of the indicated genotypes (representative of 3 samples each) that had grown in recipients treated with tamoxifen (tam) or recipients that had been left untreated (ctrl). Probing for actin was used as a loading control. Lanes 1 and 2 are the same samples as shown in lanes 1 and 2 of Figure 5C and are presented here for comparison.
Acute loss of both alleles of Mcl-1 significantly prolongs survival of mice bearing p53−/− lymphomas. This experiment was conducted as described in Figure 5A. (A) Lymphoma-free survival of the mice that had been transplanted with lymphoma cells of the genotypes indicated and treated with either tamoxifen (solid line to delete the floxed Mcl-1 alleles) or that had been left untreated (dashed line; negative control). (B) Analysis of the proteins indicated by western blotting of transplanted thymic lymphoma samples of the indicated genotypes (representative of 3 samples each) that had grown in recipients treated with tamoxifen (tam) or recipients that had been left untreated (ctrl). Probing for actin was used as a loading control. Lanes 1 and 2 are the same samples as shown in lanes 1 and 2 of Figure 5C and are presented here for comparison.
Mice transplanted with p53−/−;RosaCreERT2 or p53−/−;RosaCreERT2;Mcl-1fl/+ lymphomas had similar thymus, spleen, and liver weights and similar lymphoma dissemination in the abdomen regardless of whether they had been treated with tamoxifen (to delete 1 Mcl-1 allele in the latter tumors) or had been left untreated (supplemental Figure 6A-C; data not shown). In contrast, thymus (P = .0077) and spleen (P = .0347) but not liver weights (P = .1016) of mice transplanted with p53−/−;RosaCreERT2;Mcl-1fl/fl lymphomas treated with tamoxifen were significantly lower compared with those of untreated control recipients transplanted with the same lymphomas (supplemental Figure 6A-C). Notably, internal organs (eg, ovaries and pancreas) of tamoxifen-treated mice transplanted with p53−/−;RosaCreERT2;Mcl-1fl/fl lymphomas were of healthy appearance at the end point of the study (90-day lymphoma-free survival).
Western blot analysis showed that p53−/−;RosaCreERT2;Mcl-1fl/+ lymphomas that were isolated from recipients that had been treated with tamoxifen had slightly lower levels of MCL-1 compared with the same lymphoma collected from untreated control recipients (Figure 6B). This indicates that p53−/− lymphomas can survive and expand with a minor reduction in MCL-1 but not in the complete absence of this pro-survival protein. Collectively, these results demonstrate that MCL-1 is essential for the sustained survival and expansion of p53−/− thymic lymphomas.
Discussion
Mutations in p53 are found in ∼50% of sporadic cancers in humans and inheritance of heterozygous mutations in p53 results in the Li-Fraumeni cancer predisposition syndrome. Due to the critical role of p53 in DNA damage-induced apoptosis,32-34 tumors-bearing mutations in p53 are refractory to diverse anticancer therapeutics that elicit DNA lesions. With the intent to identify cancer vulnerabilities, we performed genetic studies to determine which pro-survival BCL-2 family member is critical for the development of lymphomas from nascent neoplastic cells in p53−/− mice and which one is essential for the sustained expansion of such malignant tumors. For this we used the p53-deficient (p53−/−) mouse model; these animals are highly predisposed to develop thymic lymphoma (∼80% incidence by ∼150 days in p53−/− mice), sarcoma, and certain other cancers with a 100% cancer-related mortality by ∼300 days.5,6,35
Loss of BCL-XL from the early TN3 T-cell progenitor stage (TCRβ−CD3−CD4−CD8−CD25+CD44+, using Lck-Cre to delete the floxed Bcl-x alleles) onward had no impact on thymic lymphoma development driven by loss of p53. This is consistent with our previous observation that combined inhibition of BCL-2, BCL-XL, and BCL-W with the BH3 mimetic ABT-737 did not delay tumor development in p53−/− mice,36 although antagonism of BCL-XL function was most likely not complete in that study. These results demonstrate that BCL-XL is dispensable for the survival of nascent neoplastic cells that give rise to thymic lymphoma (usually TCRβ+CD4+CD8+), although this pro-survival BCL-2 family member is critical for the survival of nontransformed TCR+CD4+CD8+ immature thymocytes.37 This suggests that the thymic lymphoma initiating cells in p53−/− mice are probably found in a more immature progenitor population (see below for further discussion).
Interestingly, loss of even a single Mcl-1 allele substantially reduced the incidence and delayed the onset of thymic lymphoma in p53−/− mice. Thus, the malignant lymphoma cells may be highly dependent on MCL-1 or the tumor initiating cells in p53−/− mice may rely on MCL-1 for their survival. The tumor initiating cells in p53−/− mice have not yet been identified, but in γ radiation-induced thymic lymphoma, the cell of origin is an immature progenitor (Lin−Sca-1+c-Kit+ cells or common lymphoid progenitor cells) in the bone marrow.38 Our data indicate that the cell of origin of lymphoma in p53−/− mice is probably not a hematopoietic stem/progenitor cell but a T-lineage committed progenitor. This would explain the potent selection against loss of MCL-1 during lymphoma development that occurred in all p53−/−;Lck-Cre;Mcl-1fl/fl and in ∼50% of p53−/−;Lck-Cre;Mcl-1fl/+ mice; note that Lck-Cre becomes competent at recombining floxed genes at the TN3 TCRβ−CD3−CD4−CD8−CD25+CD44+ T cell-committed progenitor stage. Notably, these cells require MCL-1 (but not BCL-XL) for their survival39 and are rapidly proliferating and thus subject to replication errors, a potential source of oncogenic lesions that could cooperate with loss of p53 in lymphomagenesis.
Previous studies showed that MCL-1 is critical for the development of AML driven by deregulated c-MYC19 or the MLL-ENL and AML-ETO fusion oncogenes20 and BCR-ABL-driven pre-B lymphoma development.40 Conversely, BCL-XL is required for c-MYC-driven pre-B/B lymphoma development.16 Thus, there may be a cell type-specific and/or oncogenic lesion-specific dependency on distinct pro-survival BCL-2 family members for the development of different hematopoietic malignancies.
Interestingly, the thymic lymphomas in p53−/−;Mcl-1+/−, p53−/−;Lck-Cre;Mcl-1fl/+ and p53−/−;Lck-Cre;Mcl-1fl/fl mice that expressed relatively low levels of MCL-1 (in the latter 2 due to recombination of 1 Mcl-1fl allele) generally had markedly reduced levels of proapoptotic BIM compared with the lymphomas of the same genotypes that had higher MCL-1 levels. This apparent selection for reduced BIM levels in tumors containing low MCL-1 levels suggests that preventing BIM-mediated apoptosis could be the crucial role of MCL-1 in the lymphoma initiating cells in p53−/− mice. How BIM is activated in lymphoma initiating cells is unclear but could be due to oncogene activation, replication stress or limited availability of growth factors, all of which initiate apoptosis in a BIM-dependent manner.41,42
The reduction in the incidence of thymic lymphoma in p53−/− mice with constitutive loss of 1 allele of Mcl-1 or T cell-restricted loss of 1 or both Mcl-1 alleles was accompanied by a concomitant increase in the incidence of sarcoma, a tumor that develops more slowly than the lymphomas in p53−/− mice.5,6 The latter 2 strains only allow investigation of the impact of T cell-specific loss of MCL-1. In these mice the precursors of the sarcomas are unaffected by Mcl-1fl recombination, and sarcoma may thus occur at a higher frequency because the mice survive longer due to the delay in thymic lymphoma development. However, in the p53−/−;Mcl-1+/− mice, all cells including those giving rise to sarcoma, lacked 1 allele of Mcl-1. In these mice, we observed a 2.6-fold increase in sarcoma accounting for a similar-fold decrease in thymic lymphoma incidence. This indicates that MCL-1 may be less critical for sarcoma development than for lymphomagenesis. Perhaps 1 or more other pro-survival BCL-2 family members guarantee the survival of the cells undergoing neoplastic changes to become sarcomas. Alternatively, evasion of apoptosis may be less critical for sarcoma development than for lymphomagenesis. In accordance with this hypothesis, it was shown that, although restoration of p53 caused apoptosis in p53−/− thymic lymphomas, this caused cellular senescence in p53−/− sarcomas.43
For the development of novel therapeutic strategies, it is essential to know which pro-survival BCL-2 family member is critical for the sustained survival and expansion of a particular tumor. This can be investigated by using specific drugs (if they exist, such as ABT-199 to inhibit BCL-2) or by conditional deletion of pro-survival Bcl-2 family genes in malignant tumors by using the tamoxifen-regulated CreERT2 recombinase. By performing tumor transplant experiments and then inducing acute deletion of either Mcl-1 or Bcl-x floxed alleles, we found that MCL-1 but not BCL-XL is critical for sustained survival and expansion of p53-deficient thymic lymphomas. This is reminiscent of previous reports, which showed that MCL-1 is also critical for the sustained growth of AML driven by various fusion oncogenes,19,20 as well as c-MYC-driven pre-B/B lymphoma.31 Collectively, these observations indicate that the transformed state driven by diverse oncogenic lesions (eg, deregulated MYC, loss of p53, and MLL-ENL) renders a broad range of malignant hematopoietic cells highly dependent on MCL-1. The reasons for this are presently not clear. Differences in expression of different pro-survival BCL-2 family members does not appear to be the answer, because the aforementioned tumors all express readily detectable levels of not only MCL-1 but also other pro-survival BCL-2 family members. These considerations are important to decide which cancers should be treated with which type of BH3 mimetic drugs. Although BCL-2 inhibition alone (using ABT-199) shows promise in clinical trials of chronic lymphocytic leukemia (CLL),44 our data here suggest that MCL-1 blockade (if this does not cause unacceptable collateral damage) may be the treatment of choice for T-cell lymphomas, particularly those containing defects in the p53 pathway.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs M. J. Herold, G. L. Kelly, and D. H. Gray for advice, P. Bouillet and L. A. O’Reilly for reagents, J. Mansheim, C. Gatt, L. Reid, S. Allan, K. Landells, and G. Siciliano for expert animal care, B. Helbert, H. Ierino, K. Mackwell, and C. Young for genotyping, and J. Corbin for automated blood analysis.
This work was supported by grants and fellowships from the Cancer Council of Victoria (postdoctoral fellowship to S.G., Sydney Parker Smith postdoctoral research fellowship to A.R.D.D., and postgraduate research scholarship to L.J.V.), the Lady Tata Memorial Trust (postdoctoral research award to S.G.), National Health and Medical Research Council (program grant 1016701; National Health and Medical Research Council fellowship 1020363 (to A.S.), the Leukemia and Lymphoma Society (Specialized Centres of Research grant 7001-03, to A.S.), University of Melbourne International Research Scholarship (to S.G.), University of Melbourne International Fee Remission scholarship (to S.G.), Australian Postgraduate award (to A.R.D.D.), Cancer Therapeutics CRC Top-up scholarship (to S.G. and A.R.D.D.), and the operational infrastructure grants through the Australian Government Independent Research Institutes Infrastructure Support Scheme and the Victorian State Government Operational Infrastructure Support.
Authorship
Contribution: S.G. conceived ideas, planned and conducted the majority of experiments, and wrote the manuscript; A.R.D.D. and L.J.V. helped with some experiments and manuscript writing; and A.S. provided experimental ideas and intellectual guidance and helped with manuscript writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andreas Strasser, The Walter and Eliza Hall Institute, 1G Royal Parade, Parkville, 3052 VIC, Australia; e-mail: strasser@wehi.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal