Abstract

Point mutations in isocitrate dehydrogenase (IDH) define distinct subsets of acute myelogenous leukemia (AML). IDH is a metabolic enzyme that interconverts isocitrate and α-ketoglutarate (α-KG), but cancer-associated point mutations in IDH1 and IDH2 confer a neomorphic activity that allows reduction of α-KG to the oncometabolite R-2-hydroxyglutarate (2-HG). High levels of 2-HG have been shown to inhibit α-KG-dependent dioxygenases including histone and DNA demethylases, which play a key role in regulating the epigenetic state of cells, but the relationship between 2-HG and oncogenesis is not completely understood. Consistent with 2-HG promoting cancer via an effect on chromatin structure, patients harboring IDH mutations display a CpG island methylator phenotype (CIMP) and several studies have shown that overexpression of IDH mutant enzymes can induce histone and DNA hypermethylation as well as block cellular differentiation. In addition, mice engineered to express IDH1-R132H in hematopoietic tissue have increased early hematopoietic progenitors, splenomegaly, anemia, hypermethylated histones and altered DNA methylation patterns similar to those found in AML patients harboring IDH1/2 mutations.[i] Taken together, these data suggest that cancer-associated IDH mutations may induce a block in cellular differentiation to promote tumorigenesis.
To investigate whether selective pharmacological inhibition of the mutant IDH1 enzyme could provide an effective way to lower intracellular 2-HG levels and restore normal differentiation, we treated TF-1 cells or primary human AML patient samples expressing mutant IDH1 with AG-120, an oral, selective, first-in-class, potent IDH1 mutant inhibitor currently in phase I clinical trials. Treatment with AG-120 decreased intracellular 2-HG levels, inhibited growth factor independent proliferation and restored erythropoietin (EPO)-induced differentiation in TF-1 IDH1-R132H cells. Similarly, pharmacological inhibition of mutant IDH1 enzyme with AG-120 in primary human blast cells cultured ex vivo provided an effective way to lower intracellular 2-HG levels and induced myeloid differentiation. Taken together, these data demonstrate that AG-120 is effective at lowering 2-HG levels and restoring cellular differentiation, and support further clinical development of this compound.
Diagnosis and karyotypes of primary AML patient samples used in ex vivo studies
Diagnosis and karyotypes of primary AML patient samples used in ex vivo studies
PB = peripheral blood, BM = bone marrow
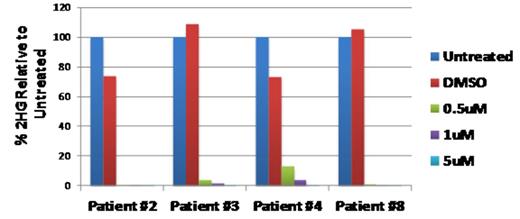
Percent 2-HG remaining relative to DMSO control after 6-day treatment with AG-120 in IDH1 R132H or IDH1 R132C patient samples
Percent 2-HG remaining relative to DMSO control after 6-day treatment with AG-120 in IDH1 R132H or IDH1 R132C patient samples
or following 6 days of treatment with control (DMSO) or AG-120 (0.5, 1.0, and 5.0 μM)
Relative proportion of cell types in human AML bone marrow samples untreated
Relative proportion of cell types in human AML bone marrow samples untreated
[i] M. Sasaki et al., IDH1(R132H) mutation increases murine haematopoietic progenitors and alters epigenetics. Nature 488(7413):656-9, 2012.
Hansen:Agios Pharmaceuticals: Employment, Stockholder Other. Quivoron:Institut National de la Santé Et de la Recherche Médicale (INSERM): Grant Other; Association Laurette Fugain: Grant, Grant Other; Institut National du Cancer (INCa): Grant, Grant Other; Association pour la recherche contre le Cancer (ARC): Grant, Grant Other; AGIOS: Grant Other. Straley:Agios Pharmaceuticals: Employment, Stockholder Other. Lemieux:Agios Pharmaceuticals: Employment, Stockholder Other, US20130190249 (pending) Patents & Royalties. Popovici-Muller:Agios Pharmaceuticals: Employment, Stockholder Other. Fathi:Agios Pharmaceuticals: Advisory board participation Other. Gliser:Agios Pharmaceuticals: Employment, Stockholder Other. David:Institut National de la Santé Et de la Recherche Médicale (INSERM): Grant Other; Institut National du Cancer (INCa): Grant, Grant Other; Association pour la Recherche contre le Cancer (ARC): Grant, Grant Other; Association Laurette Fugain: Grant, Grant Other; AGIOS: Grant Other. Bernard:Institut National de la Santé Et de la Recherche Médicale (INSERM): Grant Other; Association Laurette Fugain: Grant, Grant Other; Institut National du Cancer (INCa): Grant, Grant Other; Ligue Nationale contre le cancer (LNCC): Grant, Grant Other; AGIOS: Grant Other. Dorsch:Agios Pharmaceuticals: Employment, Stockholder Other. Yang:Agios Pharmaceuticals: Employment, Stockholder Other. Su:Agios Pharmaceuticals: Employment, Stockholder Other. Agresta:Agios Pharmaceuticals: Employment, Stockholder Other. de Botton:AGIOS: Grant Other. Penard-Lacronique:Institut National de la Santé Et de la Recherche Médicale (INSERM): Grant Other; Association Laurette Fugain: Grant, Grant Other; Institut National du Cancer (INCa): Grant, Grant Other; Association pour la recherche contre le Cancer (ARC): Grant, Grant Other; AGIOS: Grant Other. Yen:Agios Pharmaceuticals: Employment, Stockholder Other.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal